Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.


steels and other metals are often subjected to a ‘‘pickling’’ treatment in which acids are
used to dissolve the oxide scale. In some metals, such as tungsten (W) and beryllium
(Be), hot-working must be done in a protective atmosphere to prevent oxidation.
The dimensional accuracy is also more di‰cult to control during hot working. A
greater elastic strain must be considered, since the modulus of elasticity is low at hot-
working temperatures. In addition, the metal contracts as it cools from the hot-working
temperature. The combination of elastic strain and thermal contraction requires that
the part be made oversi ze during deformation; forming dies must be carefully designed,
and precise temperature control is necessary if accurate dimensions are to be obtained.
SUMMARY
V When a metallic material is deformed by cold working, strain hardening occurs
as additional dislocations are introduced into the structure. Very large increases
in strength may be obtained in this manner. The ductility of the strain hardened
metallic material is reduced.
V Wire drawing, stamping, rolling, and extrusion are some examples of manufactur-
ing methods for shaping metallic materials. Some of the underlying principles for
these processes can also be used for the manufacturing of polymeric materials.
V Annealing of metallic materials is a heat treatment intended to eliminate all, or a
portion of, the e¤ects of strain hardening. The annealing process may involve as
many as three steps.
V Recovery occurs at low temperatures, eliminating residual stresses and restoring
electrical conductivity without reducing the strength. A ‘‘stress relief anneal’’ refers
to recovery.
V Recrystallization occurs at higher temperatures and eliminates almost all of the
e¤ects of strain hardening. The disloc ation density decreases dramatically during
recrystallization as new grains nucleate and grow.
V Grain growth, which normally should be avoided, occurs at still higher temper-
atures. In cold-worked metallic materials, grain growth follows recovery and re-
crystallization.
V Hot working combines plastic deformation and annealing in a single step, permit-
ting large amounts of plastic deformation without embrittling the material.
V Residual stresses in materials need to be controlled. In cold-worked metallic mate-
rials, residual stresses can be eliminated using a stress-relief anneal.
GLOSSARY
Annealing In the context of metallic material, annealing is a heat treatment used to eliminate
part or all of the e¤ects of cold working. For glasses, annealing is a heat treatment that removes
thermally induced stresses.
Bauschinger effect A material previously plastically deformed under tension shows decreased
flow stress when tested again under compression or vice versa.
Cold working Deformation of a metal below the recrystallization temperature. During cold
working, the number of dislocations increases, causing the metal to be strengthened as its shape is
changed.
C HA P T E R 8 Strain Hardening and Annealing250
Deformation processing Techniques for the manufacturing of metallic and other materials using
such processes as rolling, extrusion, drawing, etc.
Drawing A deformation processing technique in which a material is pulled through an opening
in a die (e.g., wire drawing).
Extrusion A deformation processing technique in which a material is pushed through an open-
ing in a die. Used for metallic and polymeric materials.
Fiber texture A preferred orientation of grains obtained during the wire drawing process. Cer-
tain crystallographic directions in each elongated grain line up with the drawing direction, caus-
ing anisotropic behavior.
Formability The ability of a material to stretch and bend without breaking. Forming diagrams
describe the ability to stretch and bend materials.
Frank-Read source A pinned dislocation that, under an applied stress, produces additional dis-
locations. This mechanism is at least partly responsible for strain hardening.
Heat-affected zone (HAZ) The volume of material adjacent to a weld that is heated during the
welding process above some critical temperature at which a change in the structure, such as grain
growth or recrystallization, occurs.
Hot working Deformation of a metal above the recrystallization temperature. During hot
working, only the shape of the metal changes; the strength remains relatively unchanged because
no strain hardening occurs.
Polygonized subgrain structure A subgrain structure produced in the early stages of annealing.
The subgrain boundaries are a network of dislocations rearranged during heating.
Recovery A low-temperature annealing heat treatment designed to eliminate residual stresses
introduced during deformation without reducing the strength of the cold-worked material. This is
the same as a stress-relief anneal.
Recrystallization A medium-temperature annealing heat treatment designed to eliminate all of
the e¤ects of the strain hardening produced during cold working.
Recrystallization temperature A temperature above which essentially dislocation-free and new
grains emerge from a material that was previously cold worked. This depends upon the extent of
cold work, time of heat treatment, etc., and is not a fixed temperature.
Residual stresses Stresses introduced in a material during processing. These can originate as a
result of cold working or di¤erential thermal expansion and contraction. A stress-relief anneal in
metallic materials and the annealing of glasses minimize residual stresses. Compressive residual
stresses deliberately introduced on the surface by the tempering of glasses or shot peening of
metallic materials improve their mechanical properties.
Sheet texture A preferred orientation of grains obtained during the rolling process. Certain
crystallographic directions line up with the rolling direction, and certain preferred crystallo-
graphic planes become parallel to the sheet surface.
Shot peening Introducing compressive residual stresses at the surface of a part by bombarding
that surface with steel shot. The residual stresses may improve the overall performance of the
material.
Strain hardening Strengthening of a material by increasing the number of dislocations by de-
formation, or cold working. Also known as ‘‘work hardening.’’
Glossary 251

Strain-hardening exponent (n) A parameter that describes susceptibility of a material to cold
working. It describes the e¤ect that strain has on the resulting strength of the material. A material
with a high strain-hardening coe‰cient obtains high strength with only small amounts of de-
formation or strain.
Strain rate The rate at which a material is deformed.
Strain-rate sensitivity (m) The rate at which stress develops changes as a function of strain rate.
A material may behave much di¤erently if it is slowly pressed into a shape rather than trans-
formed rapidly into a shape by an impact loading.
Stress-relief anneal The recovery stage of the annealing heat treatment during which residual
stresses are relieved without reducing the mechanical properties of the material.
Texture strengthening Increase in the yield strength of a material as a result of preferred crys-
tallographic texture.
Thermomechanical processing Processes involved in the manufacturing of metallic components
using mechanical deformation and various heat treatments.
Thermoplastics A class of polymers that consist of large, long spaghetti-like molecules that are
intertwined (e.g., polyethylene, nylon, PET, etc.).
Warm working A term used to indicate the processing of metallic materials in a temperature
range that is between those that define cold and hot working (usually a temperature between 0.3
to 0.6 of melting temperature in K).
Work hardening A term sometimes used instead of strain hardening or cold working to describe
the e¤ect of deformation on the strengthening of metallic materials.
PROBLEMS
3
Section 8-1 Relationship of Cold Working to the
Stress-Strain Curve
8-1 A 1.263-cm-diameter metal bar with a 5-cm gage
length l
0
is subjected to a tensile test. The following
measurements are made in the plastic region:
Force (N)
Change in Gage
length (cm) (Dl )
Diameter
(cm)
122,320 0.5258 1.2
120,100 1.107 1.1415
114,310 1.7493 1.0858
Determine the strain-hardening exponent for the
metal. Is the metal most likely to be FCC, BCC, or
HCP? Explain.
8-2 Define the following terms: strain-hardening ex-
ponent (n), strain-rate sensitivity (m), and plastic
strain ratio (r). Use appropriate equations.
8-3 A 1.5-cm-diameter metal bar with a 3-cm gage
length (l
0
) is subjected to a tensile test. The fol-
lowing measurements are made:
Force (N)
Change in Gage
Length (cm) (Dl )
Diameter
(cm)
16,240 0.6642 1.2028
19,066 1.4754 1.0884
19,273 2.4663 0.9848
Determine the strain-hardening coe‰cient for the
metal. Is the metal most likely to be FCC, BCC,
or HCP? Explain.
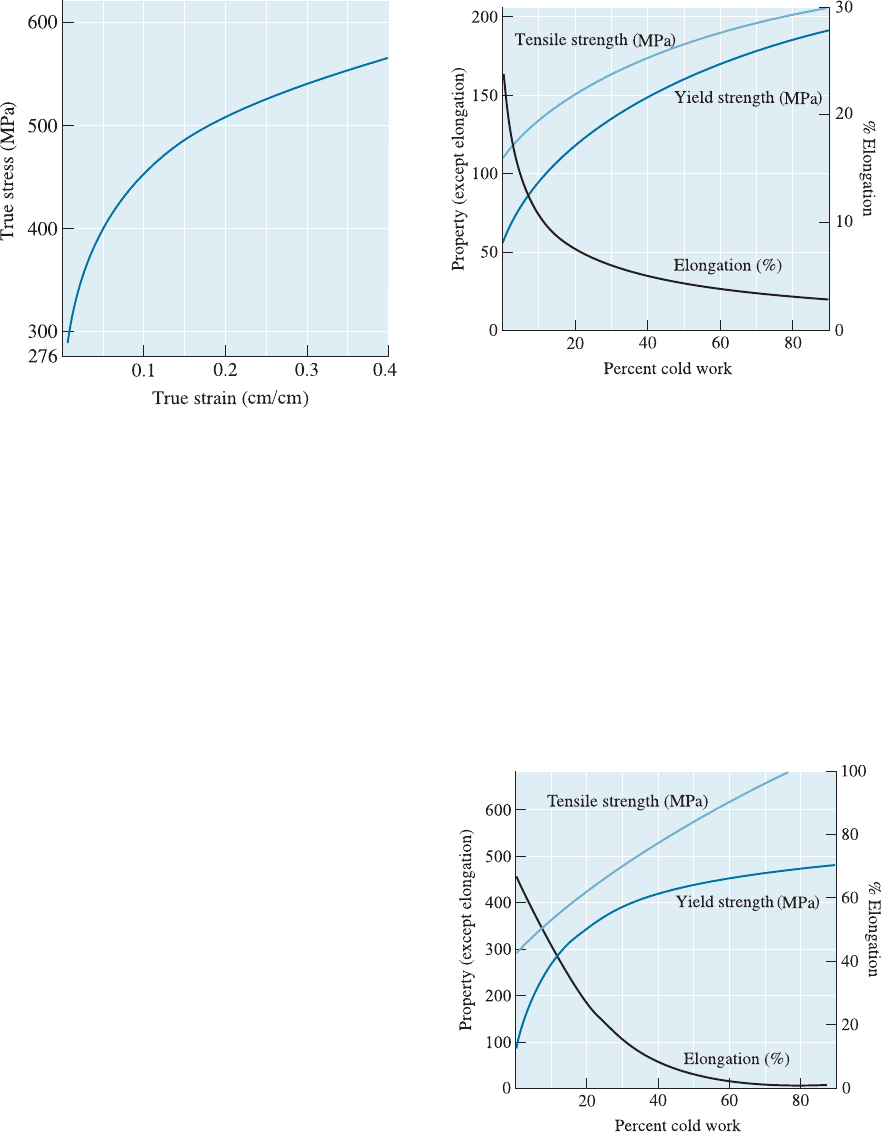
8-4 A true stress-true strain curve is shown in Figure
8-20. Determine the strain-hardening exponent for
the metal.
8-5 A Cu-30% Zn alloy tensile bar has a strain-
hardening coe‰cient of 0.50. The bar, which has
an initial diameter of 1 cm and an initial gage
C HA P T E R 8 Strain Hardening and Annealing252
length of 3 cm, fails at an engineering stress of
120 MPa. After fracture, the gage length is 3.5 cm
and the diameter is 0.926 cm. No necking oc-
curred. Calculate the true stress when the true
strain is 0.05 cm/cm.
Section 8-2 Strain-Hardening Mechanisms
Section 8-3 Properties versus Percent Cold Work
8-6 A 0.625-cm-thick copper plate is to be cold
worked 63%. Find the final thickness.
8-7 A 0.625-cm-diameter copper bar is to be cold
worked 63%. Find the final diameter.
8-8 A 5-cm-diameter copper rod is reduced to a 3.75-cm
diameter, then reduced again to a final diameter of
2.5 cm. In a second case, the 5-cm-diameter rod is
reduced in one step from a 5 cm to a 2.5-cm
diameter. Calculate the % CW for both cases.
8-9 A 3105 aluminum plate is reduced from 4.38 cm
to 2.88 cm. Determine the final properties of the
plate. Note 3105 designates a special composition
of aluminum alloy. (See Figure 8-21.)
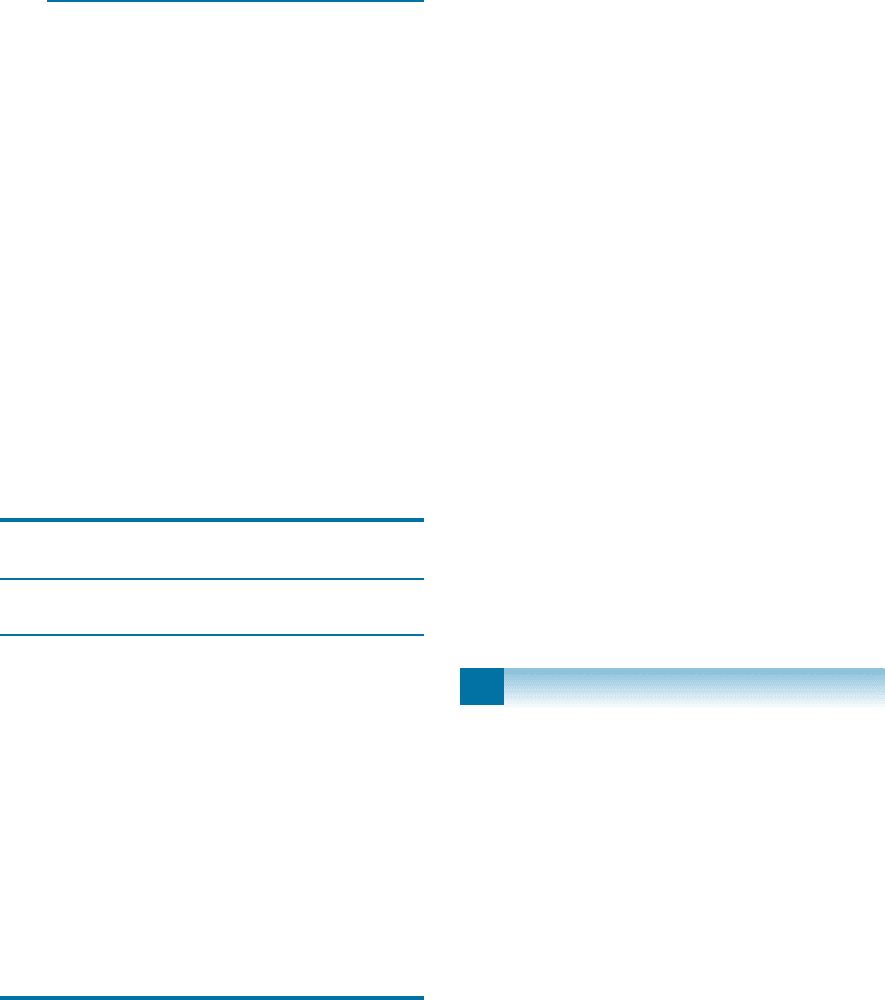
8-10 A Cu-30% Zn brass bar is reduced from a 2.5-cm
diameter to a 1.13-cm diameter. Determine the
final properties of the bar. (See Figure 8-22.)
8-11 A 3105 aluminum bar is reduced from a 2.5-cm
diameter, to a 2-cm diameter, to a 1.5-cm diame-
ter, to a final 1-cm diameter. Determine the %
CW and the properties after each step of the
process. Calculate the total percent cold work.
Note 3105 designates a special composition of
aluminum alloy. (See Figure 8-21.)
8-12 We want a Cu-30% Zn brass plate originally
3-cm thick to have a yield strength greater than
345 MPa and a % elongation of at least 10%.
What range of final thicknesses must be ob-
tained? (See Figure 8-22.)
Figure 8-20 True stress-true strain curve (for Problem
8-4).
Figure 8-21 The effect of percent cold work on the
properties of a 3105 aluminum alloy (for Problems
8-9 and 8-11).
Figure 8-22 The effect of percent cold work on the
properties of a Cu-30% Zn brass (for Problems 8-10
and 8-12).
Problems 253
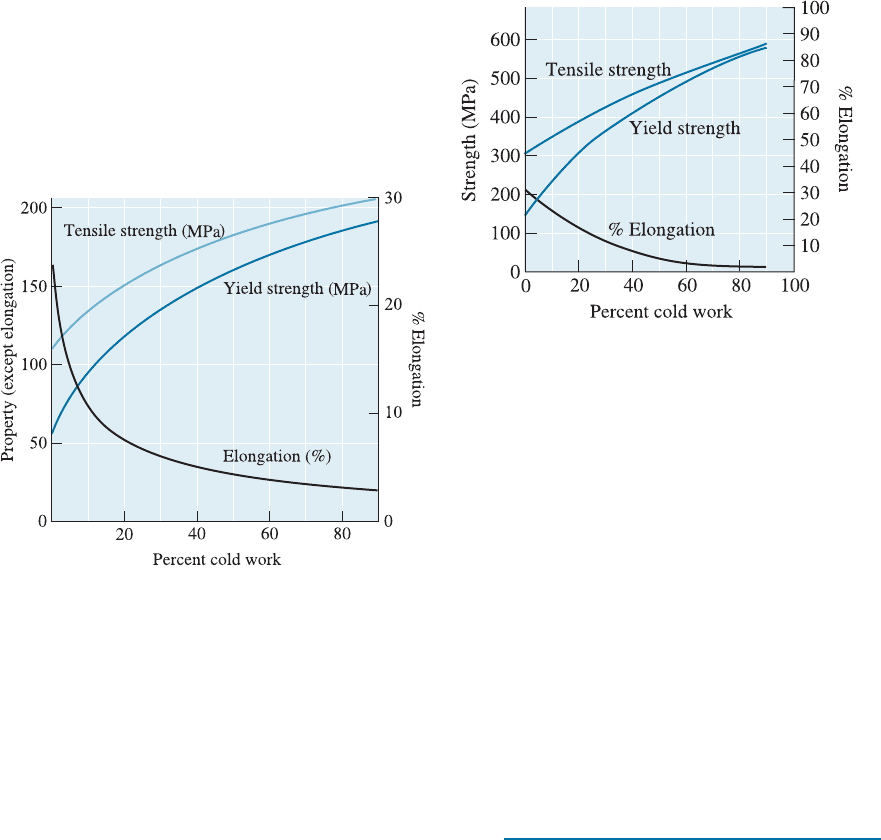
8-13 A 3105 aluminum plate previously cold worked
20% is 5-cm thick. It is then cold worked further
to 3.25 cm. Calculate the total percent cold work
and determine the final properties of the plate.
Note 3105 designates a special composition of
aluminum alloy. (See Figure 8-21.)
Section 8-4 Microstructure, Texture
Strengthening, and Residual Stresses
Section 8-5 Characteristics of Cold Working
8-14 Aluminum cans made by deep drawing derive
considerable strength during their fabrication.
Explain why.
8-15 Such metals as magnesium can not be e¤ectively
strengthened using cold working. Explain why.
8-16 We want to draw a 0.75-cm-diameter copper wire
having yield strength of 138 MPa into a 0.625-
cm-diameter wire.
(a) Find the draw force, assuming no friction;
(b) Will the drawn wire break during the draw-
ing process? Show why. (See Figure 8-5.)
8-17 A 3105 aluminum wire is to be drawn to give a
1-mm-diameter wire having yield strength of
138 MPa. Note 3105 designates a special compo-
sition of aluminum alloy.
(a) Find the original diameter of the wire;
(b) Calculate the draw force required; and
(c) Determine whether the as-drawn wire will
break during the process. (See Figure 8-21.)
Section 8-6 The Three Stages of Annealing
8-18 The following data were obtained when a cold-
worked metal was annealed.
(a) Estimate the recovery, recrystallization, and
grain growth temperatures;
(b) Recommend a suitable temperature for a
stress-relief heat treatment;
(c) Recommend a suitable temperature for a hot-
working process; and
(d) Estimate the melting temperature of the
alloy.
Annealing
Temperature
(
˚
C)
Electrical
Conductivity
(ohm
C1
·cm
C1
)
Yield
Strength
(MPa)
Grain
Size
(mm)
400 3:04 10
5
86 0.10
500 3:05 10
5
85 0.10
600 3:36 10
5
84 0.10
700 3:45 10
5
83 0.098
800 3:46 10
5
52 0.030
900 3:46 10
5
47 0.031
1000 3:47 10
5
44 0.070
1100 3:47 10
5
42 0.120
8-19 The following data were obtained when a cold-
worked metallic material was annealed.
(a) Estimate the recovery, recrystallization, and
grain growth temperatures;
(b) Recommend a suitable temperature for
obtaining a high-strength, high-electrical-
conductivity wire;
Figure 8-21 (Repeated for Problems 8-13, 8-17 and
8-22.) The effect of percent cold work on the
properties of a 3105 aluminum alloy.
Figure 8-5 (Repeated for Problems 8-16 and 8-23.)
The effect of cold work on the mechanical properties
of copper.
C HA P T E R 8 Strain Hardening and Annealing254
(c) Recommend a suitable temperature for a hot
working process; and
(d) Estimate the melting temperature of the alloy.
Annealing
Temperature
(
˚
C)
Residual
Stresses
(MPa)
Tensile
Strength
(MPa)
Grain
Size
(cm)
250 145 359 0.0075
275 145 359 0.0075
300 35 359 0.0075
325 0 359 0.0075
350 0 234 0.0025
375 0 207 0.0025
400 0 186 0.0088
425 0 172 0.0018
Section 8-7 Control of Annealing
8-20 Two sheets of steel have cold works of 20% and
80%, respectively. Which one would likely have a
lower recrystallization temperature? Why?
Section 8-8 Annealing and Materials Processing
8-21 Using the data in Table 8-3, plot the recrystalli-
zation temperature versus the melting temper-
ature of each metal, using absolute temperatures
(Kelvin). Measure the slope and compare with
the expected relationship between these two tem-
peratures. Is our approximation a good one?
8-22 We wish to produce a 0.75-cm-thick plate of 3105
aluminum having a tensile strength of at least
172 MPa and a % elongation of at least 5%. The
original thickness of the plate is 7.5 cm. The max-
imum cold work in each step is 80%. Describe
the cold working and annealing steps required to
make this product. Compare this process with
what you would recommend if you could do the
initial deformation by hot working. (See Figure
8-21.)
8-23 We wish to produce a 0.5-cm-diameter wire of
copper having a minimum yield strength of
414 MPa and a minimum % elongation of 5%.
The original diameter of the rod is 5 cm and the
maximum cold work in each step is 80%. Describe
the cold working and annealing steps required to
make this product. Compare this process with
what you would recommend if you could do the
initial deformation by hot working. (See Figure
8-5.)
8-24 What is a heat-a¤ected zone? Why do some
welding processes result in a joint where the ma-
terial in the heat-a¤ected zone is weaker than the
base metal?
8-25 What welding techniques can be used to avoid
loss of strength in the material in the heat-
a¤ected zone? Explain why these techniques are
e¤ective.
Section 8-9 Hot Working
8-26 The amount of plastic deformation that can
be performed during hot working is almost un-
limited. Justify this statement.
8-27 Compare and contrast hot working and cold
working.
Design Problems
g
8-28 Design, using one of the processes discussed in
this chapter, a method to produce each of the
following products. Should the process include
hot working, cold working, annealing, or some
combination of these? Explain your decisions.
(a) paper clips;
(b) I-beams that will be welded to produce a
portion of a bridge;
(c) copper tubing that will connect a water fau-
cet to the main copper plumbing;
(d) the steel tape in a tape measure; and
(e) a head for a carpenter’s hammer formed
from a round rod.
8-29 We plan to join two sheets of cold-worked copper
by soldering. Soldering involves heating the metal
TABLE 8-3 9 Typical recrystallization temperatures for
selected metals (Repeated for Problem 8-21)
Metal
Melting Temperature
(
˚
C)
Recrystallization
Temperature (
˚
C)
Sn 232 4
Pb 327 4
Zn 420 10
Al 660 150
Mg 650 200
Ag 962 200
Cu 1085 200
Fe 1538 450
Ni 1453 600
Mo 2610 900
W 3410 1200
(Source: Adapted from Structure and Properties of Engineering
Materials, by R. Brick, A. Pense, and R. Gordon, 1977.
Copyright > 1977 The McGraw-Hill Companies. Adapted by
permission.)
Problems 255
to a high enough temperature that a filler mate-
rial melts and is drawn into the joint (Chapter 9).
Design a soldering process that will not soften the
copper. Explain. Could we use higher soldering
temperatures if the sheet material were a Cu-30%
Zn alloy? Explain.
8-30 We wish to produce a 1-mm-diameter copper
wire having a minimum yield strength of
414 MPa and a minimum % elongation of 5%.
We start with a 20-mm-diameter rod. Design the
process by which the wire can be drawn. Include
all-important details and explain.
C HA P T E R 8 Strain Hardening and Annealing256

9
Principles and Applications of
Solidification
Have You Ever Wondered?
9 Whether water really does ‘‘freeze’’ at 0
C and ‘‘boil’’ at 100
C?
9 What is the process used to produce several million kilograms of steels and other alloys?
9 What factors determine the strength of a cast product?
9 What is a Liquidmetal
TM
?
Of all the processing techniques used in the
manufacturing of materials, solidification is
probably the most important. All metallic mate-
rials, as well as many ceramics, inorganic
glasses, and thermoplastic polymers, are liquid
or molten at some point during processing. Like
water freezes to ice, molten materials solidify as
they cool below their freezing temperature. In
Chapter 2, we learned how materials are classi-
fied based on their atomic, ionic, or molecular
order. During the solidification of materials that
crystallize, the atomic arrangement changes from
a short-range order (SRO) in a liquid to a long-
range order (LRO) in the crystalline solid. The
257

solidification of materials that crystallize requires
two steps: In the first step, ultra-fine crystallites,
known as the nuclei of a solid phase, form from
the liquid. In the second step, which can overlap
with the first, the ultra-fine solid crystallites be-
gin to grow as atoms from the liquid are attached
to the nuclei until no liquid remains. Some ma-
terials, such as inorganic silicate glasses, will
turn into a solid without developing a long-range
order (i.e., they remain amorphous). Many poly-
meric materials may develop partial crystallinity
during solidification or processing.
The solidification of metallic, polymeric, and
ceramic materials is an important process to study
because of its effect on the properties of the ma-
terials involved. In Chapter 8, we examined how
strain hardening can be used to strengthen and
shape metallic materials. We learned in Chapter 4
that grain size plays an important role in de-
termining the strength of metallic materials. In
this chapter, we will study the principles of solid-
ification as they apply to pure metals. We will dis-
cuss solidification of alloys and more complex
materials in subsequent chapters. We will first
discuss the technological significance of solid-
ification, and then examine the mechanisms by
which solidification occurs. This will be followed
by an examination of the microstructure of cast
metallic materials and its effect on the material’s
mechanical properties. We will also examine the
role of casting as a materials shaping process. We
will examine how techniques such as welding,
brazing, and soldering are used for joining metallic
materials. Applications of the solidification process
in single crystal growth and the solidification of
glasses and polymers also will be discussed.
9-1 Technological Significance
The ability to use fire to produce, melt, and cast metals such as copper, bronze, and
steel indeed is regarded as an important hallmark in the development of mankind. The
use of fire for reducing naturally occurring ores into metals and alloys led to the pro-
duction of useful tools and other products. Today, thousands of years later, solidifi-
cation is still considered one of the most important manufacturing processes. Several
million pounds of steel, aluminum alloys, copper, and zinc are produced through the
casting process. The solidification process is also used to manufacture specific compo-
nents (e.g., aluminum alloy for automotive wheels) (Figure 9-1). Industry also uses the
solidification process as a primary processing step to produce metallic slabs or ingots (a
simple, and often large casting that is processed later into useful shapes). The ingots or
slabs are then hot and cold worked through secondary processing steps into more useful
shapes (i.e., sheets, wires, rods, plates, etc.). Solidification also is applied when joining
metallic materials using techniques such as welding, brazing, and soldering.
We also use solidification for processing inorganic glasses; silicate glass, for exam-
ple, is processed using the float-glass process. High-quality optical fibers and other
materials, such as fiberglass fibers, also are produced from the solidification of molten
glasses. During the solidification of inorganic glasses, amorphous rather than crystal-
line, materials are produced. In the manufacture of glass-ceramics, we first shape the
materials by casting amorphous glasses, and then crystallize them using a heat treat-
ment to enhance their strength. Many thermoplastic materials such as polyethylene,
polyvinyl chloride (PVC), polypropylene, and the like are processed into useful shapes
(i.e., fibers, tubes, bottles, toys, utensils, etc.) using a process that involves melting and
C H A P TE R 9 Principles and Applications of Solidification258

solidification. Therefore, solidification is an extremely important technology used to
control the properties of many melt-derived products as well as a tool for the manu-
facturing of modern engineered materials. In the sections that follow, we first discuss
the nucleation and growth processes.
9-2 Nucleation
In the context of solidification, the term nucleation refers to the formation of the first
nano-sized crystallites from molten material. For example, as water begins to freeze,
nano-sized ice crystals, known as nuclei, form first. In a broader sense, the term nucle-
ation refers to the initial stage of formation of one phase from another phase. When a
vapor condenses into liquid, the nanoscale sized drops of liquid that appear when the
condensation begins are referred to as nuclei. Later, we will also see that there are many
systems in which the nuclei of a solid (b) will form a second solid material (a) (i.e., a-to
b-phase transformation). What is interesting about these transformations is that, in
most engineered materials, many of them occur while the material is in the solid state
(i.e., there is no melting involved). Therefore, although we discuss nucleation from a
solidification perspective, it is important to note that the phenomenon of nucleation is
general and is associated with phase transformations.
We expect a material to solidify when the liquid cools to just below its freezing (or
melting) temperature, because the energy associated with the crystalline structure of the
solid is then less than the energy of the liquid. This energy di¤erence between the liquid
and the solid is the free energy per unit volume ðDG
v
Þ and is the driving force for
solidification.
When the solid forms a solid-liquid interface is created (Figure 9-2). A surface free
energy s
sl
is associated with this interface; the larger the solid, the greater the increase
in surface energy. Thus, the total change in energy DG, shown in Figure 9-3, is:
Figure 9-1
Aluminum alloy wheels for
automobiles. (Courtesy of Simon
Askham/Stockphoto.)
9-2 Nucleation 259
