Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.

are prepared in order to minimize the likelihood of a fatigue failure. Shot peening is also
a process that is used very e¤ecti vely to enhance fatigue life of materials. Small metal
spheres are shot at the component. This leads to a residual compressive stress at the
surface similar to strengthening of inorganic glasses by tempering (Section 7-9).
EXAMPLE 7-11
Design of a Rotating Shaft
A solid shaft for a cement kiln produced from tool steel must be 240 cm long
and must survive continuous operation for one year with an applied load of
55,600 N. The shaft makes one revolution per minute during operation. Design
a shaft that will satisfy these requirements. The S-N curve for the tool steel is
shown in Figure 7-17.
SOLUTION
The fatigue life required for our design is the total number of cycles N that the
shaft will experience in one year:
N ¼ð1 cycle=minÞð60 min=hÞð24 h=d Þð365 d=yÞ
N ¼ 5:256 10
5
cycles=y
where y ¼ year, d ¼ day, and h ¼ hour.
From Figure 7-17, the applied stress therefore must be less than about
496 MPa. From Equation 7-13, the diameter of the shaft must be:
Gs ¼
16FL
pd
3
¼ 5:09
FL
d
3
496 MPa ¼
ð5:09Þð240 cmÞð55;600 NÞ
d
3
d ¼ 11:1cm
A shaft with a diameter of 11.1 cm should operate for one year under these
conditions. However, a significant margin of safety might be incorporated in
the design. In additi on, we might consider producin g a shaft that would never
fail.
Let us assume the factor of safety to be 2 (i.e., we will assume that the
maximum allowed stress level will be 496/2 ¼ 248 MPa). The minimum diam-
eter required to prevent failure would now be:
248 MPa ¼
ð5:09Þð240 cmÞð55;600 NÞ
d
3
d ¼ 14 cm
Selection of a larger shaft reduces the stress level and makes fatigue less likely
to occur or delays the failure. Other considerations might, of course, be im-
portant. High temperatures and corrosive conditions are inherent in producing
cement. If the shaft is heated or attacked by the corrosive environment, fatigue
is accelerated. Thus, in the applications involving fatigue of components regu-
lar inspections of the components go a long way toward avoiding a catastro-
phic failure.
C HA P T E R 7 Fracture Mechanics, Fatigue, and Creep Behavior210
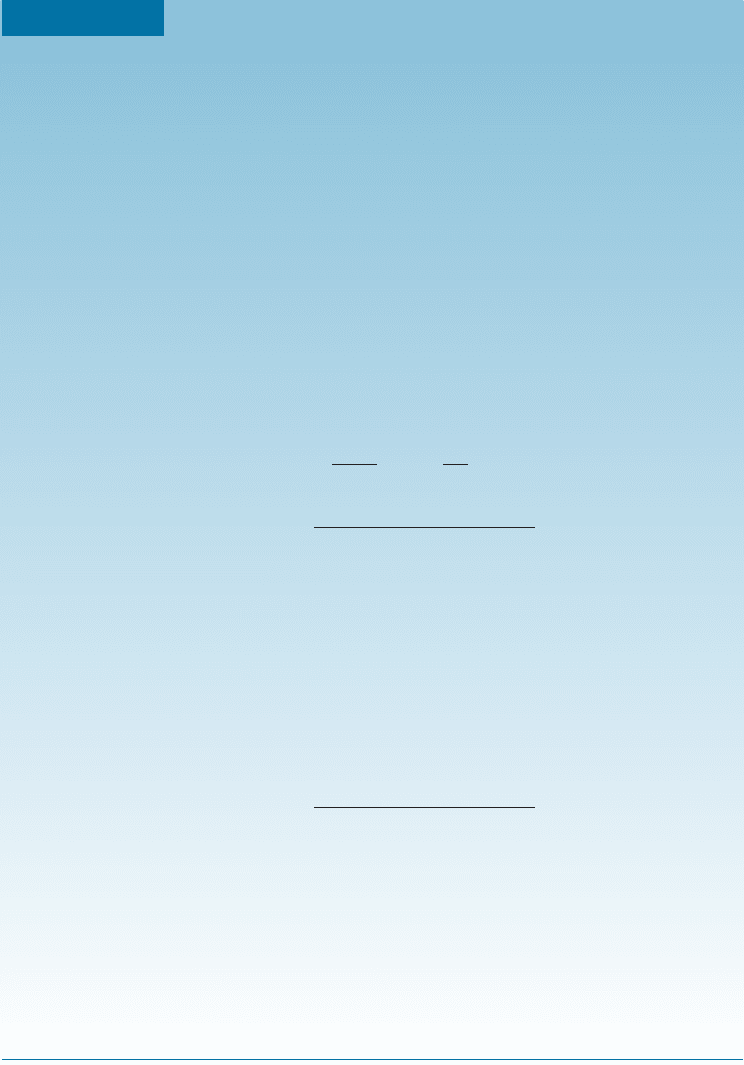
EXAMPLE 7-12 Do Materials have an Infinite Fatigue Life?
Typically, engineering materials such as alloys and composites are tested for
fatigue up to about 10
7
cycles (known as low-cycle fatigue). Following are the
data for an aluminum alloy designated as 7075T73 (the solid curve labeled as
A), used for making helicopter propellers. The solid circles are the data for an
aluminum matrix composite reinforced with SiC fibers (labeled as the B-dashed
curve). (a) Based on these low-cycle fatigue data, which material appears to
have better fati gue resistance? (b) What appears to be the fatigue strength of
the aluminum matrix composites? (c) Can these data be used to predict the fa-
tigue life of these materials for longer tests involving fatigue cycles up to 10
10
?
SOLUTION
(a) From the low-cycle (i.e., up to 10
7
cycles) fatigue data, it is clear that the
aluminum alloy reinforced with SiC fibers (material B) has a better fatigue
behavior at a low number of cycles.
(b) The fatigue curve for material B appears to show a plateau at around 10
5
cycles. This would suggest a fatigue strength of @300 MPa.
(c) If we examine the data between 10
6
and 10
7
cycles, it appears that the
stress to failure for the SiC reinforced composite (material B) now is lower
and is approaching that for the alloy 7075T73 (material A). Thus, it prob-
ably would not be safe to assume that over a longer period of time (up to
10
10
cycles) that the SiC reinforced material will have a better fatigue life
than alloy 7075T73.
In fact, measurements conducted by Bathias and co-workers show that
maximum stress that can be supported for the SiC reinforced alloy decreases
even more to almost 200 MPa at @10
10
cycles.
Thus, an observation is that we must make fatigue measurements in the
giga-cycle range to see the fatigue behavior and not just extend what is seen
only in the low cycle fatigue regime. In fact, most materials may not have an
infinite fatigue life, as is mostly suggested by the low-cycle fatigue data. These
giga-cycle fatigue tests can be done on modern fatigue machines that are driven
by piezoelectric materials at a frequency of @20 kHz, compared to 100 Hz.
Figure 7-18
Low-cycle fatigue data for an
aluminum alloy and an
aluminum-alloy matrix
composite reinforced with SiC.
(Source: Ref. Bathias, C.,
Fatigue and Fracture in
Engineering Materials
Structures, Blackwell
Publishing, page 559–565,
Vol. 22 (1999).)
7-7 Results of the Fatigue Test 211
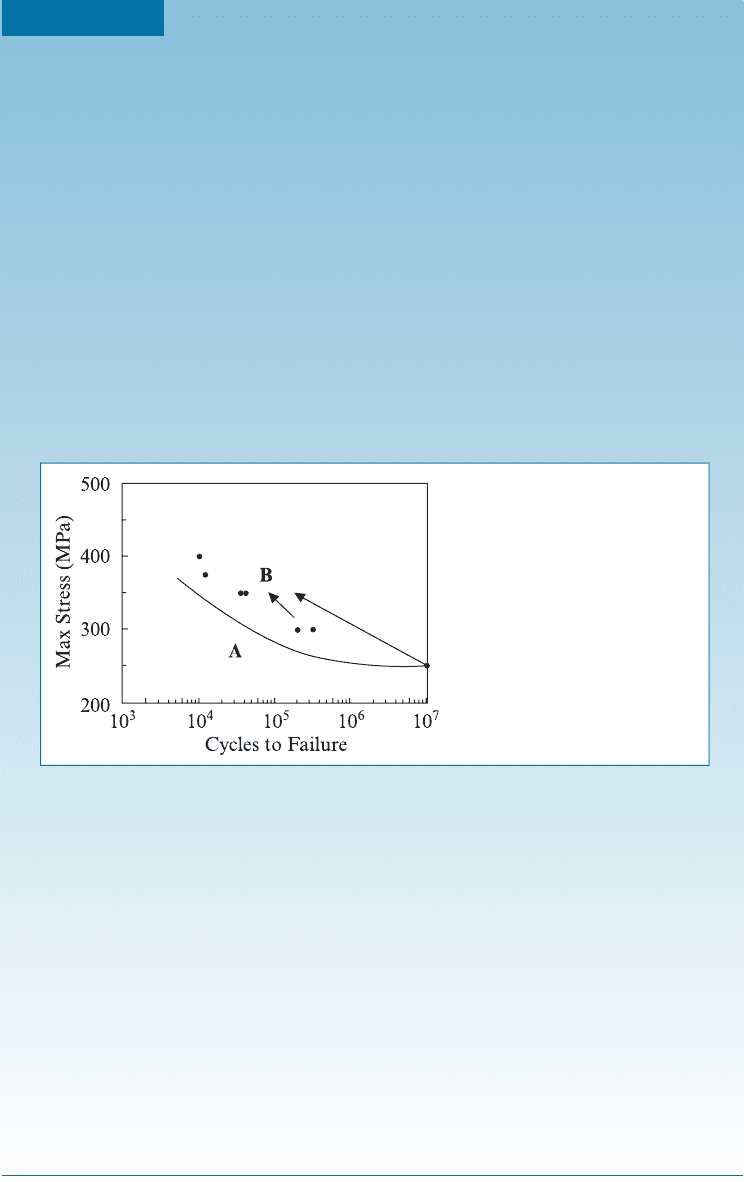
7-8 Application of Fatigue Testing
Material components are often subjected to loading conditions that do not give equal
stresses in tension and compression (Figure 7-19). For example, the maximum stress
during compression may be less than the maximum tensile stress. In other cases, the
loading may be between a maximum and a minimum tensile stress; here the S-N curve
is presented as the stress amplitude versus number of cycles to failure. Stress amplitude
(s
a
) is defined as half of the di¤erence between the maximum and minimum stresses;
Figure 7-19
Examples of stress cycles. (a) Equal
stress in tension and compression,
(b) greater tensile stress than
compressive stress, and (c) all of the
stress is tensile.
C HA P T E R 7 Fracture Mechanics, Fatigue, and Creep Behavior212
mean stress (s
m
) is defined as the average between the maximum and minimum stresses:
s
a
¼
s
max
s
min
2
ð7-15Þ
s
m
¼
s
max
þ s
min
2
ð7-16Þ
A compressive stress is considered a ‘‘negative’’ stress. Thus, if the maximum tensile stress
is 345 MPa and the minimum stress is a 69 MPa compressive stress, using Equa-
tions 7-15 and 7-16 the stress amplitude is 138 MPa and the mean stress is 207 MPa.
As the mean stress increases, the stress amplitude must decrease in order for the
material to withstand the applied stresses. This condition can be summarized by the
Goodman relationship:
s
a
¼ s
fs
1
s
m
s
TS
ð7-17Þ
where s
fs
is the desired fatigue strength for zero mean stress and s
TS
is the tensile
strength of the material. Therefore, in a typical rotating cantilever beam fatigue test,
where the mean stress is zero, a relatively large stress amplitude can be tolerated with-
out fatigue. If, however, an airplane wing is loaded near its yield strength, vibrations of
even a small amplitude may cause a fatigue crack to initiate and grow.
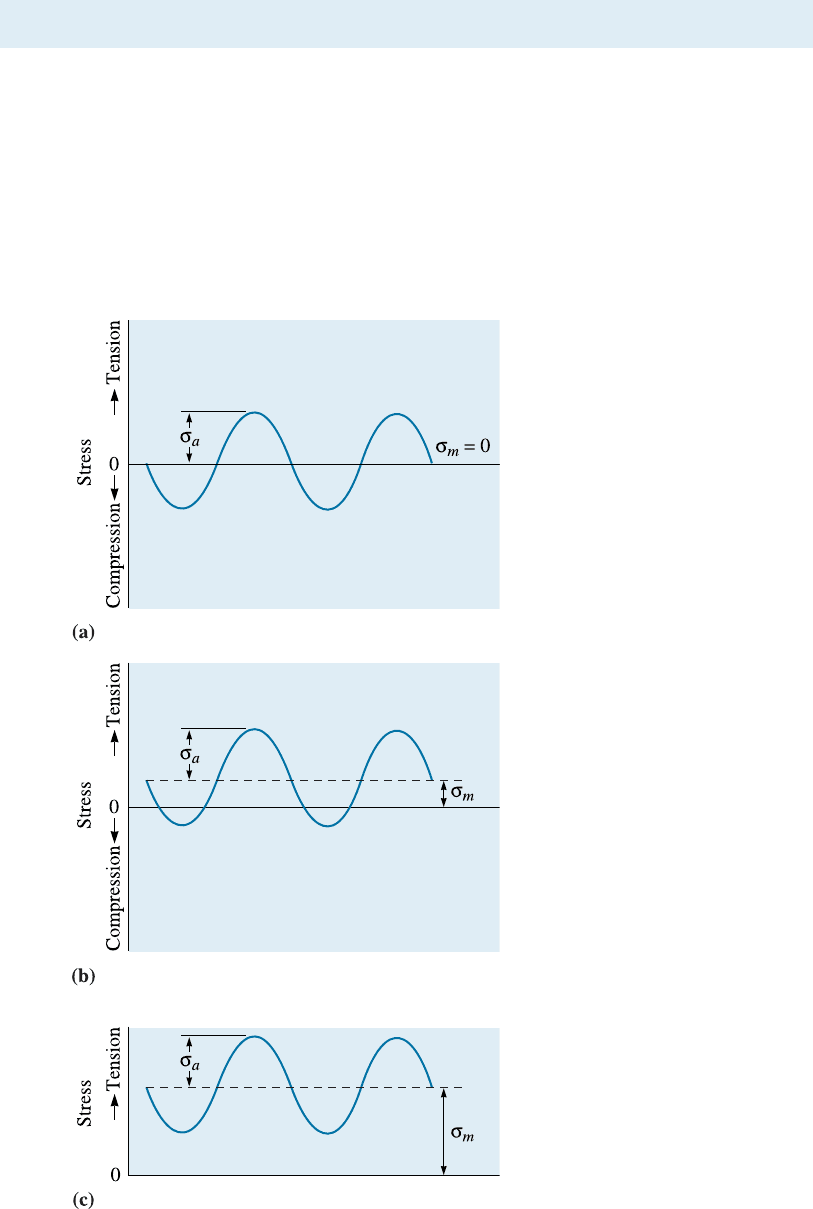
Crack Growth Rate In many cases, a component may not be in danger of failure even
when a crack is present. To estimate when failure might occur, the rate of propagation
of a crack becomes important. Figure 7-20 shows the crack growth rate versus the
range of the stress-intensity factor DK, which characterizes crack geometry and the
stress amplitude. Below a threshold DK, a crack does not grow; for somewhat higher
Figure 7-20
Crack growth rate versus stress-
intensity factor range for a high-
strength steel. For this steel,
C ¼ 1:62 10
12
and n ¼ 3: 2 for
the units shown.
7-8 Application of Fatigue Testing 213
stress-intensities, cracks grow slowly; and at still higher stress-intensities , a crack grows
at a rate given by:
da
dN
¼ CðDKÞ
n
ð7-18Þ
In this equation, C and n are empirical constants that depend upon the material. Fi-
nally, when DK is still higher, cracks grow in a rapid and unstable manner until fracture
occurs.
The rate of crack growth increases as a crack increases in size, as predicted from
the stress intensity factor (Equation 7-1):
DK ¼ K
max
K
min
¼ fs
max
ffiffiffiffiffiffi
pa
p
fs
min
ffiffiffiffiffiffi
pa
p
¼ fDs
ffiffiffiffiffiffi
pa
p
ð7-19Þ
If the cyclical stress Dsðs
max
s
min
Þ is not changed, then as crack length a increases,
DK and the crack growth rate da=dN increase. In using this expression, however, one
should note that a crack will not propagate during compression. Therefore, if s
min
is
compressive, or less than zero, then s
min
should be set equal to zero.
Knowledge of crack growth rate is of assistance in designing components and in
nondestructive evaluation to determine if a crack poses imminent danger to the struc-
ture. One approach to this problem is to estimate the number of cycles required before
failure occurs. By rearranging Equation 7-18 and substituting for DK:
dN ¼
1
Cf
n
Ds
n
p
n=2
da
a
n=2
If we integrate this expression between the initial size of a crack and the crack size re-
quired for fracture to occur, we find that
N ¼
2½ða
c
Þ
ð2nÞ=2
ða
i
Þ
ð2nÞ=2
ð2 nÞCf
n
Ds
n
p
n=2
ð7-20Þ
where a
i
is the initial flaw size and a
c
is the flaw size required for fracture. If we know
the material constants n and C in Equation 7-18, we can estimate the number of cycles
required for failure for a given cyclical stress (Example 7-13).
EXAMPLE 7-13
Design of a Fatigue-Resistant Plate
A high-strength steel plate (Figure 7-20), which has a plane strain fracture
toughness of 80 MPa
ffiffiffiffi
m
p
, is alternately loaded in tension to 500 MPa and in
compression to 60 MPa. The plate is to survive for 10 years, with the stress
being applied at a frequency of once every 5 minutes. Design a manufacturing
and testing procedure that assures that the component will serve as intended.
SOLUTION
To design our manufacturing and testing capability, we must determine the
maximum size of any flaws that might lead to failure within the 10-year period.
The critical crack size (a
c
), using the fracture toughness and the maximum
stress, is:
K
Ic
¼ fs
ffiffiffiffiffiffiffi
pa
c
p
80 MPa
ffiffiffiffi
m
p
¼ð1Þð500 MPaÞ
ffiffiffiffiffiffiffi
pa
c
p
a
c
¼ 0:0081 m ¼ 8:1mm
C HA P T E R 7 Fracture Mechanics, Fatigue, and Creep Behavior214

The maximum stress is 500 MPa; however, the minimum stress is zero, not
60 MPa in compression, because cracks do not propagate in compression.
Thus, Ds is
Ds ¼ s
max
s
min
¼ 500 0 ¼ 500 MPa
We need to determine the minimum number of cycles that the plate must
withstand:
N ¼ð1 cycle=5 minÞð60 min=hÞð24 h=dÞð365 d=yÞð10 yÞ
N ¼ 1;051;200 cycles
If we assume that f ¼ 1 for all crack lengths and note that C ¼ 1: 62 10
12
and n ¼ 3:2 in Equation 7-20, then
1;051;200 ¼
2½ð0:008Þ
ð23:2Þ=2
ða
i
Þ
ð23:2Þ=2
ð2 3:2Þð1:62 10
12
Þð1Þ
3:2
ð500Þ
3:2
p
3:2=2
1;051;200 ¼
2½18 a
0:6
i
ð1:2Þð1:62 10
12
Þð1Þð4:332 10
8
Þð6:244Þ
a
0:6
i
¼ 18 þ 2764 ¼ 2782
a
i
¼ 1:82 10
6
m ¼ 0: 00182 mm for surface flaws
2a
i
¼ 0:00364 mm for internal flaws
The manufacturing process must produce surface flaws smaller than 0.00182 mm
in length. We can conduct a similar calculation for specifying a limit on edge
cracks. In addition, nondestructive tests must be available to assure that cracks
exceeding this length are not present.
Effect of Temperature As the material’s temperature increases, both fatigue life and
endurance limit decrease. Furthermore, a cyclical temperature change encourages fail-
ure by thermal fatigue; when the material heats in a nonuniform manner, some parts of
the structure expand more than others. This nonuniform expansion introduces a stress
within the material, and when the structure later cools and contracts, stresses of the
opposite sign are imposed. As a consequence of the thermally induced stresses and
strains, fatigue may eventually occur. The frequency with which the stress is applied
also influences fatigue behavior. In particular, high-frequency stresses may cause poly-
mer materials to heat; at increased temperature, polymers fail more quickly. Chemical
e¤ects of temperature (e.g., oxidation) must also be considered.
7-9 Creep, Stress Rupture, and Stress Corrosion
If we apply stress to a material at an elevated tempe rature, the material may stretch and
eventually fail, even though the applied stress is less than the yield strength at that
temperature. A time dependent per manent deformation under a constant load or con-
stant stress and at high temperatures is known as creep. A large number of failures
occurring in components used at high temperatures can be attributed to creep or a
combination of creep and fatigue. Essentially, in creep the material begins to flow
slowly. Di¤usion, dislocation glide or climb, or grain boundary sliding can contribute
7-9 Creep, Stress Rupture, and Stress Corrosion 215
to the creep of metallic materials. Polymeric materials also show creep. In ductile met-
als and alloys subjected to creep, fracture is accompanied by necking, void nucleation
and coalescence, or grain boundary sliding.
A material is considered failed by creep even if it has not actually fractured. When
a material does actually creep and then ultimately break the fracture is defined as stress
rupture. Normally, ductile stress-rupture fractures include necking and the presence of
many cracks that did not have an opportunity to produce final fracture. Furthermore,
grains near the fracture surface tend to be elongated. Ductile stress-rupture failures
generally occur at high creep rates and relatively low exposure temperatures and have
short rupture times. Brittle stress-rupture failures usually show little necking and occur
more often at smaller creep rates and high temperatures. Equiaxed grains are observed
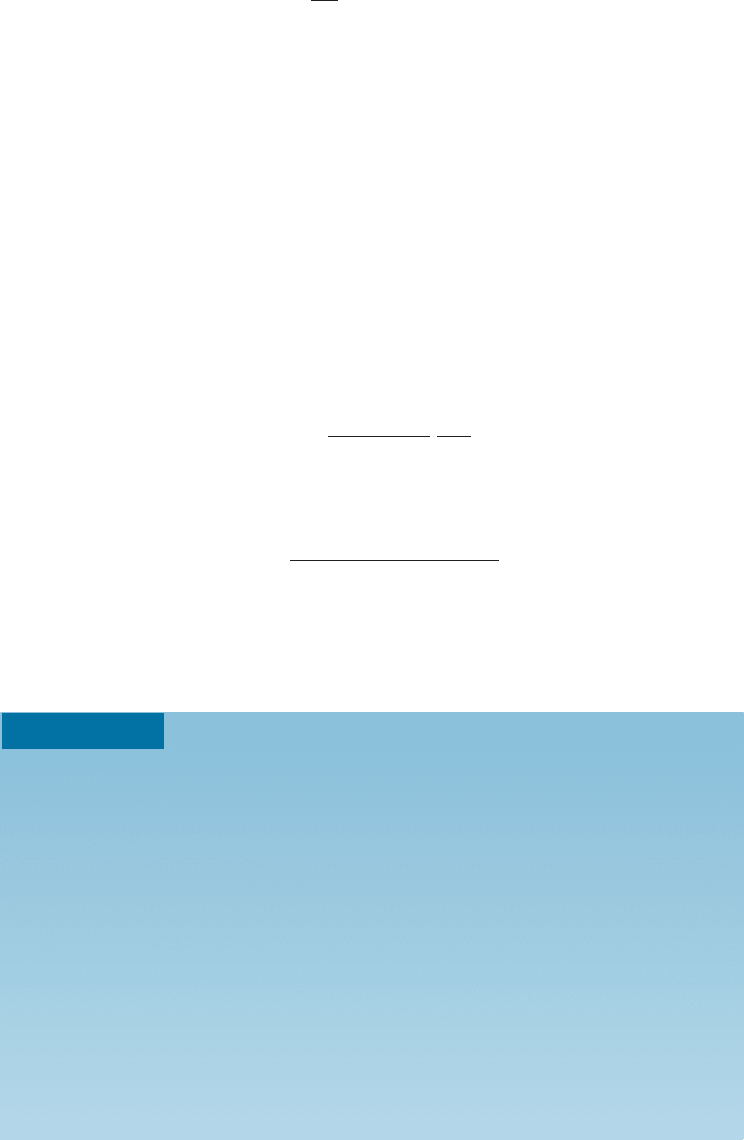
near the fracture surface. Brittle failure typically occurs by formation of voids at the
intersection of three grain boundaries and precipitation of additional voids along grain
boundaries by di¤usion processes (Figure 7-21).
Stress-Corrosion Stress-corrosion is a phenomenon in which materials react with cor-
rosive chemicals in the environment. This leads to formation of cracks and lowering
of strength. Stress-corrosion can occur at stresses well below the yield strength of the
metallic, ceramic, or glassy material due to attack by a corrosive medium. In metallic
materials, deep, fine corrosion cracks are produced, even though the metal as a whole
shows little uniform attack. The stresses can be either externally applied or stored re-
sidual stresses. Stress-corrosion failures are often identified by microstructural exami-
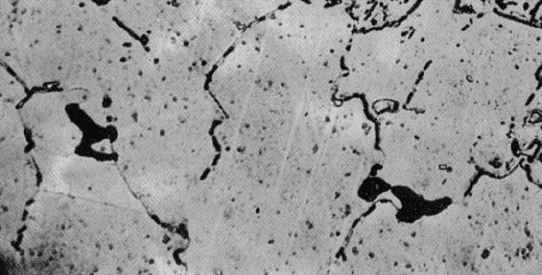
nation of the nearby metal. Ordinarily, extensive branching of the cracks along grain
boundaries is observed (Figure 7-22). The location at which cracks initiated may be
identified by the presence of a corrosion product.
Inorganic silicate glasses are especially prone to failure by reaction with water
vapor. It is well known that the strength of silica fibers or silica glass products is very
high when these materials are protected from water vapor. As the fibers or silica glass
components get exposed to water vapor, corrosion reactions begin leading to formation
of surface flaws, which ultimately cause the cracks to grow when stress is applied.
Polymeric coatings are applied to optical fibers to prevent them from reacting with
water vapor. For bulk glasses, special heat treatments such as tempering are used.
Tempering produces an overall compressive stress on the surface of glass. Thus, even if
the glass surface reacts with water vapor the cracks do not grow since the overall stress
at the surface is compressive. If we create a flaw that will penetrate the compressive
Figure 7-21 Creep cavities formed at grain boundaries in an austentic stainless steel (500).
(From ASM Handbook, Vol. 7, (1972) ASM International, Materials Park, OH 44073.)
C HA P T E R 7 Fracture Mechanics, Fatigue, and Creep Behavior216
stress region on the surface, tempered glass will shatter. Tempered glass is used widely
in building and automotive applications.
Figure 7-22
Photomicrograph of a metal near a stress-
corrosion fracture, showing the many
intergranular cracks formed as a result of the
corrosion process (200). (From ASM
Handbook, Vol. 7, (1972) ASM International,
Materials Park, OH 44073.)
EXAMPLE 7-14
Failure Analysis of a Pipe
A titanium pipe used to transport a corrosive material at 400
C is found to fail
after several months. How would you determine the cause for the failure?
SOLUTION
Since a period of time at a high temperature was required before failure
occurred, we might first suspect a creep or stress-corrosion mechanism for fail-
ure. Microscopic examination of the material near the fracture surface would
be advisable. If many tiny, branched cracks leading away from the surface are
noted, stress-corrosion is a strong possibility. However, if the grains near the
fracture surface are elongated, with many voids between the grains, creep is a
more likely culprit.
7-10 Evaluation of Creep Behavior
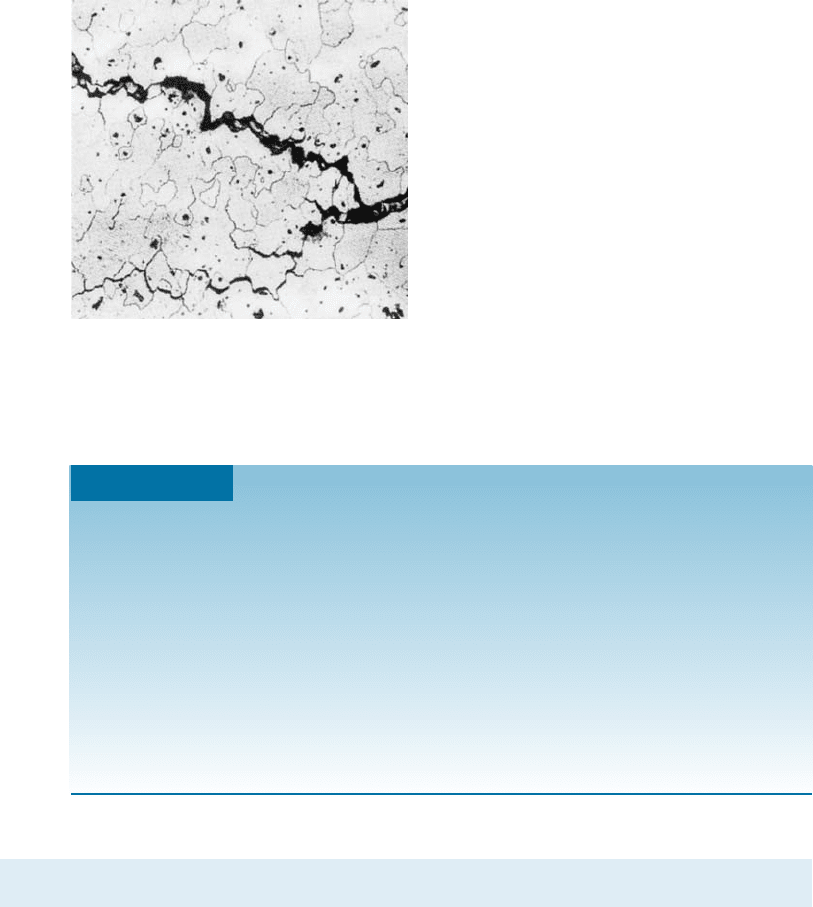
To determine the creep characteristics of a material, a constant stress is applied to a
heated specimen in a creep test . As soon as the stress is applied, the specimen stretches
elastically a small amount e
0
(Figure 7-23), depending on the applied stress and the
modulus of elasticity of the material at the high temperature. Creep testing can also be
conducted under a constant load and is important from an engineering design view -
point.
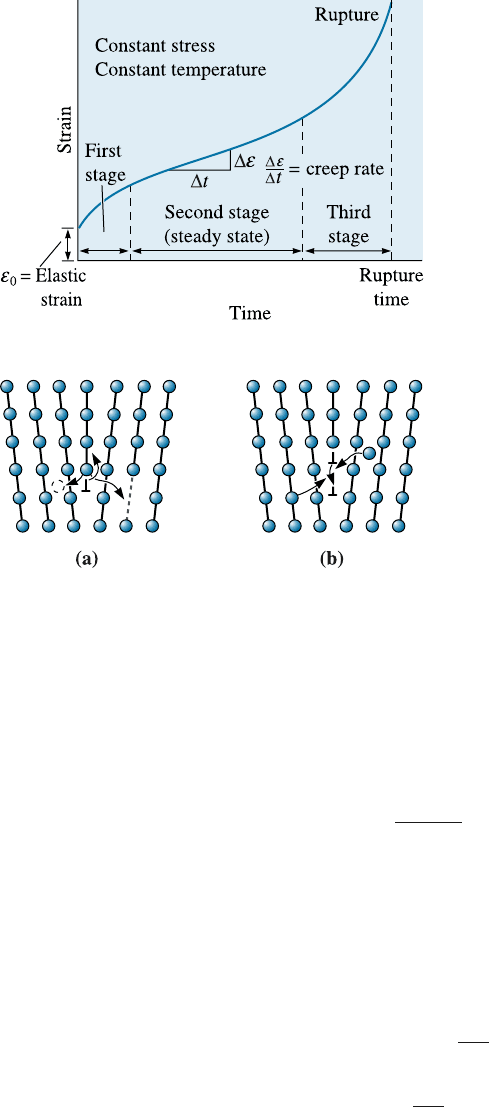
Dislocation Climb High temperatures permit dislocations in a metallic material to
climb. In climb, atoms move either to or from the dislocation line by di¤usion, causing
the dislocation to move in a direction that is perpendicular, not parallel, to the slip
plane (Figure 7-24). The dislocation escapes from lattice imperfections, continues to
slip, and causes additional deformation of the specimen even at low applied stresses.
7-10 Evaluation of Creep Behavior 217
Creep Rate and Rupture Times During the creep test, strain or elongation is measured
as a function of time and plotted to give the creep curve (Figure 7-23). In the first stage
of creep of metals, many dislocations climb away from obstacles, slip, and contribute
to deformation. Eventually, the rate at which dislocations climb away from obstacles
equals the rate at which dislocations are blocked by other imperfections. This leads to
second-stage, or steady-state, creep. The slope of the steady-state portion of the creep
curve is the creep rate:
Creep rate ¼
D strain
D time
ð7-21Þ
Eventually, during third-stage creep, necking begins, the stress increases, and the speci-
men deforms at an accelerated rate until failure occurs. The time required for failure
to occur is the rupture time. Either a higher stress or a higher temperature reduces the
rupture time and increases the creep rate (Figure 7-25).
The combined influence of applied stress and temperature on the creep rate and
rupture time (t
r
) follows an Arrhenius relationship:
Creep rate ¼ Cs
n
exp
Q
c
RT
ð7-22Þ
t
r
¼ Ks
m
exp
Q
r
RT
ð7-23Þ
where R is the gas constant, T is the temperature in Kelvin, C, K, n, and m are con-
stants for the material, Q
c
is the activation energy for creep, and Q
r
is the activation
energy for rupture. In particular, Q
c
is related to the activation energy for self-di¤usion
Figure 7-23
A typical creep curve showing the
strain produced as a function of
time for a constant stress and
temperature.
Figure 7-24
Dislocations can climb (a) when
atoms leave the dislocation line to
create interstitials or to fill vacancies
or (b) when atoms are attached to the
dislocation line by creating vacancies
or eliminating interstitials.
C HA P T E R 7 Fracture Mechanics, Fatigue, and Creep Behavior218
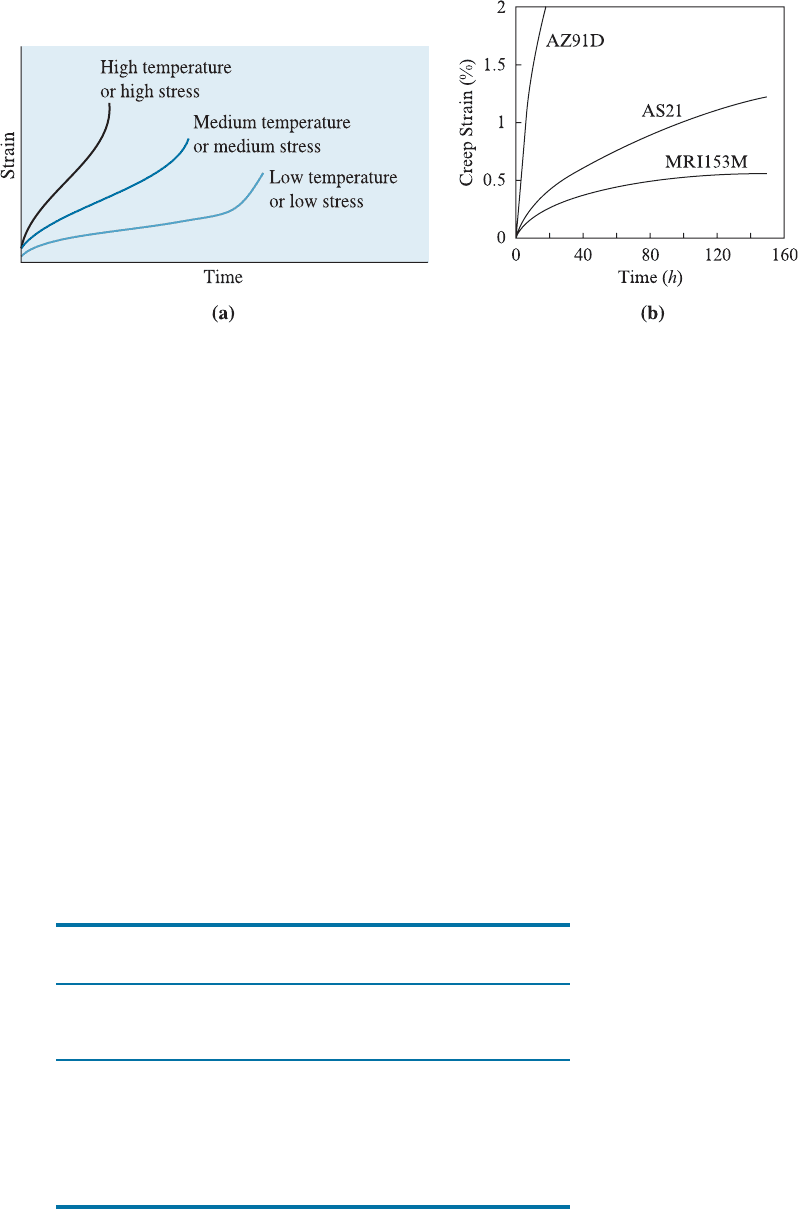
when dislocation climb is important. Relative creep resistance of three magnesium
alloys, namely AZ91D, AS21, and MRI153M is shown in Figure 7-25(b).
In creep of polycrystalline ceramics, other factors—including grain boundary slid-
ing and nucleation of microcracks—are particularly important. Often, a noncrystalline
or glassy material is present at the grain boundaries; the activation energy required for
the glass to deform is low, leading to high creep rates compared with completely crys-
talline ceramics. For the same reason, creep occurs at a rapid rate in ceramic glasses
and amorphous polymers.
The stress exponent ðnÞ and the creep activation energy ðQ
c
Þ encountered in Equa-
tion 7-22 for Mg alloys MRI 151, MRI 153, and As21 are shown in Table 7-2.
In Mg alloys containing Al, an intermetallic compound Mg
12
Al
12
can form at grain
boundaries, and this causes the creep resistance to be lowered. The creep resistance is
enhanced by adding small concentrations of alkaline earth metals, such as Ca or Mg.
These metals react with Al preferentially and form other intermetallics (i.e., Al
2
Ca and
Al
2
Mg). These intermetallics have melting temperatures greater than 1000
C. This
translates into enhanced creep resistance, as grain boundary sliding is suppressed.
Figure 7-25 (a) The effect of temperature or applied stress on the creep curve. (b) Relative
creep resistance of Mg alloys AZ91D, AS21, and MRI153M. (Ref. A.M. Russell and K.L. Lee
in Structure-Property Relations in Nonferrous Metals, Publ. Wiley, (2005), page 176.)
TABLE 7-2 9 Creep exponent (n) and activation energy (Q
c
) for
some Mg Alloys
Alloy
Stress Exponent (n)
T F 135˚ C, stress
85–110 MPa
Activation energy (Q
c
)
kJ/mol (90 MPa,
130–150˚ C)
MRI 151 7.0 175
MRI 153 7.6 181
AS21 19.5 166
(Source: A.M. Russell and K.L. Lee in Structure-Property Relations in
Nonferrous Metals, Publ. Wiley, (2005), page 176.)
7-10 Evaluation of Creep Behavior 219
