Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.

Note that in these calculations, the fractions have been rounded o¤ to the
nearest percent. This can pose problems if we were to calculate masses of dif-
ferent phases, in that you may not be able to preserve mass balance. It is usu-
ally a good idea not to round these percentages if you are going to perform
calculations concerning amounts of di¤erent phases or masses of elements in
di¤erent phases.
We call the solid a phase that forms when the liquid cooled from the liquidus to the
eutectic the primary or proeutectic microconstituent. This solid a did not take part in the
eutectic reaction. Thus, the morphology and appearance of this proeutectic a phase is
very distinct from that of the a phase that appears in the eutectic microconstituent. Of-
ten we find that the amounts and compositions of the microconstituents are of more use
to us than the amounts and compositions of the phases.
EXAMPLE 11-5
Microconstituent Amount and Composition for a Hypoeutectic
Alloy
Determine the amounts and compositions of each microconstituent in a Pb-30%
Sn alloy immediately after the eutectic reaction has been completed.
SOLUTION
This is a hypoeutectic composition. Therefore, the microconstituents expected
are primary a and eutectic. Note that we still have only two phases (a and b).
We can determine the amounts and compositions of the microconstituents if
we look at how they form. The primary a microconstituent is all of the solid
a that forms before the alloy cools to the eutectic temperature; the eutectic
microconstituent is all of the liquid that goes through the eutectic reaction. At
a temperature just above the eutectic—say, 184
C—the amounts and compo-
sitions of the two phases are:
a: 19% Sn % a ¼
61:9 30
61:9 19
100 ¼ 74% ¼ % primary a
L:61:9% Sn % L ¼
30 19
61:9 19
100 ¼ 26% ¼ % eutectic at 182
C
Thus, the primary alpha microconstituent is obtained by determining the
amount of a present at the temperature just above the eutectic. The amount of
eutectic microconstituent at a temperature just below the eutectic (e.g., 182
C)
is determined by calculating the amount of liquid just abov e the eutectic tem-
perature (e.g., at 184
C), since all of this liquid of eutectic composition is
transformed into the eutectic microconstituent. Note that at the eutectic tem-
perature (183
C) the eutectic reaction is in progress (formation of the pro-
eutectic a is complete); hence, the amount of the eutectic microconstituent at
183
C will change with time (starting at 0% and ending at 26% eutectic, in this
case). Please be certain that you understand this example since many students
tend to miss how this calculation is performed.
When the alloy cools below the eutectic to 182
C, all of the liquid at 184
C
transforms to eutectic and the composition of the eutectic microconstituent is
61.9% Sn. The solid a phase present at 184
C remains unchanged after cooling
to 182
C and is the primary microconstituent.
C H A P TE R 1 1 Dispersion Strengthening and Eutectic Phase Diagrams340
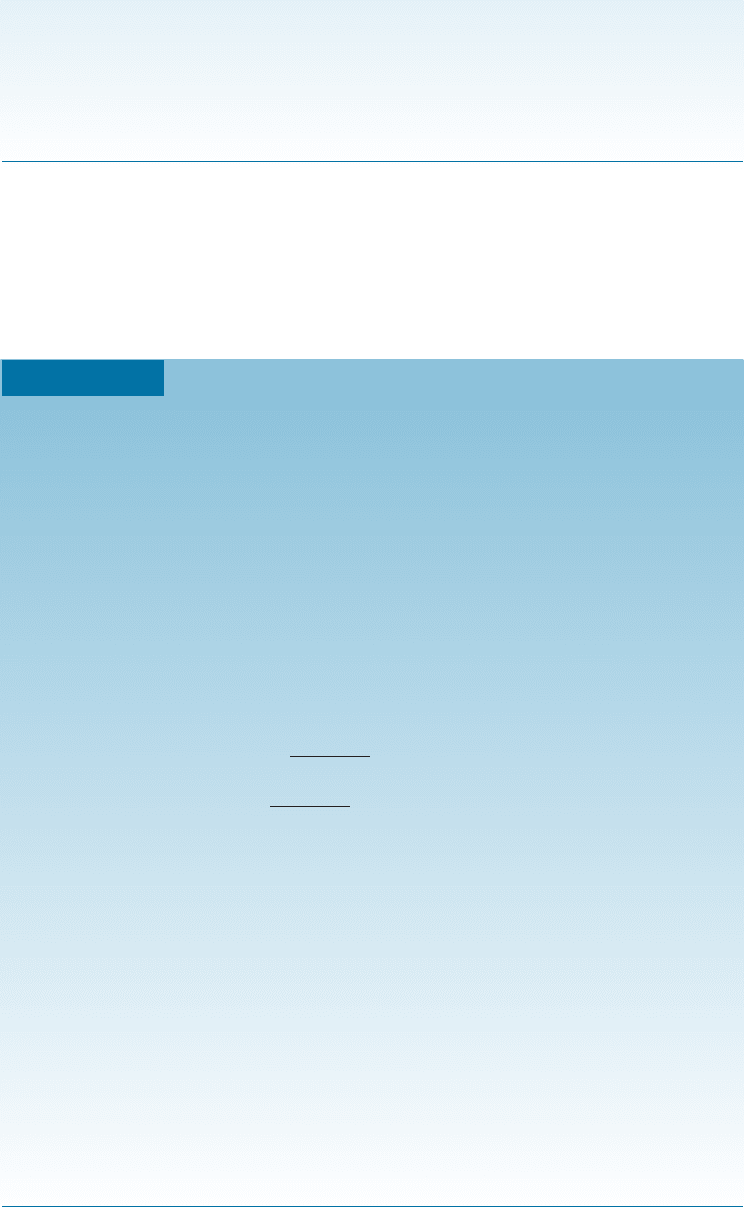
The cooling curve for a hypoeutectic alloy is a composite of those for solid-solution
alloys and ‘‘straight’’ eutectic alloys (Figure 11-15). A change in slope occurs at the
liquidus as primary a beg ins to form. Evolution of the latent heat of fusion slows the
cooling rate as the solid a grows. When the alloy cools to the eutectic temperature, a
thermal arrest is produced as the eutectic reaction proceeds at 183
C. The solidifica-
tion sequence is similar in a hypereutectic alloy, giving the microstructure shown in
Figure 11-14(b).
11-5 Strength of Eutectic Alloys
Each phase in the eutectic alloy is, to some degree, solid-solution strengthened. In the
lead-tin system , a, which is a solid solution of tin in lead, is stronger than pure lead
(Chapter 10). Some eutectic alloys can be strengthened by cold working. We also con-
trol grain size by adding appropriate inoculants or grain refiners during solidification.
Finally, we can influence the properties by controlling the amount and microstructure
of the eutectic.
Eutectic Colony Size Eutectic colonies, or grains, each nucleate and grow indepen-
dently. Within each colony, the orientation of the lamellae in the eutectic microcon-
stituent is identical. The orientatio n changes on crossing a colony boundary [Figure
11-16(a)]. We can refine the eutectic colonies and improve the strength of the eutectic
alloy by inoculation (Chapter 9).
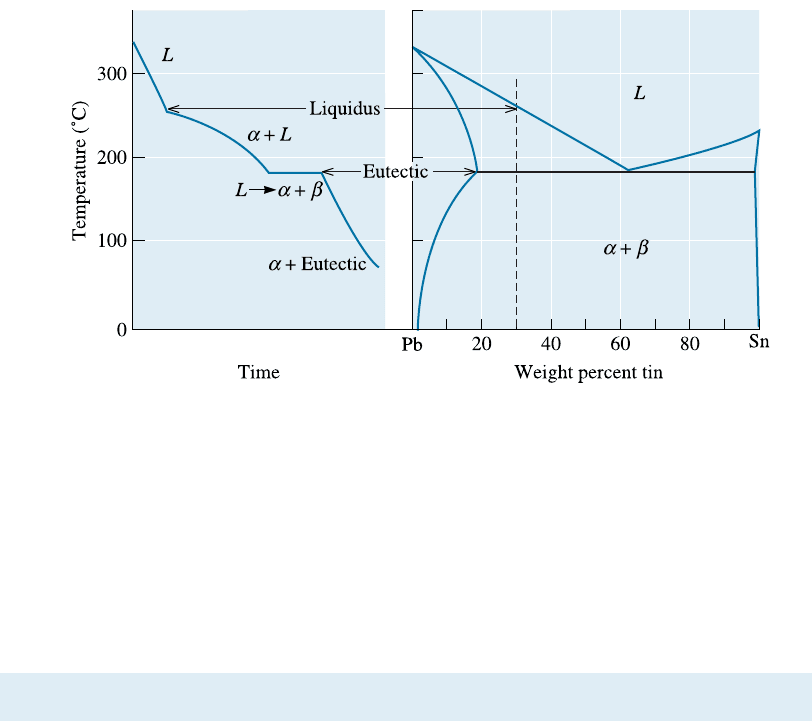
Interlamellar Spacing The interlamellar spacing of a eutectic is the distance from the
center of one a lamella to the center of the next a lamella [Figure 11-16(b)]. A small
interlamellar spacing indicates that the amount of a–b interfac e area is large. A small
interlamellar spacing therefore increases the strength of the eutectic.
The interlamellar spacing ðlÞ is determined primarily by the growth rate of the
eutectic,
l ¼ cR
1=2
ð11-2Þ
Figure 11-15 The cooling curve for a hypoeutectic Pb-30% Sn alloy.
11-5 Strength of Eutectic Alloys 341
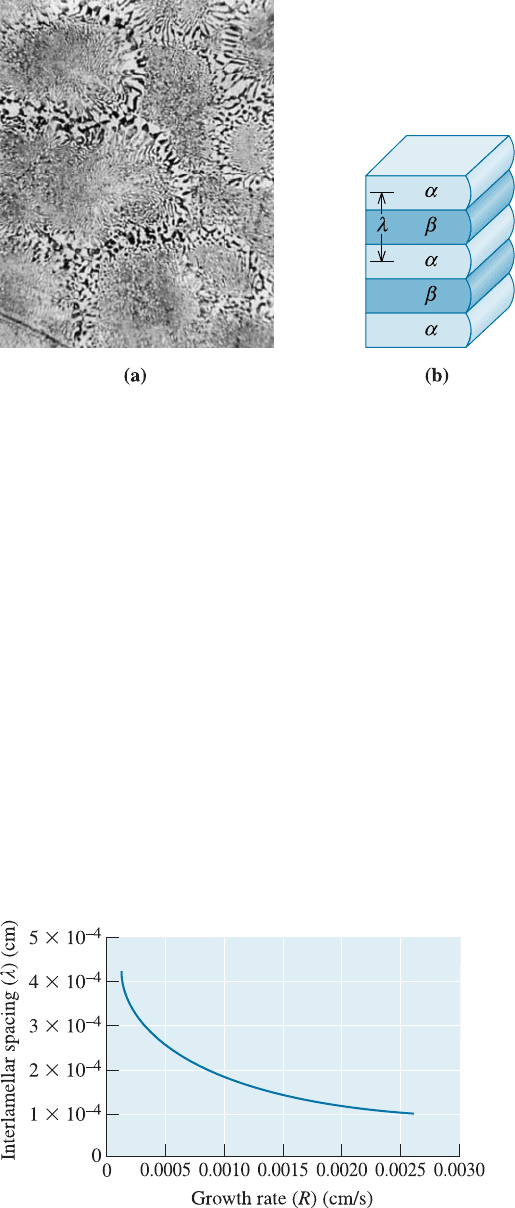
where R is the growth rate (cm/s) and c is a constant. The interlamellar spacing for the
lead-tin eutectic is shown in Figure 11-17. We can increase the growth rate R, and
consequently reduce the interlamellar spacing, by increasing the cooling rate or re-
ducing the solidification time.
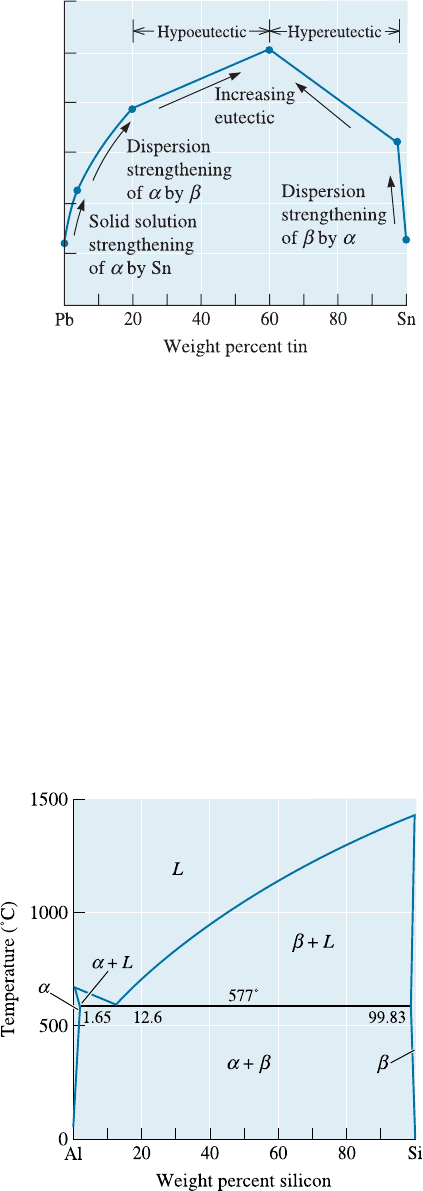
Amount of Eutectic We also control the properties by the relative amounts of the
primary microconstituent and the eutectic. In the lead-tin system, the amount of the
eutectic microconstituent changes from 0% to 100% when the tin content increases from
19% to 61.9%. With increasing amounts of the stronger eutectic microconstituent, the
strength of the alloy increases (Figure 11-18). Similarly, when we increase the lead
added to tin from 2.5% to 38.1% Pb, the amo unt of primar y b in the hypereutectic alloy
decreases, the amount of the strong eutectic increases, and the strength increases. When
both individual phases have about the same strength, the eutectic alloy is expected to
have the highest strength due to e¤ective dispersion strengthening.
Microstructure of the Eutectic Not all eutectics give a lamellar structure. The shapes
of the two phases in the microconstituent are influenced by the cooling rate, the pres-
ence of impurity elements, and the nature of the alloy.
Figure 11-16
(a) Colonies in the lead-
tin eutectic (300).
(b) The interlamellar
spacing in a eutectic
microstructure.
Figure 11-17
The effect of growth rate ðRÞ
on the interlamellar spacing
ðlÞ in the lead-tin eutectic.
C H A P TE R 1 1 Dispersion Strengthening and Eutectic Phase Diagrams342
The aluminum-silicon eutectic phase diagram (Figure 11-19) forms the basis for a
number of important commercial alloys. However, the silicon portion of the eutectic
grows as thin, flat plates that appear needle-like in a photomicrograph [Figure 11-20(a)].
The brittle silicon platelets concentrate stresses and reduce ductility and toughness.
The eutectic microstructure in aluminum-silicon alloys is altered by a process
known as modification. Modification causes the silicon phase to grow as thin, inter-
connected rods betwee n aluminum dendrites [Figure 11-20(b)], improving both tensile
strength and % elongation. In two dimensions, the modified silicon appears to be com-
posed of tiny, round particles. Rapidly cooled alloys, such as those in die casting, are
naturally modified during solidification. At slower cooling rates, however, about 0.02%
Na or 0.01% Sr must be added to cause modification.
10
20
30
40
50
60
Tensile strength (MPa)
0
Figure 11-18
The effect of the composition and
strengthening mechanism on the
tensile strength of lead-tin alloys.
Figure 11-19
The aluminum-silicon phase
diagram.
11-5 Strength of Eutectic Alloys 343

The shape of the primary phase is also important. Often the primary phase grows
in a dendritic manner; decreasing the secondary dendrite arm spacing of the primary
phase may improve the properties of the alloy. However, in hypereutectic aluminum-
silicon alloys, coarse b is the primary phase [Figure 11-21(a)]. Bec ause b phase is hard,
the hypereutectic alloys are wear-resistant and are used to produce automotive engine
parts. However, the coarse b causes poor machinability and gravity segregation (where
the primary b floats to the surface of the casting during freezing). Addition of 0.05%
phosphorus (P) encourages nucleation of primary silicon, refines its size, and minimizes
its deleterious qualities [Figure 11-21(b)]. The two examples following show how eu-
tectic compositions can be designed to achieve certain levels of mechanical properties.
Figure 11-20 Typical eutectic microstructures: (a) needle-like silicon plates in the aluminum-
silicon eutectic (100), and (b) rounded silicon rods in the modified aluminum-silicon eutectic
(100).
Figure 11-21 The effect of hardening with phosphorus on the microstructure of hypereutectic
aluminum-silicon alloys: (a) coarse primary silicon, and (b) fine primary silicon, as refined by
phosphorus addition (75). (From ASM Handbook, Vol. 7, (1972), ASM International,
Materials Park, OH 44073.)
C H A P TE R 1 1 Dispersion Strengthening and Eutectic Phase Diagrams344

EXAMPLE 11-6 Design of Materials for a Wiping Solder
One way to repair dents in a metal is to wipe a partly liquid-partly solid mate-
rial into the dent, then allow this filler material to solidify. For our application,
the wiping material should have the following specifications: (1) a melting tem-
perature below 230
C, (2) a tensile strength in excess of 41 MPa, (3) be 60% to
70% liquid during application, and (4) the lowest possible cost. Design an alloy
and repair procedure that will meet these specifications.
SOLUTION
Let’s see if one of the Pb-Sn alloys will satisfy these conditions. First, the alloy
must contain more than 40% Sn in order to have a melting temperature below
230
C (Figure 11-6). This low temperature will make it easier for the person
doing the repairs to apply the filler.
Second, Figure 11-18 indicates that the tin content must lie between 23%
and 80% to achieve the required 41 MPa tensile strength. In combination with
the first requirement, any alloy containing between 40 and 80% Sn will be sat-
isfactory.
Third, the cost of tin is about $5500/ton whereas that of lead is $550/ton.
Thus, an alloy of Pb-40% Sn might be the most economical choice. There are
other considerations, as well, such as: What is the geometry? Can the alloy flow
well under that geometry (i.e., the viscosity of the molten metal)?
Finally, the filler material must be at the correct temperature in order to be
60% to 70% liquid. As the calculations below show, the temperature must be
between 200
C and 210
C:
% L
200
¼
40 18
55 18
100 ¼ 60%
% L
210
¼
40 17
50 17
100 ¼ 70%
Our recommendation, therefore, is to use a Pb-40% Sn alloy applied at 205
C,
a temperature at which there will be 65% liquid and 35% primary a. As men-
tioned before, we should also pay attention to the toxicity of lead and any legal
liabilities the use of such materials may cause. A number of new lead free sol-
ders have been developed.
EXAMPLE 11-7
Design of a Wear-Resistant Part
Design a lightweight, cylindrical component that will provide excellent wear-
resistance at the inner wall, yet still have reasonable ductility and toughness
overall. Such a product might be used as a cylinder liner in an automotive
engine.
11-5 Strength of Eutectic Alloys 345
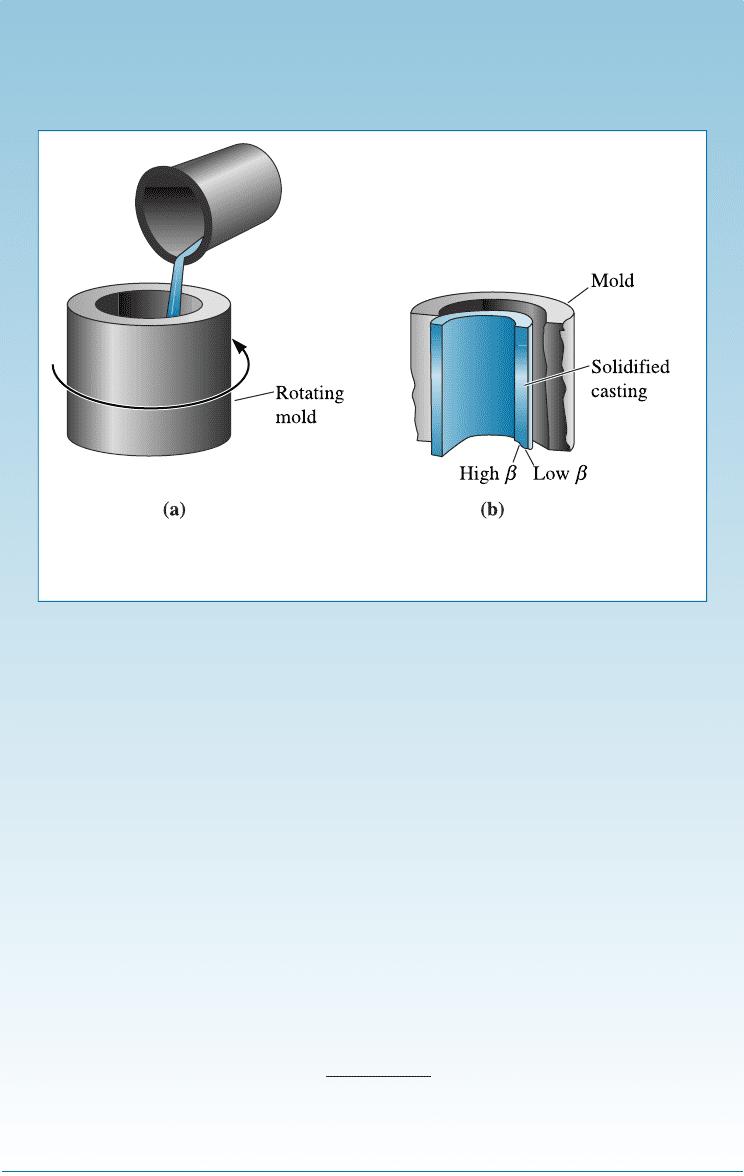
SOLUTION
Many wear-resistant parts are produced from steels, which have a relatively
high density, but the hypereutectic Al-Si alloys containing primary b may
provide the wear-resistance that we wish at one-third the weight of the steel.
Since the part to be produced is cylindrical in shape, centrifugal casting
(Figure 11-22) might be a unique method for producing it. In centrifugal cast-
ing, liquid metal is poured into a rotating mold and the centrifugal force pro-
duces a hollow shape. In addition, material that has a high density is spun to
the outside wall of the casting, while material that has a lower density than the
liquid migrates to the inner wall.
When we centrifugally cast a hypereutectic Al-Si alloy, primary b nucleates
and grows. The density of the b phase (if we assume it to be same as that of pure
Si) is, according to Appendix A, 2.33 g/cm
3
, compared with a density near 2.7
g/cm
3
for aluminum. As the primary b particles precipitate from the liquid,
they are spun to the inner surface. The result is a casting that is composed of
eutectic microconstituent (with reasonable ductility) at the outer wall and a hy-
pereutectic composition, containing large amounts of primary b , at the inner
wall.
A typical al loy used to produce aluminum engine components is Al-17% Si.
From Figure 11-19, the total amount of primary b that can form is calculated at
578
C, just above the eutectic temperature:
% Primary b ¼
17 12:6
99:83 12:6
100 ¼ 5: 0%
Although only 5.0% primary b is expected to form, the centrifugal action can
double or triple the amount of b at the inner wall of the casting.
Figure 11-22 Centrifugal casting of a hypereutectic Al-Si alloy: (a) Liquid alloy is
poured into a rotating mold, and (b) the solidified casting is hypereutectic at the
inner diameter and eutectic at the outer diameter (for Example 11-7).
C H A P TE R 1 1 Dispersion Strengthening and Eutectic Phase Diagrams346

11-6 Eutectics and Materials Processing
Several manufacturing processes take advantage of the low melting temperature asso-
ciated with the eutectic reaction. The Pb-Sn alloys are the basis for a series of alloys
used to produce filler materials for soldering (Chapter 9). If, for exam ple, we wish to
join copper pipe, individual segments can be joined by introducing the low-melting-
point eutectic Pb-Sn alloy into the joint [Figure 11-23(a)]. The copper is heated just
above the eutectic temperature. The heated copper melts the Pb-Sn alloy, which is then
drawn into the thin gap by capillary action. When the Pb-Sn alloy cools and solidifies,
the copper is joined. The prospects of corrosion of such pipes and the introduction of
lead (Pb) into water must also be factored in.
Many casting alloys are also based on eutectic alloys. Liquid can be melted and
solidified into a mold at low and at constant temperatures, reducing energy costs in-
volved in melting, minimizing casting defects such as gas porosity, and preventing liq-
uid metal-mold reactions. Cast iron and many aluminum alloys are based on eutectic
compositions.
Although most of this discussion has been centered around metallic materials, it is
important to recognize that the eutectics are very important in many ceramic systems as
well (Chapter 15). Formation of eutectics played a role in the successful formation of
glass-like materials known as the Egyptian faience. The sands of the Nile River Valley
contained appreciable amounts of limestone (CaCO
3
). Plant ash contains considerable
amounts of potassium oxide and sodium oxide and is used to cause the sand to melt at
lower temperatures by the formation of the eutectics.
Silica and alumina are the most widely used ceramic materials. Figure 11-23(b)
shows a phase diagram for the Al
2
O
3
-SiO
2
. Notice the eutectic at @1587
C. The
dashed lines on this diagram show metastable extensions of the liquidus and metastable
miscibility gaps. As mentioned before, the existence of these gaps makes it possible to
make technologically useful products such as the Vycor
TM
and the Pyrex
8
glasses. A
Vycor
TM
glass is made by first melting (approximately at 1500
C) silica (63%), boron
oxide (27%), sodium oxide (7%), and alumina (3%). The glass is then formed into de-
sired shapes. During glass formation, the glass has phase separated (because of the
metastable miscibility gap) into boron oxide rich and silica rich regions. The boron
oxide rich regions are dissolved using an acid. The porous object is sintered to form
Vycor
TM
glass that contains 95% silica, 4% boron oxide, and 1% sodium oxide. It
would be very di‰cult to achieve a high silica glass such as this without resorting to the
technique described above. Pyrex
8
contains about 80% silica, 13% boron oxide, 4%
sodium oxide, 2% alumina. These are used widely in making laboratory ware (i.e.,
beakers, etc.) and household products.
Figure 11-23(c) shows a binary phase diagram for the CaO-SiO
2
system. Compo-
sitions known as the E-glass or S-glass are used to make the fibers that go into fiber-
reinforced plastics. These glasses are made by melting silica sand, limestone, boric acid
at about 1260
C. The glass is then drawn into fibers. The E-glass (the letter ‘‘E’’ stands
for ‘‘electrical’’, as the glass was originally made for electrical insulation) contains ap-
proximately 52–56 wt.% silica, 12–16% Al
2
O
3
, 5–10% B
2
O
3
, 0–5% MgO, 0–2% Na
2
O,
0–2% K
2
O. The ‘‘S’’-glass (the letter ‘‘S’’ represents ‘‘strength’’) contains approximately
65 wt.% silica, 12–25% Al
2
O
3
, 10% MgO, 0–2% Na
2
O, 0–2% K
2
O.
11-6 Eutectics and Materials Processing 347
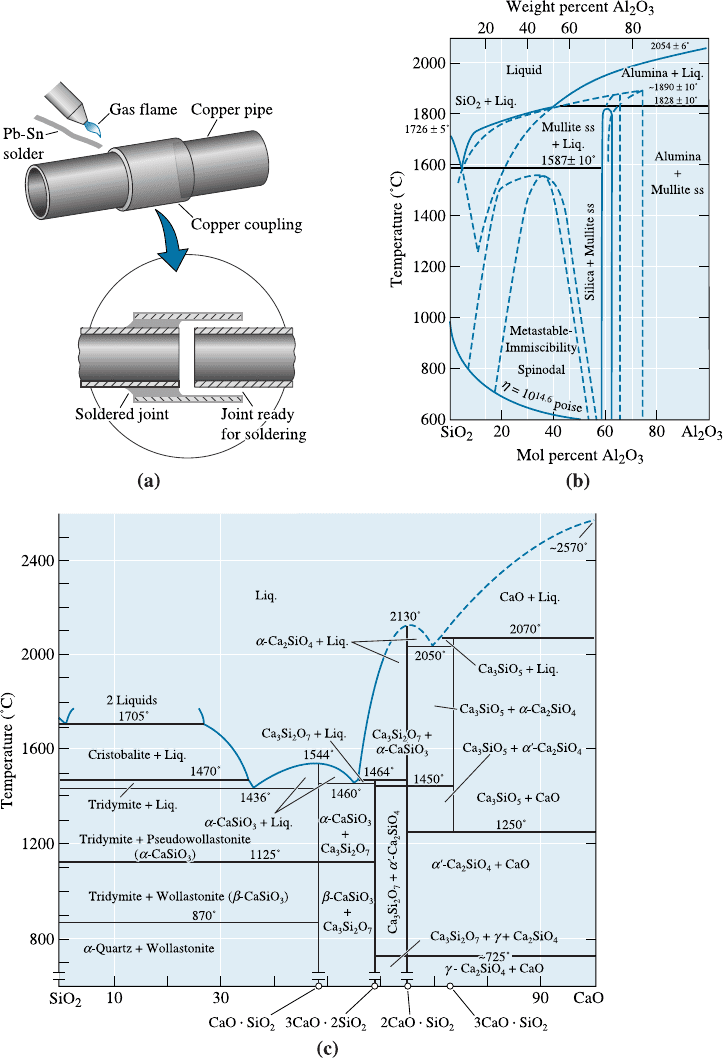
Figure 11-23 (a) A Pb-Sn eutectic alloy is often used during soldering to assemble parts.
A heat source, such as a gas flame, heats both the parts and the filler material. The filler
is drawn into the joint and solidifies. (b) A phase diagram for Al
2
O
3
-SiO
2
. (Adapted from
Introduction to Phase Equilibria in Ceramics, by Bergeron, C.G. and Risbud, S.H., The
American Ceramic Society, Inc., 1984, page 44.) (c) A phase diagram for the CaO-SiO
2
system. (Source: Adapted from Introduction to Phase Equilibria, by C.G. Bergeron and S.H.
Risbud, pp. 44 and 45, Figs. 3-36 and 3-37. Copyright > 1984 American Ceramic Society.)
C H A P TE R 1 1 Dispersion Strengthening and Eutectic Phase Diagrams348
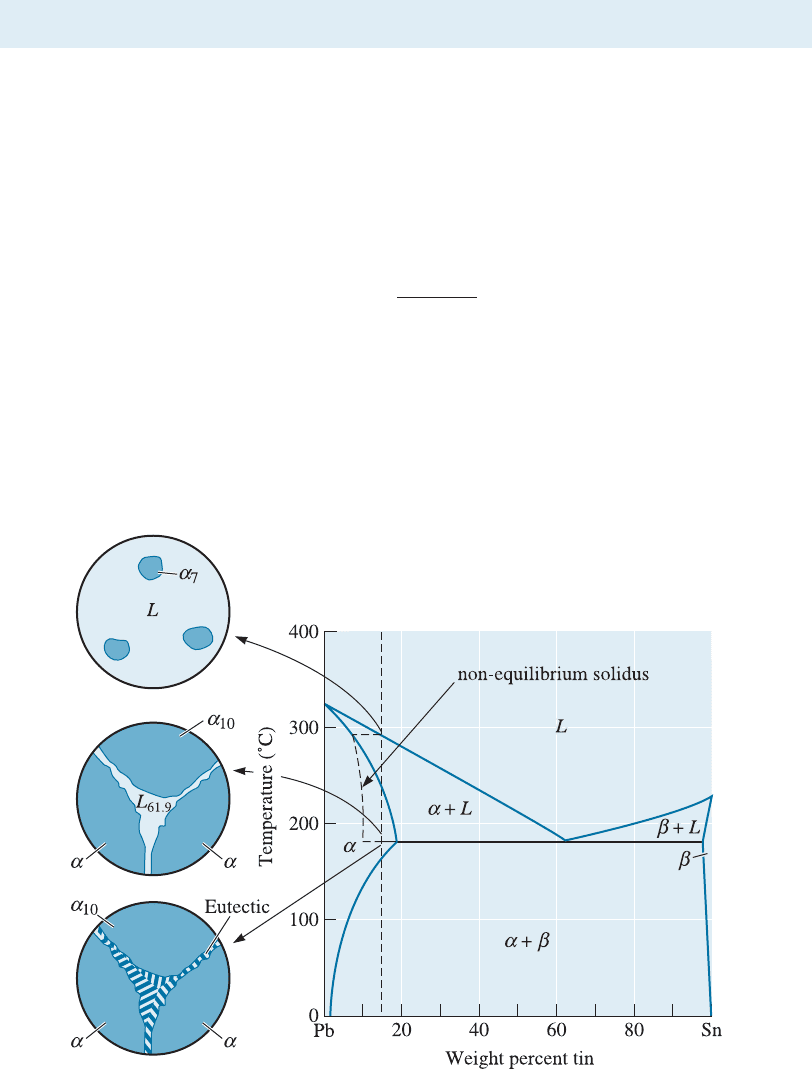
11-7 Nonequilibrium Freezing in the Eutectic System
Suppose we have an alloy, such as Pb-15% Sn, that ordinarily solidifies as a solid solution
alloy. The last liquid should freeze near 230
C, well above the eutectic. However, if the
alloy cools too quickly, a nonequilibrium solidus curve is produced (Figure 11-24). The
primary a continues to grow until, just above 183
C, the remaining nonequilibrium
liquid contains 61.9% Sn. This liquid then transforms to the eutectic microconstituent,
surrounding the primary a. For the conditions shown in Figure 11-24, the amount of
nonequilibrium eutectic is:
% eutectic ¼
15 10
61:9 10
100 ¼ 9: 6%
When heat treating an alloy such as Pb-15% Sn, we must keep the maximum temper-
ature below the eutectic temperature of 183
C to prevent hot shortness or partial melt-
ing. This concept is very important in the precipitation, or age, hardening of metallic
alloys such as those in the Al-Cu system.
Figure 11-24 Nonequilibrium solidification and microstructure of a Pb-15% Sn alloy.
A nonequilibrium eutectic microconstituent can form if the solidification is too rapid.
11-7 Nonequilibrium Freezing in the Eutectic System 349
