Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.


SUMMARY
V Toughness refers to the ability of materials to absorb energy before they fracture.
Tensile toughness is equal to the area under the true or engineering stress-true
strain curve. The impact toughness is measured using the impact test. This could be
very di¤erent from the tensile toughness. Fracture toughness describes how easily a
crack or flaw in a material propagates. The plane strain fracture toughness K
Ic
is a
common result of these tests.
V Weibull statistics are used to describe and characterize the variability in the
strength of brittle materials. The Weibull modulus is a measure of the variability of
the strength of a material.
V The fatigue test permits us to understand how a material performs when a cyclical
stress is applied. Knowledge of the rate of crack growth can help determine fatigue
life.
V Microstructural analysis of fractured surfaces can lead to better insights into the
origin and cause of fracture. Di¤erent microstructural features are associated with
ductile and brittle fracture as well as fatigue failure.
V The creep test provides information on the load-carrying ability of a material at
high temperatures. Creep rate and rupture time are important properties obtained
from these tests.
GLOSSARY
Beach or clamshell marks Patterns often seen on a component subjected to fatigue. Normally
formed when the load is changed during service or when the loading is intermittent, perhaps per-
mitting time for oxidation inside the crack.
Chevron pattern A common fracture feature produced by separate crack fronts propagating at
di¤erent levels in the material.
Climb Movement of a dislocation perpendicular to its slip plane by the di¤usion of atoms to or
from the dislocation line.
Conchoidal fracture Fracture surface containing a very smooth mirror zone near the origin of
the fracture, with tear lines comprising the remainder of the surface. This is typical of amorphous
materials.
Creep A time dependent, permanent deformation at high temperatures, occurring at constant
load or constant stress.
Creep rate The rate at which a material deforms when a stress is applied at a high temperature.
Creep test Measures the resistance of a material to deformation and failure when subjected to a
static load below the yield strength at an elevated temperature.
Delamination The process by which di¤erent layers in a composite will begin to debond.
Endurance limit An older concept that defined a stress below which a material will not fail in a
fatigue test. Factors as corrosion or occasional overloading can cause materials to fail at stresses
below the assumed endurance limit.
Endurance ratio The endurance limit divided by the tensile strength of the material. The ratio is
about 0.5 for many ferrous metals. See the cautionary note on endurance limit.
Fatigue life The number of cycles permitted at a particular stress before a material fails by
fatigue.
C HA P T E R 7 Fracture Mechanics, Fatigue, and Creep Behavior220
Fatigue strength The stress required to cause failure by fatigue in a given number of cycles, such
as 500 million cycles.
Fatigue test Measures the resistance of a material to failure when a stress below the yield
strength is repeatedly applied.
Fracture mechanics The study of a material’s ability to withstand stress in the presence of
a flaw.
Fracture toughness The resistance of a material to failure in the presence of a flaw.
Griffith flaw A crack or flaw in a material that concentrates and magnifies the applied stress.
Impact test Measures the ability of a material to absorb the sudden application of a load
without breaking. The Charpy and Izod tests are commonly used impact tests.
Intergranular In between grains or along the grain boundaries.
Microvoids Development of small holes in a material. These form when a high stress causes
separation of the metal at grain boundaries or interfaces between the metal and inclusions.
Notch sensitivity Measures the e¤ect of a notch, scratch, or other imperfection on a material’s
properties, such as toughness or fatigue life.
Rotating cantilever beam test An older test for fatigue testing.
Rupture time The time required for a specimen to fail by creep at a particular temperature and
stress.
S-N curve (also known as the W
€
ohler curve) A graph showing stress as a function of number of
cycles in fatigue.
Shot peening A process in which metal spheres are shot at a component. This leads to a re-
sidual compressive stress at the surface of a component and this enhances fatigue life.
Stress-corrosion A phenomenon in which materials react with corrosive chemicals in the envi-
ronment, leading to the formation of cracks and lowering of strength.
Striations Patterns seen on a fractured surface of a fatigued sample. These are on a much finer
scale than beach marks and show the position of the crack tip after each cycle.
Tempering A glass heat treatment that makes the glass safer; it does so by creating a com-
pressive stress layer at the surface.
Toughness A qualitative measure of the energy required to cause fracture of a material. A ma-
terial that resists failure by impact is said to be tough. One measure of toughness is the area under
the true stress-strain curve (tensile toughness), another is the impact energy measured during an
impact test (impact toughness). The ability of materials containing flaws to withstand load is
known as fracture toughness.
Transgranular Meaning across the grains (e.g., a transgranular fracture would be fracture in
which cracks would go through the grains).
Weibull distribution A mathematical distribution showing the probability of failure or survival
of a material as a function of the stress.
Weibull modulus (m) A parameter related to the Weibull distribution. It is an indicator of the
variability of the strength of materials resulting from a distribution of flaw sizes.
W
€
ohler curve Graph showing fatigue stress as a function of number of cycles (also known as the
S-N curve).
Glossary 221

PROBLEMS
3
Section 7-1 Fracture Mechanics
Section 7-2 The Importance of Fracture
Mechanics
7-1 Alumina Al
2
O
3
is a brittle ceramic with low
toughness. Suppose that fibers of silicon carbide
SiC, another brittle ceramic with low toughness,
could be embedded within the alumina. Would
doing this a¤ect the toughness of the ceramic ma-
trix composite? Explain.
7-2 A ceramic matrix composite contains internal flaws
as large as 0.001 cm in length. The plane strain
fracture toughness of the composite is 45 MPa
ffiffiffiffi
m
p
and the tensile strength is 550 MPa. Will the stress
cause the composite to fail before the tensile
strength is reached? Assume that f ¼ 1.
7-3 An aluminum alloy that has a plane strain fracture
toughness of 27.5 MPa
ffiffiffiffi
m
p
fails when a stress of
290 MPa is applied. Observation of the fracture
surface indicates that fracture began at the surface
of the part. Estimate the size of the flaw that ini-
tiated fracture. Assume that f ¼ 1:1.
7-4 A polymer that contains internal flaws 1 mm in
length fails at a stress of 25 MPa. Determine the
plane strain fracture toughness of the polymer.
Assume that f ¼ 1.
7-5 A ceramic part for a jet engine has a yield strength
of 517 MPa and a plane strain fracture toughness
of 5.5 MPa
ffiffiffiffi
m
p
. To be sure that the part does not
fail, we plan to assure that the maximum applied
stress is only one-third the yield strength. We use a
nondestructive test that will detect any internal
flaws greater than 0.125 cm long. Assuming that
f ¼ 1:4, does our nondestructive test have the re-
quired sensitivity? Explain.
7-6 Assume that the critical stress intensity factor or
fracture toughness ðK
Ic
Þ for a partially stabilized
zirconia is 10 MPa-m
1=2
. If there is a plate of this
ceramic with a sharp edge notch 100 mm deep and
subjected to a stress of 300 MPa, will this plate be
able to withstand this stress?
7-7 Assume that the critical stress intensity factor or
fracture toughness ðK
Ic
Þ for a partially stabilized
zirconia is 10 MPa-m
1=2
. If there is a plate of this
ceramic with an internal notch 100 mm deep and
subjected to a stress of 300 MPa, will this plate be
able to withstand this stress?
Section 7-3 Microstructural Features of Fracture
in Metallic Materials
Section 7-4 Microstructural Features of Fracture
in Ceramics, Glasses, and Composites
7-8 Concrete has exceptional strength in compression
but it fails rather easily in tension. Explain why.
7-9 What controls the strength of glasses? What can be
done to enhance the strength of silicate glasses?
Section 7-5 Weibull Statistics for Failure
Strength Analysis
7-10 Sketch a schematic of the strength of ceramics
and that of metals and alloys as a function of
probability of failure. Explain the di¤erences you
anticipate.
7-11 Why does the strength of ceramics vary consid-
erably with the size of ceramic components?
7-12 Explain the significance of the Weibull dis-
tribution.
Section 7-6 Fatigue
Section 7-7 Results of the Fatigue Test
Section 7-8 Application of Fatigue Testing
7-13 A cylindrical tool steel specimen that is 15 cm
long and 0.625 cm in diameter rotates as a canti-
lever beam and is to be designed so that failure
never occurs. Assuming that the maximum tensile
and compressive stresses are equal, determine the
maximum load that can be applied to the end of
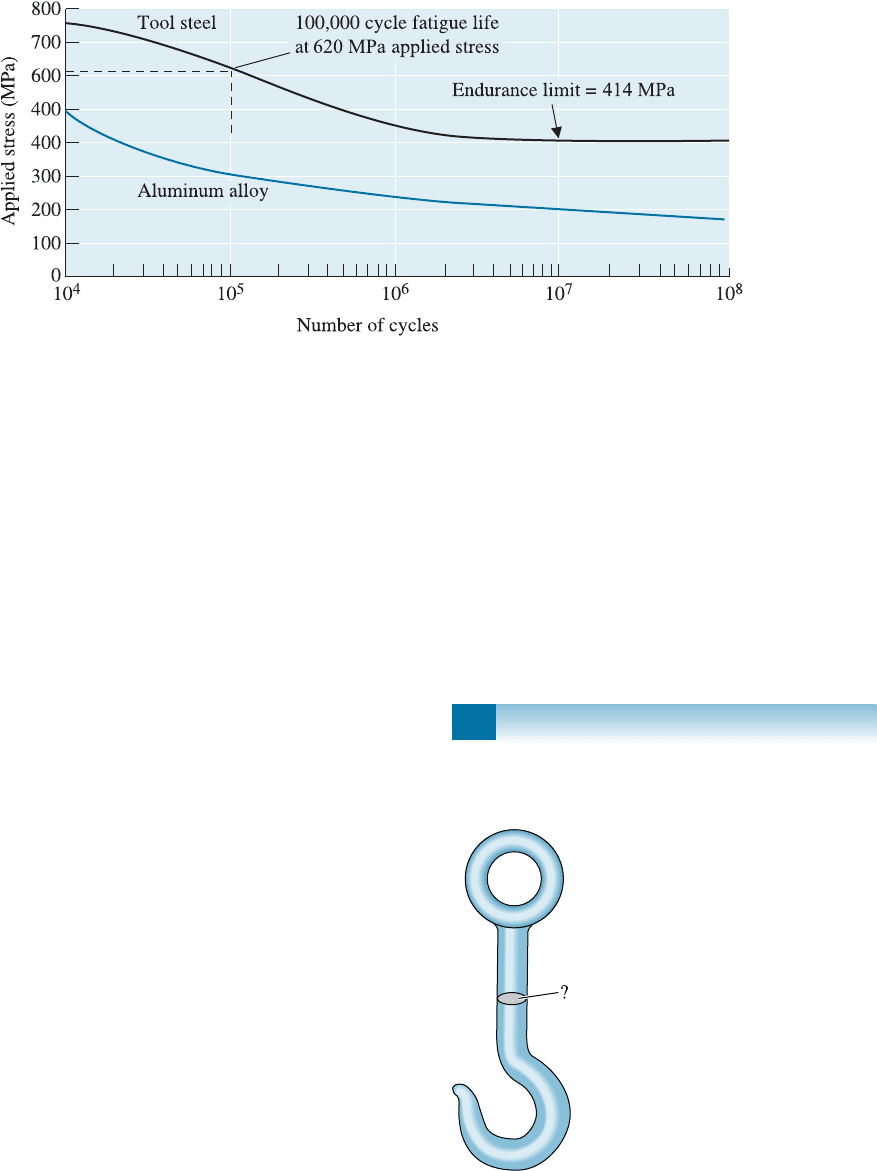
the beam. (See Figure 7-17.)
7-14 A 2-cm-diameter, 20-cm-long bar of an acetal
polymer (Figure 7-26) is loaded on one end and
Figure 7-26 The S-N fatigue curve for an acetal
polymer (for Problems 7-14, 7-16, and 7-17).
C HA P T E R 7 Fracture Mechanics, Fatigue, and Creep Behavior222
is expected to survive one million cycles of load-
ing, with equal maximum tensile and compressive
stresses, during its lifetime. What is the maximum
permissible load that can be applied?
7-15 A cyclical load of 6672 N is to be exerted at the
end of a 25-cm-long aluminum beam (Figure
7-17). The bar must survive for at least 10
6
cycles. What is the minimum diameter of the bar?
7-16 A cylindrical acetal polymer bar 20 cm long and
1.5 cm in diameter is subjected to a vibrational
load at a frequency of 500 vibrations per minute,
with a load of 50 N. How many hours will the
part survive before breaking? (See Figure 7-26.)
7-17 Suppose that we would like a part produced from
the acetal polymer shown in Figure 7-26 to sur-
vive for one million cycles under conditions that
provide for equal compressive and tensile
stresses. What is the fatigue strength, or max-
imum stress amplitude, required? What are the
maximum stress, the minimum stress, and the
mean stress on the part during its use? What ef-
fect would the frequency of the stress application
have on your answers? Explain.
7-18 Explain how fatigue failure occurs even if the
material does not see overall stress levels higher
than the yield strength.
7-19 A fatigue test is conducted on an aluminum alloy
at a frequency of 100 Hz. If the number of cycles
is 10
7
, how much time will this fatigue test take?
How much will be the time if this test were con-
ducted for 10
8
cycles?
7-20 How much time will a piezoelectric fatigue test-
ing machine take to conduct a fatigue test on a
titanium alloy for 10
10
cycles? Assume that the
frequency of this test is 10 kHz.
Section 7-9 Creep, Stress Rupture, and Stress
Corrosion
7-21 Define the term ‘‘creep’’ and di¤erentiate creep
from stress relaxation.
7-22 What is meant by the terms ‘‘stress rupture’’
and ‘‘stress corrosion?’’
7-23 What is the di¤erence between failure of a ma-
terial by creep and that by stress rupture?
Design Problems
g
7-24 A hook (Figure 7-27) for hoisting containers
of ore in a mine is to be designed using a non-
Figure 7-17 (Repeated for Problems 7-13 and 7-15) The stress-number of cycles to failure (S-N) curves for a
tool steel and an aluminum alloy.
Figure 7-27 Schematic of a hook (for Problem 7-24).
Problems 223

ferrous (not based on iron) material. (A non-
ferrous material is used because iron and steel
could cause a spark that would ignite explosive
gases in the mine.) The hook must support a
load of 111,200 N, and a factor of safety of 2
should be used. We have determined that the
cross-section labeled ‘‘?’’ is the most critical
area; the rest of the device is already well over-
designed. Determine the design requirements
for this device and, based on the mechanical
property data given in Chapters 13 and 14 and
the metal/alloy prices obtained from such sour-
ces as your local newspapers, the internet web-
site of London Metal Exchange or The Wall
Street Journal, design the hook and select an
economical material for the hook.
7-25 A support rod for the landing gear of a private
airplane is subjected to a tensile load during
landing. The loads are predicted to be as high as
177,920 N. Because the rod is crucial and fail-
ure could lead to a loss of life, the rod is to be
designed with a factor of safety of 4 (that is,
designed so that the rod is capable of support-
ing loads four times as great as expected). Op-
eration of the system also produces loads that
may induce cracks in the rod. Our non-
destructive testing equipment can detect any
crack greater than 0.05 cm deep. Based on the
materials given in Table 7-1, design the support
rod and the material, and justify your answer.
7-26 A lightweight rotating shaft for a pump on the
national aerospace plane is to be designed to
support a cyclical load of 66,720 N during serv-
ice. The maximum stress is the same in both
tension and compression. The endurance limits
or fatigue strengths for several candidate mate-
rials are shown here. Design the shaft, including
an appropriate material, and justify your sol-
ution.
Material
Endurance Limit/
Fatigue Strength
(MPa)
Al-Mn alloy 110
Al-Mg-Zn alloy 225
Cu-Be alloy 295
Mg-Mn alloy 80
Be alloy 180
Tungsten alloy 320
7-27 A ductile cast-iron bar is to support a load of
177,920 N in a heat-treating furnace used to
make malleable cast iron. The bar is located in
a spot that is continuously exposed to 500
C.
Design the bar so that it can operate for at least
10 years without failing.
C HA P T E R 7 Fracture Mechanics, Fatigue, and Creep Behavior224

8
Strain Hardening and
Annealing
Have You Ever Wondered?
9 Why does bending a copper wire make it stronger?
9 What type of steel improves the crashworthiness of cars?
9 How are aluminum beverage cans made?
9 Why do thermoplastics get stronger when strained?
9 Why is it that the strength of the metallic material around a weld could be lower than that of the
surrounding material?
In this chapter, we will learn how the strength of
metals and alloys is influenced by mechanical
processing and heat treatments. In Chapter 4, we
learned about the different techniques that can
strengthen metals and alloys (e.g., enhancing dis-
location density, decreasing grain size, alloying,
etc.). In this chapter, we will learn how to enhance
the strength of metals and alloys using cold
working, a process by which a metallic material is
simultaneously deformed and strengthened. We
will also see how hot working can be used to shape
metals and alloys by deformation at high temper-
atures without strengthening. We will learn how
the annealing heat treatment can be used to
225

enhance ductility and counter the increase in
hardness caused by cold working. The strength-
ening we obtain during cold working, which is
brought about by increasing the dislocation den-
sity, is called strain hardening or work hardening.
By controlling the thermo-mechanical processing
(i.e., combinations of mechanical processing and
heat treatment), we are able to process metallic
materials into a usable shape yet still improve
and control their mechanical properties.
The topics discussed in this chapter pertain
particularly to metals and alloys. Strain hardening
(obtained by multiplication of dislocations) re-
quires that the materials have ductility. We use
strain hardening as a tool to enhance strength of
a material. We have to counter the effects of
strain hardening in manufacturing processes. For
example, when we draw a wire or extrude a tube,
strain hardening can occur and we have to ensure
that the product still has acceptable ductility.
Cars and trucks are made by stamping out a ma-
terial known as sheet steel. This process leads to
aerodynamic and aesthetically pleasing car chas-
sis. The sheet steel used must exhibit an ability
to stretch and bend easily during stamping.
However, we must ultimately produce a strong
steel that can withstand minor bumps and major
impacts. The increase in the strength of steel as a
result of strain hardening helps us in this regard.
Furthermore, for better crashworthiness we must
use steels that exhibit rapid strain hardening dur-
ing impact loading.
What about polyme rs, glasses, and ceramics?
Do they also exhibit strain hardening? We will
show that the deformation of thermoplastic poly-
mers often produces a strengthening effect.
However, the mechanism of deformation strength-
ening is completely different in polymers than
that in metallic materials. The strength of most
brittle materials such as ceramics and glasses
depends upon the flaws and flaw-size distribution
(Chapters 6 and 7). Therefore, inorganic glasses
and ceramics do not respond well to strain hard-
ening. We, therefore, consider different strategies
to strengthen these materials. In this context, we
will learn the principles of tempering and an-
nealing of glasses. These processes make glass
stronger and safer. We will also examine con-
ditions under which ceramic materials can show
large (several hundred percent) plastic deforma-
tions. Thus, all ceramic materials are not intrin-
sically brittle! There are conditions under which
many ceramics can exhibit considerable ductility.
We begin by discussing strain hardening in
metallic materials in the context of stress-strain
curves.
8-1 Relationship of Cold Working to the Stress-Strain Curve
A stress-strain curve for a ductile metallic material is shown in Figure 8-1(a). If we apply
a stress s
1
that is greater than the yield strength (s
y
), it causes a permanent deformation
or strain. When the stress is removed, it leaves behind a strain of e
1
. If we make a tensile
test sample from the metallic material that had been previously stressed to s
1
and retest
that material, we obtain the stress-strain curve shown in Figure 8-1(b). Our new test
specimen would begin to deform plastically or flow at stress level s
1
. We define flow
stress as the stress that is needed to initiate plastic flow in a previously deformed mate-
rial. Thus, s
1
is now the flow stress of the material. If we continue to apply a stress until
we reach s
2
, then release the stress and again retest the metallic material, the new flow
stress is s
2
. Each time we apply a higher stress, the flow stress and tensile strength in-
crease and the ductility decreases. We eventually strengthen the metallic material until
C HA P T E R 8 Strain Hardening and Annealing226
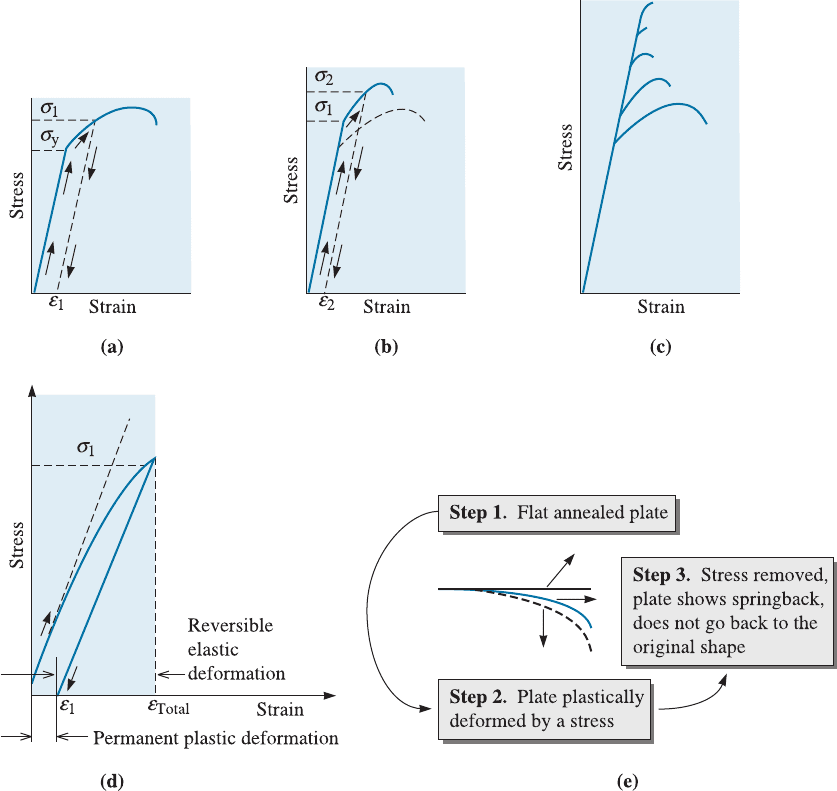
the flow stress, tensile, and breaking strengths are equal and there is no ductility [Figure
8-1(c)]. At this point, the metallic material can be plastically deformed no further. Fig-
ures 8-1(d) and (e) are related to springback, a concept that is discussed a bit later in this
section.
By applying a stress that exceeds the original yield strength of the metallic material,
we have strain hardened or cold worked the metallic material, while simultaneously
deforming it. This is the basis for many manufacturing techniques, such as wire
drawing. Figure 8-2 illustrates several manufacturing processes that make use of both
Figure 8-1 Development of strain hardening from the stress-strain diagram. (a) A specimen is
stressed beyond the yield strength before the stress is removed. (b) Now the specimen has a
higher yield strength and tensile strength, but lower ductility. (c) By repeating the procedure,
the strength continues to increase and the ductility continues to decrease until the alloy
becomes very brittle. (d) Note the total strain and the elastic strain recovery lead to remnant
plastic strain and (e) illustration of springback. (This article was published in Engineering
Materials I, Second Edition, M.F. Ashby and D.R.H. Jones. Copyright > Butterworth-
Heinemann (1996). )
8-1 Relationship of Cold Working to the Stress-Strain Curve 227

Figure 8-2 Manufacturing processes that make use of cold working as well as hot working.
Common metalworking methods. (a) Rolling. (b) Forging (open and closed die). (c) Extrusion
(direct and indirect). (d) Wire drawing. (e) Stamping. (Adapted from Mechanical Behavior of
Materials by Meyers, M.A. & Chawla, K.K. 1999, Prentice Hall.) (Source: Adapted from
Mechanical Behavior of Materials, by M.A. Meyers and K.K. Chawla, p. 292, Fig. 6-1.
Copyright > 1999 Prentice Hall. Adapted with permission of Pearson Education, Inc.,
Upper Saddle River, NJ.)
C HA P T E R 8 Strain Hardening and Annealing228
cold-working and hot-working processes. We will discuss the di¤erence between hot
working and cold working later in this chapter (Section 8-7). Many techniques are used
to simultaneously shape and strengthen a material by cold working (Figure 8-2). For
example, rolling is used to produce metal plate, sheet, or foil. Forging deforms the metal
into a die cavity, producing relatively complex shapes such as automotive crankshafts
or connecting rods. In drawing, a metallic rod is pulled through a die to produce a wire
or fiber. In extrusion, a material is pushed through a die to form products of uniform
cross-sections, including rods, tubes, or aluminum trims for doors or windows. Deep
drawing is used to form the body of aluminum beverage cans. Stretch forming and
bending are used to shape sheet material. Thus, cold working is an e¤ective way of
shaping metallic materials while simultaneously increasing their strength. The down
side of this process is the loss of ductility. If you take a metal wire and bend it re-
peatedly it will harden and eventually break because of strain hardening. Strain hard-
ening is used in many products, especially those that are not going to be exposed to
very high temperatures. For example, an aluminum beverage can derives almost 70% of
its strength as a result of strain hardening that occurs during its fabrication. Some of the
strength of aluminum cans also comes from the alloying elements (e.g., Mg) added.
Note that many of the processes such as rolling, can be conducted using both cold and
hot working. The pros and cons of using each will be discussed later in this chapter.
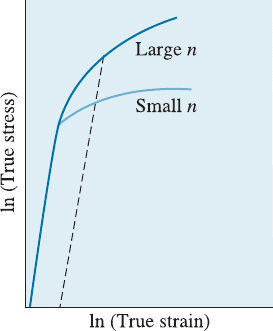
Strain-Hardening Exponent (n) The response of a metallic material to cold working is
given by the strain-hardening exponent, n, which is the slope of the plastic portion of the
true stress-true strain curve in Figure 8-3 when a logarithmic scale is used:
s
t
¼ Ke
n
t
ð8-1aÞ
or
ln s
t
¼ ln K þ n ln e
t
ð8-1bÞ
The constant K (strength coe‰cient) is equal to the stress when e
t
¼ 1. The strain-
hardening exponent is relatively low for HCP metals, but is higher for BCC and, par-
ticularly, for FCC metals (Table 8-1). Metals with a low strain-hardening exponent
respond poorly to cold working. If we take a copper wire and bend, the bent wire is
stronger as a result of strain hardening.
Figure 8-3
The true stress-true strain curves for metals with large
and small strain-hardening exponents. Larger degrees of
strengthening are obtained for a given strain for the
metal with the larger n.
8-1 Relationship of Cold Working to the Stress-Strain Curve 229
