Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.


DG ¼
4
3
pr
3
DG
v
þ 4pr
2
s
sl
ð9-1Þ
where
4
3
pr
3
is the volume of a spherical solid of radius r,4pr
2
is the surface area of a
spherical solid, s
sl
is the surface free energy of the solid-liquid interface (in this case),
and DG
v
is the free energy change for the solidification process per unit volume, which
is a negative since the phase transformation is assumed to be therm odynamically fea-
sible. Note that s
sl
is not a strong function of r and is assumed constant. The DG
v
also
does not depend on r.
An embryo is a tiny particle of solid that forms from the liquid as atoms cluster
together. The embryo is unstable, and may either grow into a stable nucleus or redis-
solve back into the liquid.
In Figure 9-3, the top curve shows the parabolic variation of the total surface en-
ergy ð 4pr
2
s
sl
Þ. The bottom most curve shows the total volume free-energy change
term ð
4
3
pr
3
DG
v
Þ. The curve in the middle shows the variation of DG. At the temper-
ature where the solid and liquid phases are predicted to be in thermodynamic equili-
brium (i.e., at the freezing temperature), the free energy of the solid phase and that of the
Figure 9-2
An interface is created when a solid forms
from the liquid.
Figure 9-3
The total free energy of the solid-
liquid system changes with the size
of the solid. The solid is an embryo
if its radius is less than the critical
radius, and is a nucleus if its radius
is greater than the critical radius.
C H A P TE R 9 Principles and Applications of Solidification260

liquid phase are equal ( DG
v
¼ 0), so the total free energy change (DG) will be positive.
When the solid is very small, with a radius less than the critical radius for nucleation (r
)
(Figure 9-3), further growth causes the total free energy to increase. The critical radius
(r
) is the minimum size of a crystal that must be formed by atoms clustering together in
the liquid before the solid particle is stable and begins to grow. But instead of growing,
the solid has a tendency to remelt, causing the free energy to decrease; thus, the bulk of
the material remains liquid leaving just a small crystal solid. At freezing temperatures,
embryos are thermodynamically unstable. So how can they grow?
The formation of embryos is a statistical process. Many embryos form and redis-
solve. If by chance, an embryo forms which has a radius that is larger than r
, further
growth causes the total free energy to decrease. The new solid is then stable and sus-
tainable since nucleation has occurred, and growth of the solid particle—which is now
called a nucleus—begins. At the thermodynamic freezing temperature, the probability
of forming stable, sustainable nuclei is extremely small. Therefore, solidification does
not begin at the thermodynamic melting or freezing temperature. If the temperature
continues to decrease below the equilibrium freezing temperature, the liquid phase that
should have transformed into a solid becomes increasingly unstable thermodynamically
speaking. Because the liquid is below the equilibrium freezing temperature, the liquid is
considered undercooled. The undercooling of DT is the equilibrium freezing temper-
ature minus the actual temperature of the liquid . As the extent of undercooling in-
creases, the thermodynamic driving force for the formation of a solid phase from liquid
overtakes the resistance to create a solid-liquid interface.
This phenomenon can be seen in many other phase transformations. When one
solid phase (a) transforms into another solid phase (b), the system has to be cooled to a
temperature that is below the thermodynamic phase transformation temperature (at
which free energies of the a and b phases are equal). When a liquid is transformed into
a vapor, a bubble of vapor is created in the liquid. In order to create the trans -
formation, though, we need to superheat the liquid above its boiling temperature!
Therefore, we can see that liquids do not really freeze at t heir freezing temperatur e and
do not really boil at their boiling point! We need to undercool the liquid for it to solid-
ify and superheat it for it to boil!
Homogeneous Nucleation As liquid cools to temperatures below the equilibrium
freezing temperature, two factors combine to favor nucleation. First, since atoms are
losing their thermal energy the probability of forming clusters to form larger embryos
increases. Second, the larger volume free energy di¤erence between the liquid and the
solid reduces the critical size (r
) of the nucleus. Homogeneous nucleation occurs when
the undercooling becomes large enough to cause the formation of a stable nucleus.
The critical radius r
is given by
r
¼
2s
sl
T
m
DH
f
DT
ð9-2Þ
where DH
f
is the latent heat of fusion, T
m
is the equilibrium solidification temperature
in Kelvin, and D T ¼ðT
m
TÞ is the undercooling when the liquid temperature is T.
The latent heat of fusion represents the heat given o¤ during the liquid-to-solid trans-
formation. As the undercool ing increases, the critical radius required for nucleation
decreases. Table 9-1 presents values for s
sl
, DH
f
, and typical undercoolings observed
experimentally for homogeneous nucleation.
The following example shows how we can calcula te the critical radius of the nucleus
for the solidification of copper.
9-2 Nucleation 261

TABLE 9-1 9 Values for freezing temperature, latent heat of fusion, surface energy, and maximum
undercooling for selected materials
Freezing
Temperature
(T
m
)
Heat of
Fusion
(DH
f
)
Solid-Liquid
Interfacial
Energy
(s
sl
)
Typical Undercooling
for Homogeneous
Nucleation
(DT )
Metal (
˚
C) (J/cm
3
) (J/cm
2
)(
˚
C)
Ga 30 488 56 10
7
76
Bi 271 543 54 10
7
90
Pb 327 237 33 10
7
80
Ag 962 965 126 10
7
250
Cu 1085 1628 177 10
7
236
Ni 1453 2756 255 10
7
480
Fe 1538 1737 204 10
7
420
NaCl 801 169
CsCl 645 152
H
2
O0 40
EXAMPLE 9-1
Calculation of Critical Radius for the Solidification of Copper
Calculate the size of the critical radius and the number of atoms in the critical
nucleus when solid copper forms by homogeneous nucleation. Comment on the
size of the nucleus and assumptions we made while deriving the equation for
the radius of the nucleus.
SOLUTION
From Table 9-1:
DT ¼ 236
C T
m
¼ 1085 þ 273 ¼ 1358 K
DH
f
¼ 1628 J=cm
3
s
sl
¼ 177 10
7
J=cm
2
r
¼
2s
sl
T
m
DH
f
DT
¼
ð2Þð177 10
7
Þð1358Þ
ð1628Þð236Þ
¼ 12:51 10
8
cm
The lattice parameter for FCC copper is a
0
¼ 0:3615 nm ¼ 3:615 10
8
cm
V
unit cell
¼ða
0
Þ
3
¼ð3:615 10
8
Þ
3
¼ 47:24 10
24
cm
3
V
r
¼
4
3
pr
3
¼
4
3
p
ð12:51 10
8
Þ
3
¼ 8200 10
24
cm
3
The number of unit cells in the critical nucleus is
8200 10
24
47:24 10
24
¼ 174 unit cells
Since there are four atoms in each unit cell of FCC metals, the number of
atoms in the critical nucleus must be:
ð4 atoms=cellÞð174 cells=nucleusÞ¼696 atoms=nucleus
C H A P TE R 9 Principles and Applications of Solidification262
In these types of calculations, we assume that a nucleus that is made from only
a few hundred atoms still exhibits properties similar to those of bulk materials.
This is not strictly correct and as such considered to be a weakness of the clas-
sical theory of nucleation.
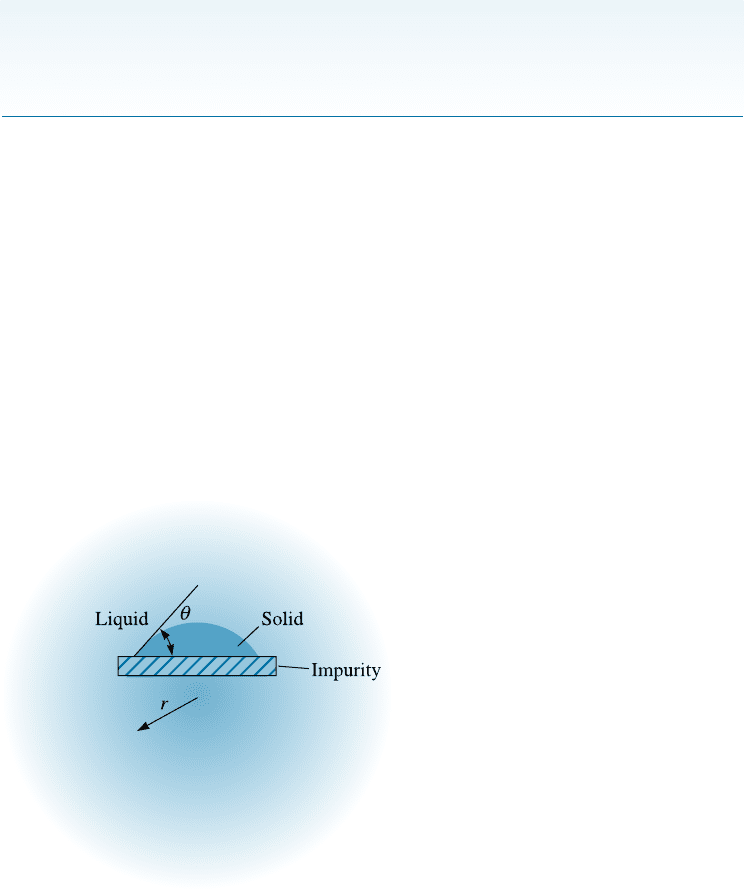
Heterogeneous Nucleation From Table 9-1, we can see that water will not solidify
into ice via homogeneous nucleation until we reach a temperature of 40
C (under-
cooling of 40
C)! Except in controlled laboratory experiments , homogeneous nuclea-
tion almost never occurs in liquids. Instead, impurities in contact with the liquid, either
suspended in the liquid or on the walls of the container that holds the liquid, provide a
surface on which the solid can form (Figure 9-4). Now, a radius of curvature greater
than the critical radius is achieved with very little total surface between the solid and
liquid. Only a few atoms must cluster together to produce a solid particle that has the
required radius of curvature. Much less undercooling is required to achieve the critical
size, so nucleation occurs more readily. Nucleation on preexisting surfaces is known as
heterogeneous nucleation. This process is dependent on the contact angle (y) for the nu-
cleating phase and the surface on which nucleation occurs. The same type of phenome-
non occurs in solid-state transformations.
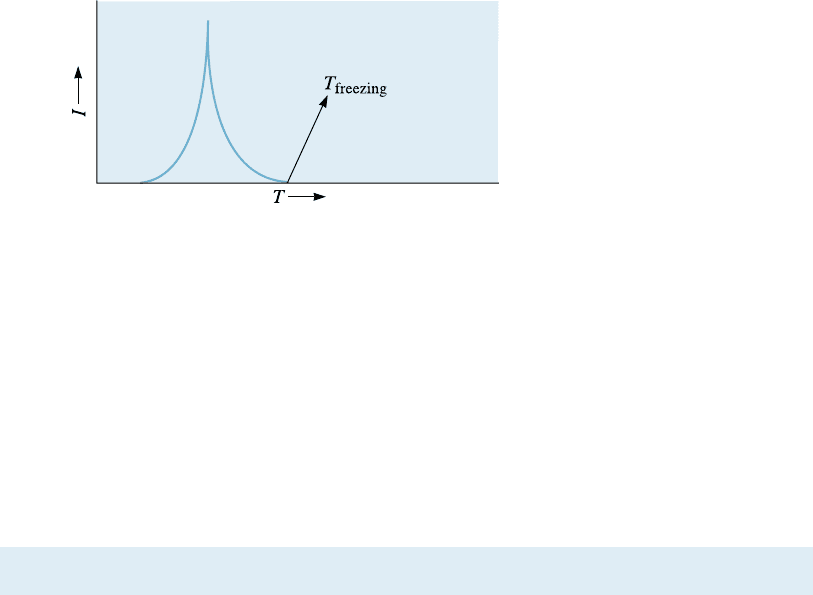
Rate of Nucleation The rate of nucleation (the number of nuclei formed per unit time)
is a function of temperatur e. Prior to solidification, of course, there is no nucleation
and, at temperatures above the freezing point, the rate of nucleation is zero. As the
temperature drops, the driving force for nucleation increases. However, as the temper-
ature becomes lower, atomic di¤usion becomes slower, hence slowing the nucleation
process. Thus, a typical rate of nucleation (I ) reaches a maximum at some temperature
below the transformation temperature (Figure 9-5). In heterogeneous nucleation, the
rate of nucleation is dictated by the concentration of the nucleating agents introduced.
By considering the rates of nucleation and growth, we can predict the overall rate of a
phase transformation.
Control of nucleation is important in the processing of metals, alloys, inorganic
glasses, and other engineered materials.
Figure 9-4
A solid forming on an impurity can
assume the critical radius with a smaller
increase in the surface energy. Thus,
heterogeneous nucleation can occur with
relatively low undercoolings.
9-2 Nucleation 263
Grain Size Strengthening When a metal casting freezes, impurities in the melt and
walls of the mold in which solidification occurs serve as heterogeneous nucleation sites.
Sometimes we intentionally introduce nucleating particles into the liquid. Such practices
are called grain refinement or inoculation. Chemicals added to molten metals to promote
nucleation and, hence, a finer grain size, are known as grain refiners or inoculants.For
example, a combination of 0.03% titanium (Ti) and 0.01% boron (B) is added to many
liquid-aluminum alloys. Tiny particles of an aluminum titanium compound (Al
3
Ti) or
titanium diboride (TiB
2
) form and serve as sites for heterogeneous nucleation. Grain re-
fining or inoculation produces a large number of grains, each beginning to grow from
one nucleus. The greater grain boundary area provides grain size strengthening in met-
allic materials. This was discussed using the Hall-Petch equation in Chapter 4.
9-3 Growth Mechanisms
Once the solid nuclei of a phase forms (in a liquid or another solid phase), growth
begins to occur as more atoms become attached to the solid surface. In this discussion,
we will concentrate on nucleation and the growth of crystals from liquid. The nature of
the growth of the solid nuclei depends on how heat is removed from the molten mate-
rial. Let’s consider casting a molten metal in a mold, for example. We assume we have
a nearly pure metal and not an alloy (as solidification of alloys is di¤erent in that, in
most cases, it occurs over a range of temperatures). In the solidification process, two
types of heat must be removed: the specific heat of the liquid and the latent heat of fu-
sion. The specific heat is the heat required to change the temperature of a unit mass of
the material by one degree. The specific heat must be removed first, either by radiation
into the surrounding atmosphere or by conduction into the surrounding mold, until the
liquid cools to its freezing temperature. This is simply the cooling of the liquid from one
temperature to a temperature at which nucleation begins.
We know that to melt a solid we need to supply heat. Therefore, when solid crystals
form from a liquid, heat is generated! This heat is the latent heat of fusion (DH
f
) and
must be removed from the solid-liquid interface before solidification is completed. The
manner in which we remove the latent heat of fusion determines the material’s growth
mechanism and final structure of a casting.
Planar Growth When a well-inoculated liquid (i.e., a liquid containing nucleating
agents) cools under equilibrium conditions, there is no need for undercooling since het-
erogeneous nucleation can occur readily. Therefore, the temperature of the liquid ahead
of the solidification front (i.e., solid–liquid interface) is greater than the freezing tem-
perature. The temperature of the solid is at or below the freezing tempe rature. During
solidification, the latent heat of fusion is removed by conduction from the solid-liquid
interface through the solid. Any small protuberance that begins to grow on the interface
Figure 9-5
Rate of nucleation (I ) as a function
of temperature of the liquid (T ).
C H A P TE R 9 Principles and Applications of Solidification264
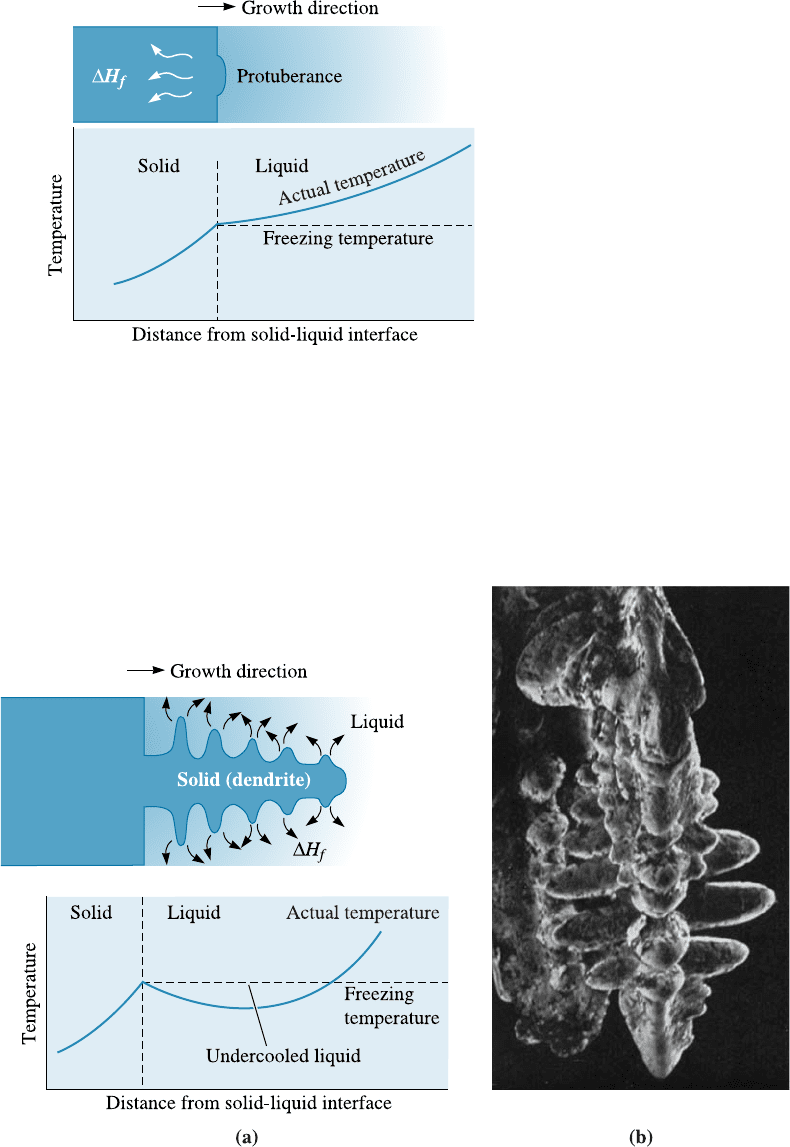
is surrounded by liquid above the freezing temperature (Figure 9-6). The growth of the
protuberance then stops until the remainder of the interface catches up. This growth
mechanism, known as planar growth, occurs by the movement of a smooth or planar
solid-liquid front into the liquid.
Dendritic Growth When the liquid is not inoculated and the nucleation is poor, the
liquid has to be undercooled before the solid forms (Figure 9-7). Under these condi-
Figure 9-6
When the temperature of the liquid
is above the freezing temperature, a
protuberance on the solid-liquid
interface will not grow, leading to
maintenance of a planar interface.
Latent heat is removed from the
interface through the solid.
Figure 9-7 (a) If the liquid is undercooled, a protuberance on the solid-liquid interface can
grow rapidly as a dendrite. The latent heat of fusion is removed by raising the temperature of
the liquid back to the freezing temperature. (b) Scanning electron micrograph of dendrites in
steel (15).
9-3 Growth Mechanisms 265

tions, a small solid protuberance called a dendrite, which forms at the interface, is
encouraged to grow since the liquid ahead of the solidification front is undercooled. The
word dendrite comes from the Greek word dend ron that means tree. As the solid den-
drite grows, the latent heat of fusion is conducted into the undercooled liquid, raising
the temperature of the liquid toward the freezing temperature. Secondary and tertiary
dendrite arms can also form on the primary stalks to speed the evolution of the latent
heat. Dendritic growth continues until the undercooled liquid warms to the freezing
temperature. Any remaining liquid then solidifies by planar growth. The di¤erence
between planar and dendritic growth arises because of the di¤erent sinks for the latent
heat of fusion. The container or mold must absorb the heat in planar growth, but the
undercooled liquid absorbs the heat in dendritic growth.
In the solidification of pure metals, dendritic growth normally represents only a
small fraction of the total growth and is given by:
Dendritic fraction ¼ f ¼
cDT
DH
f
ð9-3Þ
where c is the specific heat of the liquid. The numerator represents the heat that the
undercooled liquid can absorb, and the latent heat in the denominator represents the
total heat that must be given up during solidificatio n. As the undercooling DT in-
creases, more dendritic growth occurs. If the liquid is well inoculated, undercooling is
almost zero and growth would be mainly via the planar front solidification mechanism.
The rate at which growth of the solid occurs depends on the cooling rate, or the
rate of heat extraction. A higher cooling rate produces rapid solidification, or short
solidification times. The time t
s
required for a simple casting to solidify completely can
be calculated using Chvorinov’s rule:
t
s
¼ B
V
A
n
ð9-4Þ
where V is the volume of the casting and represents the amount of heat that must be
removed before freezing occurs, A is the surface area of the casting in contact with the
mold and represents the surface from which heat can be transferred away from the
casting, n is a constant (usually about 2), and B is the mold constant. The mold constant
depends on the properties and initial temperatures of both the metal and the mold. This
rule basically accounts for the geometry of a casting and the heat transfer conditions.
The rule states that for the same conditions a casting with a small volume and relatively
large surface area will cool more rapidly.
EXAMPLE 9-2
Redesign of a Casting for Improved Strength
Your company currently is producing a disk-shaped brass casting 5 cm thick
and 45 cm in diameter. You believe that by making the casting solidify 25%
faster, the improvement in the tensile properties of the casting will permit the
casting to be made lighter in weight. Design the casting to permit this. Assume
that the mold constant is 3.5 min/cm
2
for this process.
SOLUTION
One approach would be to use the same casting process, but reduce the
thickness of the casting. The thinner casting would solidify more quickly and,
because of the faster cooling, should have improved mechanical properties.
C H A P TE R 9 Principles and Applications of Solidification266
Chvorinov’s rule helps us calculate the required thickness. If d is the diameter
and x is the thickness of the casting, then the volume, surface area, and solid-
ification time of the 5 cm thick casting are:
V ¼ðp=4Þd
2
x ¼ðp=4Þð45Þ
2
ð5Þ¼7955:4cm
3
A ¼ 2ðp=4Þd
2
þ pdx ¼ 2ðp=4Þð45Þ
2
þ pð45Þð5Þ¼3889:2cm
2
t ¼ B
V
A
2
¼ð3:5Þ
7955:4
3889:2
2
¼ 14:71 min
The solidification time of the redesigned casting should be 25% shorter than the
current time, or t
r
¼ 0:75t, where:
t
r
¼ 0:75t ¼ð0:75Þð14:71Þ¼11:03 min
Since the casting conditions have not changed, the mold constant B is un-
changed. The V=A ratio of the new casting is:
t
r
¼ B
V
A
2
¼ð3:5Þ
V
A
2
¼ 11:03 min
V
A
2
¼ 3:1514 cm
2
or
V
A
¼ 1:775 cm
If x is the required thickness for our redesigned casting, then:
V
r
A
r
¼
ðp=4Þd
2
x
2ðp=4Þd
2
þ pdx
¼
ðp=4Þð45Þ
2
ðxÞ
2ðp=4Þð45Þ
2
þ pð45ÞðxÞ
¼ 1:775 cm
Therefore, x ¼ 4:22 cm
This thickness provides the required solidification time, while reducing the
overall weight of the casting by nearly 15%.
Solidification begins at the surface, where heat is dissipated into the surrounding
mold material. The rate of solidification of a casting can be described by how rapidly
the thickness d of the solidified skin grows:
d ¼ k
solidification
ffiffi
t
p
c
1
ð9-5Þ
where t is the time after pouring, k
solidification
is a constant for a given casting material
and mold, and c
1
is a constant related to the pouring temperature.
Effect on Structure and Properties The solidification time a¤ects the size of the den-
drites. Normally, dendrite size is characterized by measuring the distance between the
secondary dendrite arms (Figure 9-8). The secondary dendrite arm spacing (SDAS) is
reduced when the casting freezes more rapidly. The finer, more extensive dendritic net-
work serves as a more e‰cient conductor of the latent heat to the undercooled liquid.
The SDAS is related to the solidification time by
SDAS ¼ kt
m
s
ð9-6Þ
where m and k are constants depending on the composition of the metal. This relationship
9-3 Growth Mechanisms 267
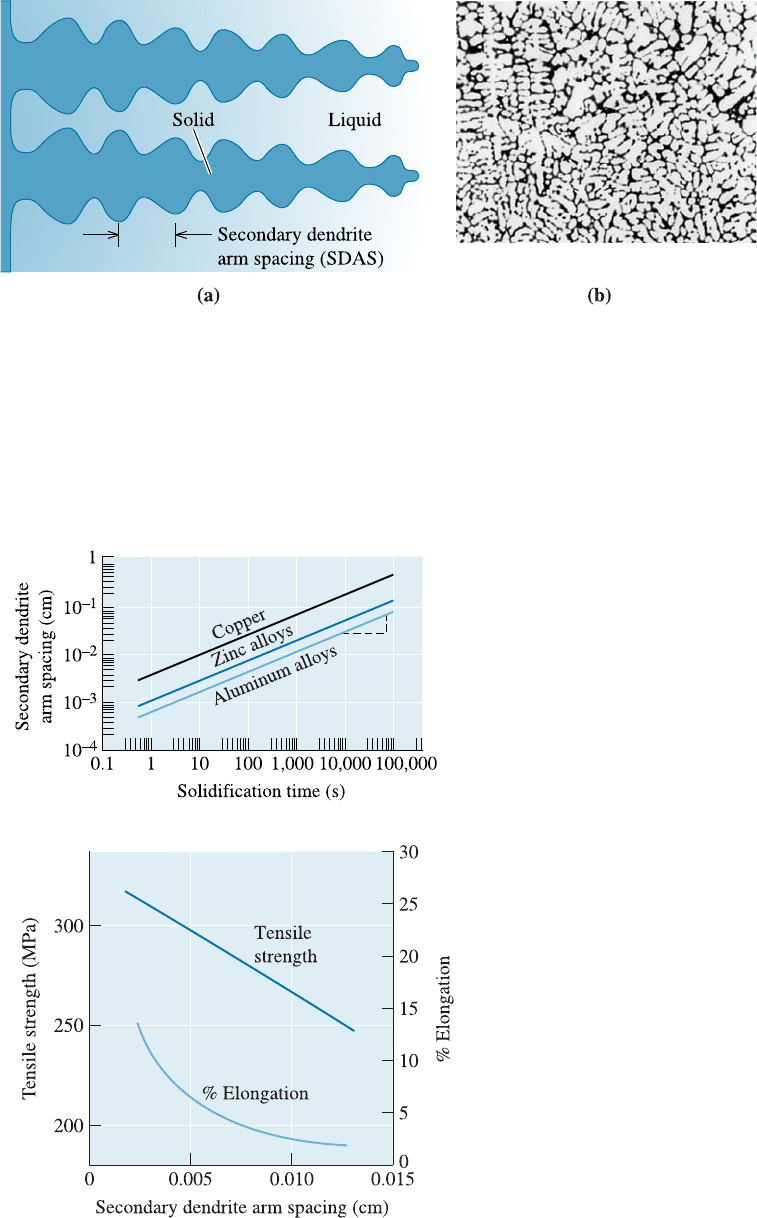
is shown in Figure 9-9 for several alloys. Small secondary dendrite arm spacings are asso-
ciated with higher strengths and improved ductility (Figure 9-10).
Rapid solidification processing is used to produce exceptionally fine secondary den-
drite arm spacings; a common method is to produce very fine liquid droplets that freeze
Figure 9-8 (a) The secondary dendrite arm spacing (SDAS). (b) Dendrites in an aluminum
alloy (50). (From ASM Handbook, Vol. 9, Metallography and Microstructure (1985), ASM
International, Materials Park, OH 44073-0002.)
Figure 9-9
The effect of solidification time on
the secondary dendrite arm
spacings of copper, zinc, and
aluminum.
Figure 9-10
The effect of the secondary dendrite
arm spacing on the properties of an
aluminum casting alloy.
C H A P TE R 9 Principles and Applications of Solidification268

into solid particles. This process is known as spray atomization. The tiny droplets freeze
at a rate of about 10
6
C/s, producing powder particles that range from @5–100 mm.
This cooling rate is not rapid enough to form a metallic glass, but does produce a fine
dendritic structure. By carefully consolidating the solid drople ts by powder metallurgy
processes, improved properties in the material can be obtained. Since the particles are
derived from melt, many complex alloy compositions can be produced in the form of
chemically homogenous powders.
The following example discusses how Chvorinov’s rule, the relationship between
SDAS and the time of solidification, and the SDAS and mechanical properties can be
used to design casting processes.
EXAMPLE 9-3
Secondary Dendrite Arm Spacing for Aluminum Alloys
Determine the constants in the equation that describe the relationship between
secondary dendrite arm spacing and solidification time for aluminum alloys
(Figure 9-9).
SOLUTION
We could obtain the value of SDAS at two times from the graph and calculate
k and m using simultaneous equations. However, if the scales on the ordinate
and abscissa are equal for powers of ten (as in Figure 9-9), we can obtain the
slope m from the log-log plot by directly measuring the slope of the graph. In
Figure 9-9, we can mark five equal units on the vertical scale and 12 equal units
on the horizontal scale. The slope is:
m ¼
5
12
¼ 0:42
The constant k is the value of SDAS when t
s
¼ 1 s, since:
log SDAS ¼ log k þ m log t
s
If t
s
¼ 1 s, m log t
s
¼ 0, and SDAS ¼ k, from Figure 9-9:
k ¼ 8 10
4
cm
9-4 Cooling Curves
A cooling curve shows how the temperature of a material (in this case, a pure metal)
changes with time [Figure 9-11(a) and (b)]. The liquid is poured into a mold at pouring
temperature, point A. The di¤erence between the pouring temperature and the freezing
temperature is the superheat. The specific heat is extracted by the mold until the liquid
reaches the freezing temperature (point B). If the liquid is not well inoculated it must be
undercooled (point B to C). The slope of the cooling curve before solidification begins is
the cooling rate
DT
Dt
. As nucleation begins (point C ) latent heat of fusion is given o¤
and the temperature rises. This increase in temperature of the undercooled liquid as a
result of nucleation is known as recalescence (point C to D). Solidification proceeds
9-4 Cooling Curves 269
