Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


5.6 Systematic Classification of Energy Conversions by Consilient Protein-based Machines
175
elastic force. Yet another interesting absorption
in the dielectric relaxation spectrum occurs in
the middle of the acoustic frequency range. All
of these absorptions are expected to be rele-
vant to electromagneto-mechanical transduc-
tion, and they would be expected to exhibit
some reversibility. The most popular aspect of
electromagneto-mechanical transduction, pho-
tomechanical transduction, as relevant to the
consilient mechanism of energy conversion
would be irreversible. A mechanical input alone
would not reversibly result in emission of a
photon of light. While stretching an elastic-con-
tractile model protein could conceivably trigger
emission of a photon, the energy recovered on
relaxation could not reconstitute a chro-
mophore capable of photon emission.
5.6.4.5.1 Photo -^ Mechanical Transduction
The examples demonstrated to date using
elastic protein-based polymers have involved
the light-driven trans to cis geometrical iso-
merization that raises the temperature of the
inverse temperature transition. This has been
seen with azobenzene^^ and with the more bio-
logically relevant cinnamide, discussed above.^^
This capacity to effect relaxation can be
reversed by heating or by a different frequency
of Ught to regain the contracted state. Although
a practical reversibility, this should not be con-
fused with the reversibility in the sense that a
mechanical energy input would result in the
emission of a photon of light.
5.6.4.5.2 Mechano
Transduction
Electromagnetic
Stretching is expected to increase the dielectric
absorption near 5 GHz and to decrease the
dielectric absorptions near
3
kHz and
5
MHz.
These effects could be used in the design of a
number of transducers. Perhaps these effects
could be referred to as mechano ^ dielectric
transduction. Furthermore, the
3
kHz dielectric
relaxation exhibits the counterpart of an
acoustic absorption."^^'^^ This means that the
mechanical oscillation of a sound wave could
be detected as a change in the
3
kHz dielectric
relaxation.
5,6.4.6 Device Reduction of a Linear
Contracting Engine to a Rotary Engine
5.6.4.6.1 Collagen-Driven Rotary Engine
of Katchalsky
The reduction of the elementary elastic strip
that contracts and relaxes to a rotary motor has
been demonstrated by Steinberg et al.^^ In that
case,
a circular elastic band was constructed. In
a continuous manner it was wrapped around a
set of pulleys and through a pair of baths, one
bath providing the correct chemical energy for
contraction and the other for relaxation. In this
way a chemomechanical rotary motor was
constructed.
5.6.4.6.2 Generalization to Input Energies
Other Than Chemical
Similarly with one bath as the reducing system
and the other as an oxidizing system, an electro-
mechanical rotary motor could be constructed.
Other means of driving contraction and relax-
ation represented in Figures 5.18 and 5.22 could
be used in a similar manner to construct rotary
motors.
5.6.5 Protein-(Tt)-Based Molecular
Machines of the Second Kind
(Molecular Machines for the
Performance of Work Other
Than Mechanical)
Discussion of each of the protein-(Tt)-based
molecular machines of the second kind is
beyond the scope of this volume. Above were
discussed the set of five pairwise energy con-
versions that constituted protein-(Tt)-based
molecular machines of the first kind. This leaves
another 13 pairwise energy conversions of the
second kind, which are listed under Axiom 4 in
section 5.6.3. Most of the protein-(Tt)-based
molecular machines of the second kind are of a
more academic interest. Because they contain
all of the coupling-of-functions, however, many
are of central interest to biology. These have
been discussed in section 5.5. Basically, when a
hydrophobic domain contains two different
vinegar-Hke R-groups, changing one to be more
oil-like can induce the other to become more

176
5.
Consilient Mechanisms for Diverse Protein-based Machines
oil-like; this is one statement of the Principle of
Le Chatelier. A favorable Gibbs free energy for
hydrophobic association, AGHA, is used to
perform work other than mechanical work, and
it does so in a way that gives rise to positive
cooperativity.
ITie sort of energy conversions that consti-
tute protein-(Tt)-based molecular machines of
the second kind have been referred to as
pumping protons and pumping electrons. There
are two aspects to chemo ^-^ chemical trans-
duction. An example would be where a partic-
ular chemical energy input caused the
elastic-contractile model protein to become
more oil-like and thereby to energize another
chemical group such as a phosphate. Another
aspect of chemo-chemical transduction could
include the classic consideration of enzyme
catalysis, in which case there occurs a decrease
in the chemical energy of reactants resulting in
an increase in the chemical energy of products.
Our focus now turns to the physical basis
whereby the energy conversions of the
hydrophobic consilient mechanism occur, and,
of course, it becomes an issue of what con-
trols the inverse temperature transition of
hydrophobic association.
5.7 What Is the Physical Basis
for the Consihent Mechanism of
Energy Conversion?
5.7.1 Answer: The Solvent-Mediated
Interaction Between Oil-like and
Vinegar-like Groups Constrained to
Coexist Along and Between Protein
Chain Molecules
Biology's machines are different from man's
machines, primarily because they evolved to
function efficiently in water. Many of man's
machines commonly operate at high tempera-
tures,
above the boiling temperature for water.
Such conditions are incompatible with life.
Other manmade machines, electric motors, and
generators and the many energy conversions of
the electronic world do not function in water.
As shown in Figure 2.16, even the chemically
driven polymeric machines that do function in
water, but that utilize the interactions of
charges in water, are very inefficient compared
with the protein-based machines available to
biology by means of inverse temperature tran-
sition of the hydrophobic consilient mechanism.
As discussed in this section, the interplay
between oil-like groups and vinegar-like groups
in an aqueous medium is the basis of the energy
conversion by the consilient mechanism. If
there were no oil-like components and no
through solvent interaction between oil-like
and vinegar-like groups, energy conversion
would have to rely on inefficient charge-charge
interactions that are shielded by the high
dielectric constant of water. Present arguments
of "conformational energy" forcing vinegar-like
groups into energetically unfavorable circum-
stances would also play a greater role. The
interactions between apolar and polar compo-
nents constrained by positions in the protein
chain molecule make for efficient protein-
based machines in water. To understand this
better, we need to understand the nature and
dynamics of hydrophobic hydration during the
function of such systems.
5.7.2 The Structure of Water
Surrounding Oil-like R-groups
Water molecules arrange in a special way
around oil-Uke groups. Stackelberg and
Miiller^^ showed this with the crystal structure
of hydrates of hydrocarbon gases (see Figure
2.8).
The waters arrange at the apices of pen-
tagons, and 12 pentagons arrange around a
small oil-like molecule. When structural detail
is sufficient, these pentagonal arrangements of
water molecules have been seen at the surface
of oil-Hke groups in protein. Martha Teeter^^
first observed hydrophobic hydration in
protein, positioned adjacent to an oil-like side
chain. Pentagonal arrangements of water mol-
ecules were observed adjacent to the side chain
of a leucine (Leu, L) residue with its oil-like,
-CH2CH(CH3)2, R-group.
More oil-like R-groups in our model protein
studies resulted in lower temperatures for the
onset of the inverse temperature transition of
hydrophobic folding and assembly (see section
5.3.2). We argued that more oil-like R-groups in

5.7 What Is the Physical Basis for the ConsiUent Mechanism of Energy Conversion?
177
polymers on the soluble side of the Tt-divide
means more hydrophobic hydration and that
more hydrophobic hydration means a lower
temperature for the Tfdivide between solubil-
ity and insolubility. In fact, we proposed that the
amount of hydrophobic hydration determines
the temperature at which the Tfdivide
occurred (section 5.3.5). In particular, as the
argument went, any process that increased the
amount of pentagonally arranged water
lowered, and any process that decreased the
amount of pentagonally arranged water neces-
sarily raised, the temperature interval for
hydrophobic association. Subsequently, the
definitive test of this perspective became pos-
sible—the capacity to measure directly the
amount of hydrophobic hydration and to
determine the effect of increasing and decreas-
ing the amount of hydrophobic hydration on
the value of Tj.The most current studies and the
most definitive studies are described immedi-
ately below in section 5.7.3. Then, the earlier
studies are described in section 5.7.9. These
latter studies preceded and, in fact, provided
the impetus to obtain the equipment required
for the definitive studies.
5.7.3 Direct Measurement of
Hydrophobic Hydration
Two decades ago, during studies of the back-
bone motions of these elastic model proteins
for the purpose of understanding the nature of
the elasticity, an associated observation sug-
gested that energy of the type produced by
microwave ovens was increasingly absorbed
as the amount of hydrophobic hydration
increased.^^'^^ The observation made possible a
more complete understanding of the physical
basis for the consiUent mechanism of energy
conversion that we call the comprehensive
hydrophobic effect and that included thermo-
dynamic characterization in terms of a Gibbs
free energy of hydrophobic association, AGHA
(see sections 5.1.3 and 5.1.7.).
5.7.3.1 Identification of
Hydrophobic Hydration
Microwave absorption by pure water as a func-
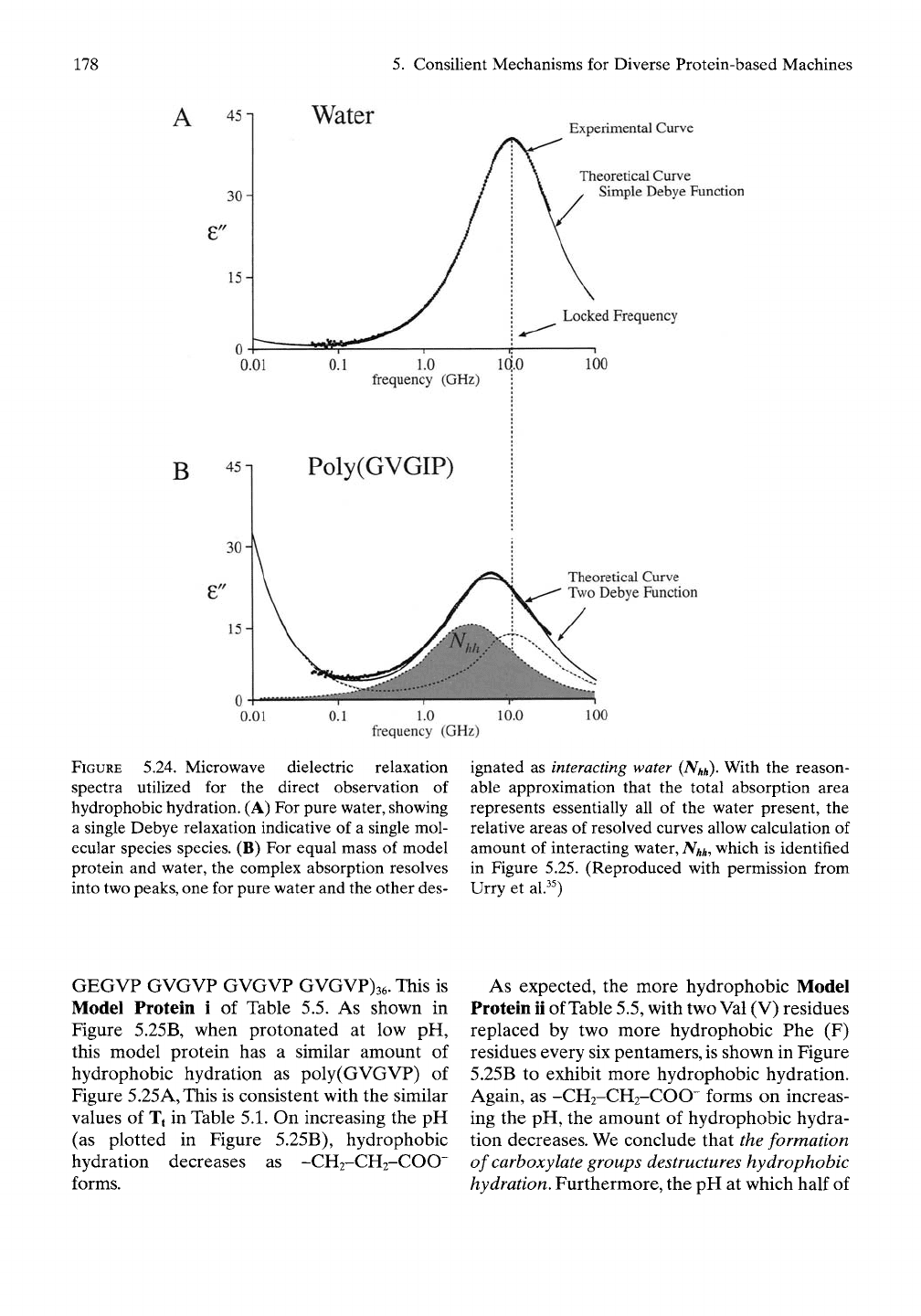
tion of frequency is shown in Figure 5.24A.^^ A
solution of approximately half water and half
model protein, (poly(GVGIP), by weight
demonstrates the presence of an absorption at
a lower frequency than that of water alone (see
Figure 5.24B). Subtracting out the peak due to
absorption by pure water leaves a peak for a
kind of water that must be interacting with the
model protein. Because the sum of both peaks
represents a reasonable estimate of the total
amount of water, and because we know the
total amount of water present, the amount of
interacting water can be calculated. Watching
the behavior of the interacting water in
response to several experimental variables pro-
vides for its identification.
How is the water interacting with the model
protein? In Figure 5.25A, the number of inter-
acting water molecules is plotted as a function
of temperature for both poly(GVGIP) and
poly(GVGVP). First, the more oil-Uke polymer,
poly(GVGIP) with an added CH2 group per
pentamer, has more of the interacting water
than the less hydrophobic poly(GVGVP), as
would be expected for hydrophobic hydration.
Most significantly, however, increasing the tem-
perature through the transition temperature
interval of hydrophobic folding and assembly,
that is, from one side to the other of the Tt-
divide, for each model protein causes the inter-
acting water to disappear. We therefore
conclude that the interacting water with its
absorption maximum near 5 GHz is hydropho-
bic hydration. Now, we address the question.
Does the previously deduced competition for
hydration between oil-like and vinegar-like
groups really occur?
5.7.3.2 Direct Observation of Competition
for Hydration
We now examine the hydrophobic hydration
of model proteins containing the vinegar-like
glutamic acid residue with the R-group,
-CH2-CH2-COOH. On decreasing the acid, H^,
concentration, the R-group ionizes to form
-CH2-CH2-COO". The common measure for a
decrease in acid (proton) concentration is an
increase in pH. Initially, the model protein has
the composition, poly[5(GVGVP),(GEGVP)],
but with a precise sequence, (GVGVP GVGVP
178
5.
Consilient Mechanisms for Diverse Protein-based Machines
A 45-
Water
0.01
0.1 1.0 IQ.
frequency (GHz)
B 45
Poly(GVGIP)
Experimental Curve
Theoretical Curve
Simple
Debye
Function
Locked Frequency
Theoretical Curve
Two
Debye Function
0.01
1.0
frequency (GHz)
FIGURE 5.24. Microwave dielectric relaxation
spectra utilized for the direct observation of
hydrophobic hydration. (A) For pure water, showing
a single Debye relaxation indicative of a single mol-
ecular species species. (B) For equal mass of model
protein and water, the complex absorption resolves
into two
peaks,
one for pure water and the other des-
ignated as
interacting
water
(Nhh)-
With the reason-
able approximation that the total absorption area
represents essentially all of the water present, the
relative areas of resolved curves allow calculation of
amount of interacting water,
Nhh,
which is identified
in Figure 5.25. (Reproduced with permission from
Urry et al.^0
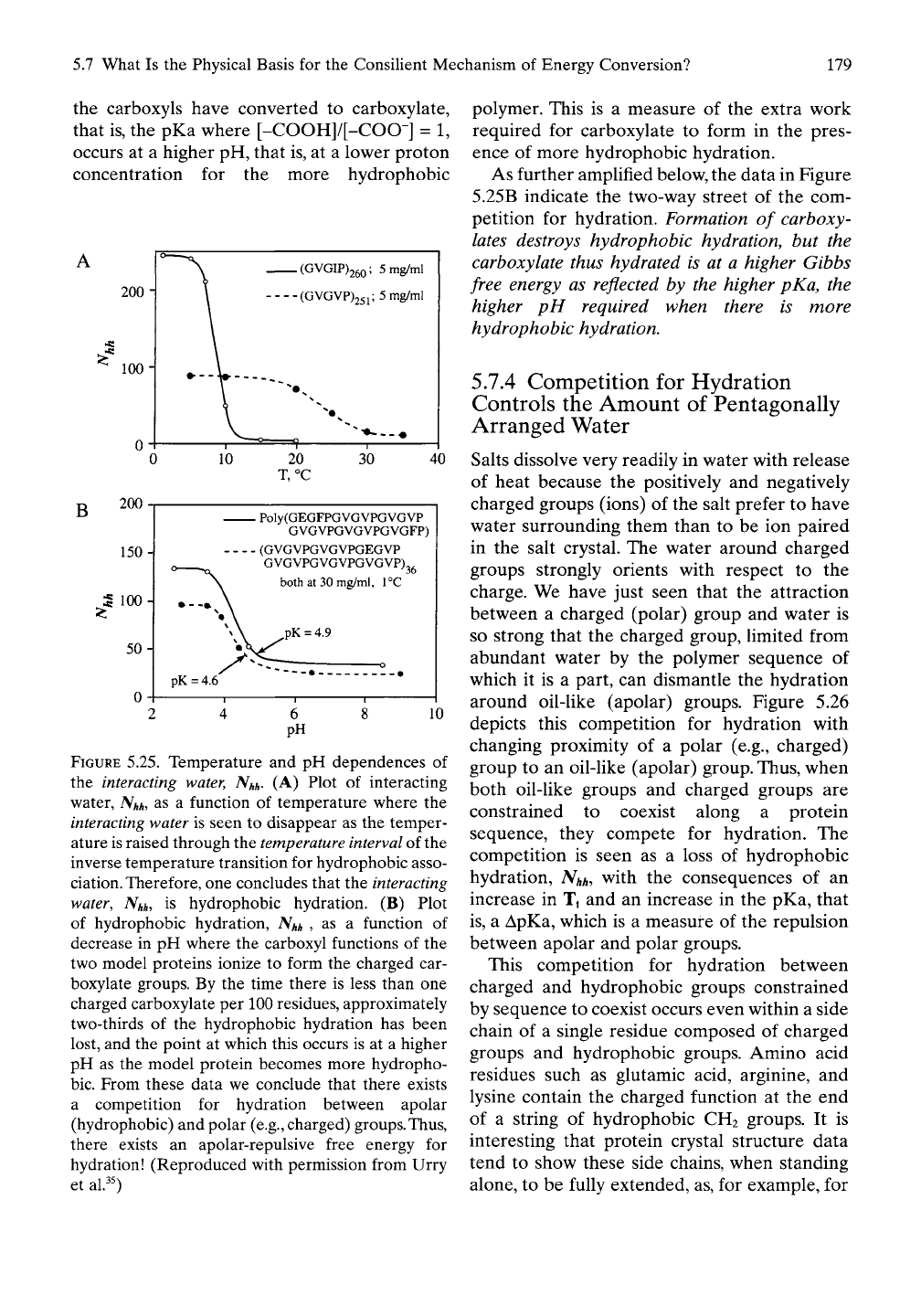
GEGVP GVGVP GVGVP GVGVP)36. This is
Model Protein i of Table 5.5. As shown in
Figure 5.25B, when protonated at low pH,
this model protein has a similar amount of
hydrophobic hydration as poly(GVGVP) of
Figure 5.25A,This is consistent with the similar
values of Tt in Table 5.1. On increasing the pH
(as plotted in Figure 5.25B), hydrophobic
hydration decreases as -CH2-CH2-COO~
forms.
As expected, the more hydrophobic Model
Protein ii of Table 5.5, with two Val (V) residues
replaced by two more hydrophobic Phe (F)
residues every six pentamers, is shown in Figure
5.25B
to exhibit more hydrophobic hydration.
Again, as -CH2-CH2-COO" forms on increas-
ing the pH, the amount of hydrophobic hydra-
tion decreases. We conclude that the formation
of carboxylate groups destructures hydrophobic
hydration. Furthermore, the pH at which half of
5.7 What Is the Physical Basis for the ConsiHent Mechanism of Energy Conversion?
179
the carboxyls have converted to carboxylate,
that is, the pKa where [-COOH]/[-COO-] = 1,
occurs at a higher pH, that is, at a lower proton
concentration for the more hydrophobic
sf
200-
100-
0 -
••--\(
(GVGIP)26o; 5mg/ml
(GVGVP)25p 5 mg/ml
10
20
T,°C
30 40
B
200
150
100
^
50
0
Poly(GEGFPGVGVPGVGVP
GVGVPGVGVPGVGFP)
- - - (GVGVPGVGVPGEGVP
GVGVPGVGVPGVGVP)3^
both at 30 mg/ml, 1°C
pK = 4.9
pK
=
4.6
^^^
» 9
6
pH
10
FIGURE 5.25. Temperature and pH dependences of
the interacting water,
Nhh-
(A) Plot of interacting
water,
Nhh,
as a function of temperature where the
interacting
water is seen to disappear as the temper-
ature is raised through the
temperature interval
of the
inverse temperature transition for hydrophobic asso-
ciation.
Therefore, one concludes that the
interacting
water, Nhh, is hydrophobic hydration. (B) Plot
of hydrophobic hydration,
Nhh
, as a function of
decrease in pH where the carboxyl functions of the
two model proteins ionize to form the charged car-
boxylate groups. By the time there is less than one
charged carboxylate per 100 residues, approximately
two-thirds of the hydrophobic hydration has been
lost, and the point at which this occurs is at a higher
pH as the model protein becomes more hydropho-
bic.
From these data we conclude that there exists
a competition for hydration between apolar
(hydrophobic) and polar
(e.g.,
charged) groups.Thus,
there exists an apolar-repulsive free energy for
hydration! (Reproduced with permission from Urry
et al.^0
polymer. This is a measure of the extra work
required for carboxylate to form in the pres-
ence of more hydrophobic hydration.
As further amplified below, the data in Figure
5.25B indicate the two-way street of the com-
petition for hydration. Formation of carboxy-
lates destroys hydrophobic hydration, but the
carboxylate thus hydrated is at a higher Gibbs
free energy as reflected by the higher pKa, the
higher pH required when there is more
hydrophobic hydration.
5,1 A Competition for Hydration
Controls the Amount of Pentagonally
Arranged Water
Salts dissolve very readily in water with release
of heat because the positively and negatively
charged groups (ions) of the salt prefer to have
water surrounding them than to be ion paired
in the salt crystal. The water around charged
groups strongly orients with respect to the
charge. We have just seen that the attraction
between a charged (polar) group and water is
so strong that the charged group, limited from
abundant water by the polymer sequence of
which it is a part, can dismantle the hydration
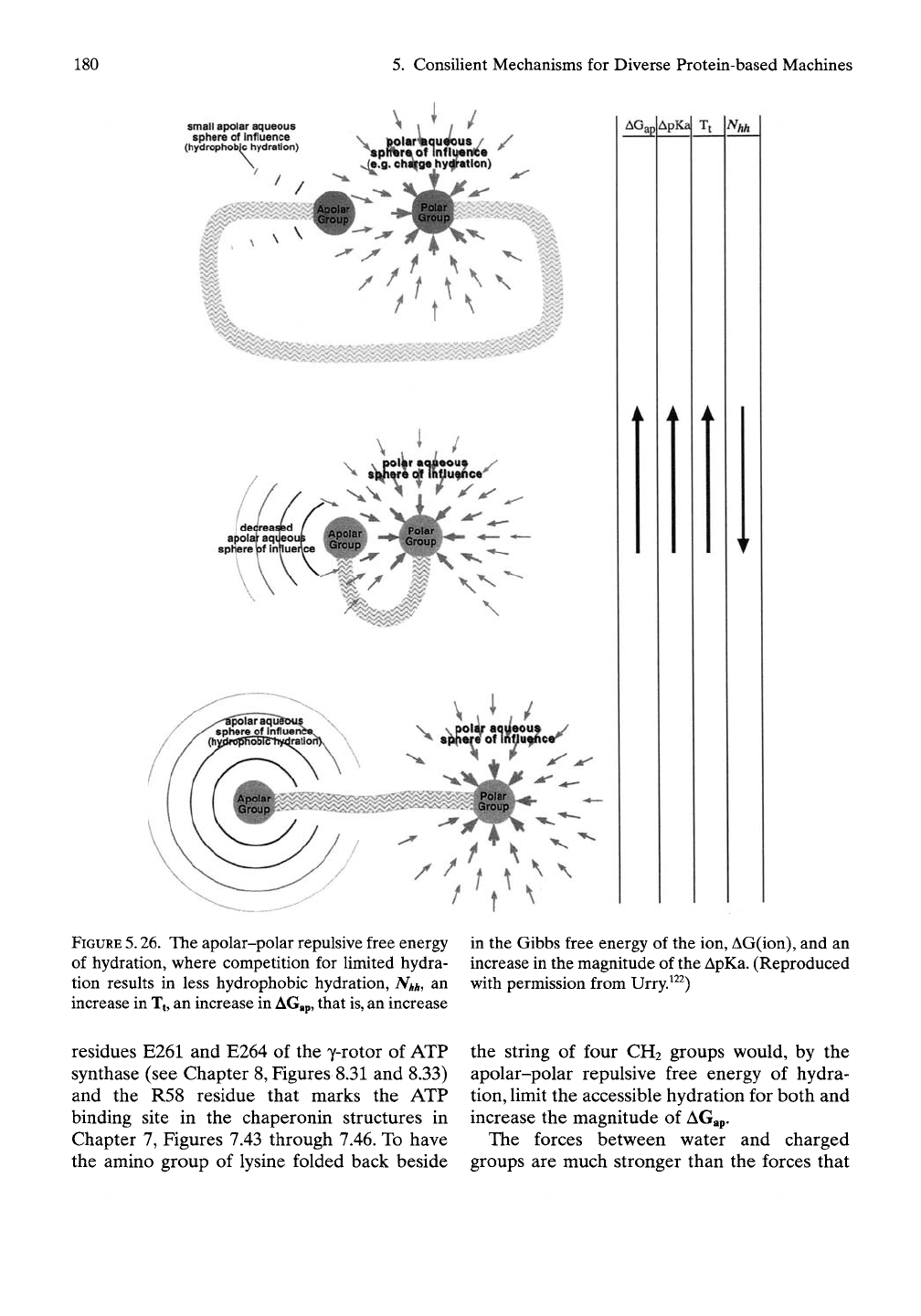
around oil-like (apolar) groups. Figure 5.26
depicts this competition for hydration with
changing proximity of a polar (e.g., charged)
group to an oil-like (apolar) group. Thus, when
both oil-like groups and charged groups are
constrained to coexist along a protein
sequence, they compete for hydration. The
competition is seen as a loss of hydrophobic
hydration, Nhh, with the consequences of an
increase in Tt and an increase in the pKa, that
is,
a ApKa, which is a measure of the repulsion
between apolar and polar groups.
This competition for hydration between
charged and hydrophobic groups constrained
by sequence to coexist occurs even within a side
chain of a single residue composed of charged
groups and hydrophobic groups. Amino acid
residues such as glutamic acid, arginine, and
lysine contain the charged function at the end
of a string of hydrophobic CH2 groups. It is
interesting that protein crystal structure data
tend to show these side chains, when standing
alone, to be fully extended, as, for example, for
180
5.
Consilient Mechanisms for Diverse Protein-based Machines
small apolar aquaoua
aphera of influanca
(hydrophobic hydration)
/
/
^•«0.
eh^||gahy^Hitlon)
^ •ifi^Jiw^^fi^
j^
^
AG,
m
ApKd Tt
\Nkh
FIGURE
5.26.
The apolar-polar repulsive free energy
of hydration, where competition for limited hydra-
tion results in less hydrophobic hydration,
NHH,
an
increase in
Tj,
an increase in
AGap,
that
is,
an increase
in the Gibbs free energy of the ion, AG(ion), and an
increase in the magnitude of the ApKa. (Reproduced
with permission from Urry.^^^)
residues E261 and E264 of the y-rotor of ATP
synthase (see Chapter 8, Figures 8.31 and 8.33)
and the R58 residue that marks the ATP
binding site in the chaperonin structures in
Chapter 7, Figures 7.43 through 7.46. To have
the amino group of lysine folded back beside
the string of four CH2 groups would, by the
apolar-polar repulsive free energy of hydra-
tion, limit the accessible hydration for both and
increase the magnitude of AGap.
The forces between water and charged
groups are much stronger than the forces that

5.7 What Is the Physical Basis for the ConsiUent Mechanism of Energy Conversion?
181
structure water adjacent to oil-like groups. A
few charged residues can destructure much of
the pentagonally arranged water. The forma-
tion of just four carboxylates in 100 residues in
poly[4(GVGVP),(GEGVP)] raises the transi-
tion temperature for oil-like separation from
24° C for -COOH to 69° C for -COO" as shown
in the upper part of Figure 5.3.
There are two sides, however, to the coin of
competition for hydration. The carboxylate
must pay a price to wrench hydration away
from hydrophobic groups and in having to do
so it never achieves the level of hydration that
it would as a dilute carboxylate in bulk water.
The price appears in the amount of hydroxyl
ion, OH", necessary in the solution before the
proton, ff, leaves the -COOH to form a car-
boxylate, that is, OH- + -COOH = -COO" +
H2O.
In the absence of the oil-like residues, the
amount of hydroxyl required to remove the
proton and form COO" is much less. When
there are only 2 carboxyls per 100 residues in
poly[9(GVGIP),(GEGIP)], it is so difficult for
the COO" to form that a pH of 6.4 is required,^^
which is a 250-fold increase in the amount of
hydroxyl ion required. WTien nearly half of the
oil-like Val residues are replaced by more oil-
like Phe residues, as in Polymer V of Table 5.5,
a million-fold increase in the amount of
hydroxyl ion was required to form the car-
boxylate ion of the aspartic acid residues."^^
5.7.5 We Now Know What Controls
Insolubility and Solubility of
Hydrophobic Domains
5.7,5,1 Loss of Solubility Due to Too
Much Hydrophobic Hydration
In general, the solubility of a model protein
such as poly(GVGVP) in water comes from the
presence of the polar peptide group, -CONH-.
The hydrogens of water, HOH, hydrogen bond
to the oxygen of the CO, that is, CO • • • HOH,
and the oxygen of water hydrogen bonds to the
NH, that
is,
NH
• • •
OH2.This hydrogen bonding
gives rise to solubility. As oil-like groups are
added to the model protein in water at a par-
ticular temperature, solubility is ultimately lost.
From Butler,^^ but also from the Tt-based
hydrophobicity scale in Table 5.1, we learn the
unique way in which this happens. Formation of
hydration surrounding oil-like groups is a
favorable exothermic reaction; heat is released;
the heat change for the hydration reaction, AH,
is negative.
Despite this and as contradictory as it may
initially sound, however, too much hydration of
oil-like groups results in loss of solubility. This
is due to the entropy change, AS, that accom-
panies hydrophobic hydration. Recall that sol-
ubility is governed by the Gibbs free energy,
AG(solubility) = AH -
TAS,
where T is the tem-
perature in degrees Kelvin, K, where K = C +
273.
The water molecules of hydrophobic
hydration, the pentagonally arranged water in
Figure 2.8, are more structured than the water
molecules in liquid or bulk water. The entropy
change for formation of hydrophobic hydration
has the opposite sign from the entropy changes
for the melting of ice and the vaporization of
liquid water in Figure 5.2. The latter changes
are to states of less order on raising the tem-
perature; so too is the loss of pentagonally
arranged water to form liquid water on raising
the temperature through the transition zone for
the inverse temperature transition. Therefore,
the entropy change, AS, is negative for forma-
tion of hydrophobic hydration on dissolution of
oil-like groups in water, such that the (-TAS)
term is positive. Now as each oil-like group is
added, the positive increment in the (-TAS)
term is greater than the negative increment in
AH. Accordingly, the oil-like additions can
occur with retention of solubility until at a
given temperature AG(solubility) becomes pos-
itive and solubility is lost.
Whether a particular hydrophobic region or
domain of a model protein or of a natural
protein associates with a second hydrophobic
domain in the same molecule or a separate mol-
ecule, the same process of loss of solubility
occurs. If the two hydrophobic domains can
associate and if together they have so much
hydrophobic hydration that their Tt is below
the temperature of the environment, they
associate; they are insoluble; AG(solubility) is
positive.

182
5.
Consilient Mechanisms for Diverse Protein-based Machines
5.7.5.2 Solubility of Oil-like Groups
Occurs Because Polar Groups Destructure
Hydrophobic Hydration
If a charged vinegar-Uke group with inadequate
hydration appears proximal to an opening fluc-
tuation of associating hydrophobic domains, it
will use the nascent hydrophobic hydration of
the opening fluctuation for its own hydration.
With less hydrophobic hydration, the magni-
tude of the positive (-TAS) term becomes less
than the magnitude of the negative AH term;
AG(solubility) is negative, and the two domains
gain solubility.
5.7.6 The Formation of Ion
Pairs Makes Model Proteins
More Hydrophobic
During the operation of many of biology's
protein-based machines, the formation and sep-
aration of ion pairs is central to function. Very
commonly the ion paired state forms as two oil-
like domains associate, one oil-like domain con-
taining the positive charge and the other the
negative charge. Examples of this are seen in
the binding and release of oxygen by hemoglo-
bin in a manner that enables the transport of
oxygen by hemoglobin from the lungs to the
tissues. Alternatively, the ion for pairing may
come from the solution. In this case, ion pair-
ing at one potentially oil-like domain makes
it more hydrophobic such that the more
hydrophobic domain can then associate with
another hydrophobic domain. Often in biology,
calcium ion is released to the solution where it
can ion pair with carboxylates of a potential
hydrophobic domain. Often, such ion pairing
provides the trigger for function, as in muscle
contraction.
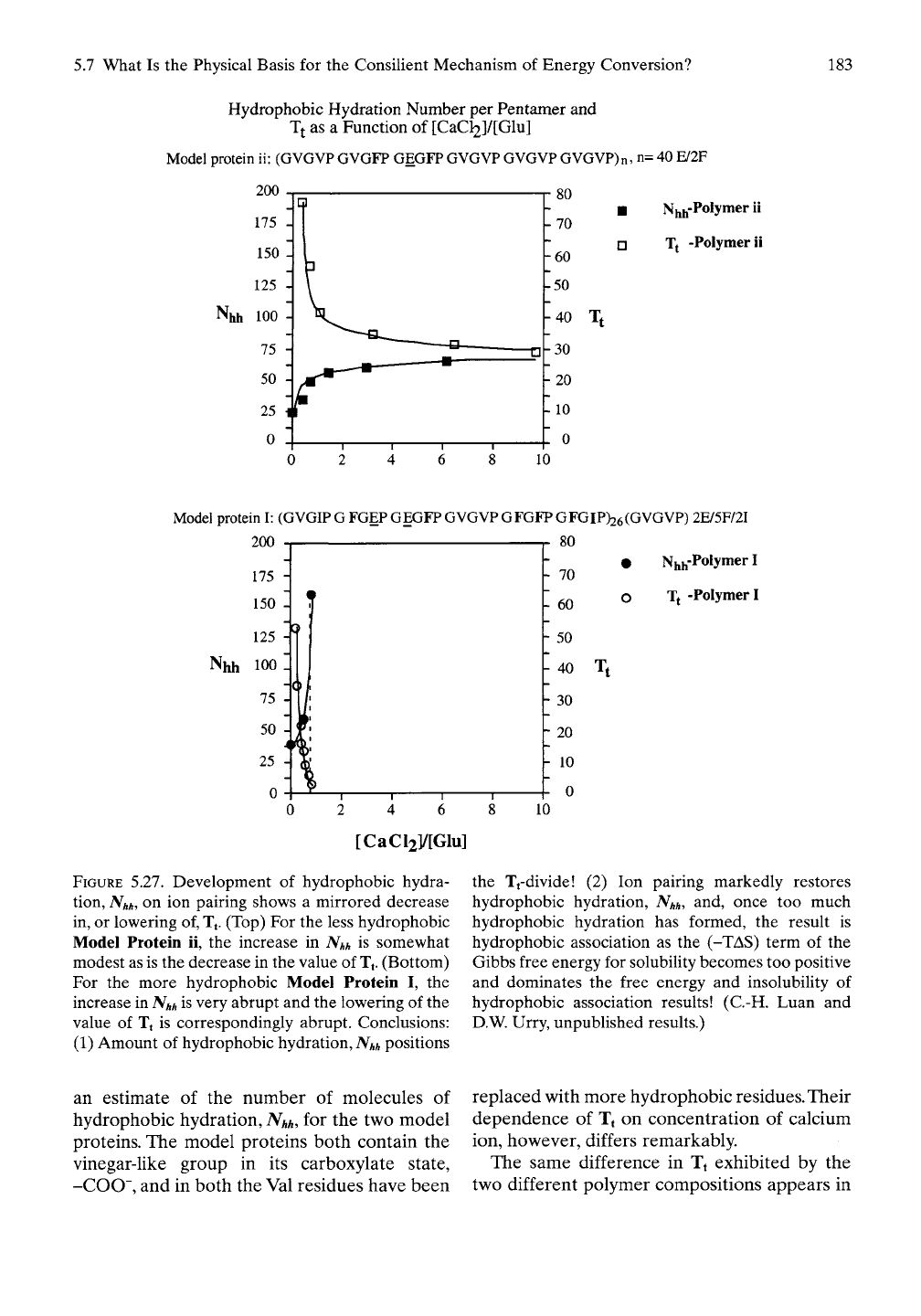
Here we report the effect of calcium ion
binding to carboxylates that exhibit
hydrophobic-induced pKa shifts. The pKa shifts
result from the work required to destructure
hydrophobic hydration in the process of ion-
ization to form the carboxylate. In Figure 5.27,
calcium ion interaction with carboxylates
makes two different model proteins more
hydrophobic to different extents, depending on
their initial oil-like composition. Figure 5.27A
considers Model Protein ii of Table 5.5, which
for every 30 residues (six pentamers) has one
carboxylate (-COO~)-containing glutamic acid
residue (Glu, E) and two, very oil-like pheny-
lalanine (Phe, F) residues with the hydrocarbon
R-group of -CH2-C6H5 having replaced two
valine (Val, V) residues with side chain
-CH2-(CH3)2. As calcium ion is added to 10
times equivalence, the Tt-value decreases, ini-
tially rapidly to about equal amounts of calcium
ion and carboxylate, then much more slowly. In
a near mirror image fashion, as calcium ion is
added, the number of waters of hydrophobic
hydration increases. Clearly, calcium ion pairing
with carboxylates increases hydrophobicity, as
indicated by the decrease in the value of Tf
Clearly, the decrease in Tt correlates with an
increase in hydrophobic hydration. For this
composition, however, not until a twofold
excess of calcium has been added does
hydrophobic association begin at body
temperature.
Figure 5.27B shows the same data for Model
Protein I of Table 5.5 with two proximal car-
boxylate (COO")-containing glutamic acid
residues (Glu, E), with five very hydrophobic F
residues and two more hydrophobic I residues
with the side chain -CH2(CH3)CH2-CH3,
having replaced nine of the valine (Val, V)
residues with side chain -CH2-(CH3)2, in every
30 residues. In this more hydrophobic model
protein, before there is one calcium ion per
carboxylate the temperature for hydrophobic
association drops below 0° C. These polymers,
of course, are the Model Proteins ii and I con-
sidered in section
5.5.3.1
and included in the
plots in Figure 5.20B.
5.7.7 The Amount of Hydrophobic
Hydration Determines the Value of Tt
The data in Figure 5.27 experimentally estab-
lish the relationship between the temperature,
Tt, for the onset of the inverse temperature
transition and the number of molecules of
hydrophobic hydration,
A^^/^.
Without going in to
the details of the experiments, it is possible to
appreciate the relationship by visual inspection.
Microwave absorption by hydrophobic
hydration as a function of frequency provides
5.7 What Is the Physical Basis for the Consihent Mechanism of Energy Conversion?
183
Hydrophobic Hydration Number per Pentamer and
Tj as a Function of [CaCl2]/[Glu]
Model protein
ii:
(GVGVP GVGFP GEGFP GVGVP GVGVP
GVGVP) n,
n= 40
E/2F
200 -r- n 80
T* -Polymer ii
Model protein
I:
(GVGIP G FGEP GEGFP GVGVP GFGFP
GFGIP)26(GVGVP) 2E/5F/2I
_j
175
j
150
J
125
J]
100
J
75
J
^4
25
T
0 +
L
^
• r
il
U
>
J
L
]r
i
I
\
1 1 1 r
80
70
60
50
40
30
20
h 10
0
O
Nhh-Polymer I
Tj -Polymer I
[CaCl2]/[Glu]
FIGURE 5.27. Development of hydrophobic hydra-
tion,
Nhh,
on ion pairing shows a mirrored decrease
in, or lowering of, Tf (Top) For the less hydrophobic
Model Protein ii, the increase in
Nhh
is somewhat
modest as is the decrease in the value of
Tj.
(Bottom)
For the more hydrophobic Model Protein I, the
increase in
Nhh
is very abrupt and the lowering of the
value of Tt is correspondingly abrupt. Conclusions:
(1) Amount of hydrophobic hydration,
Nhh
positions
10
the Tfdivide! (2) Ion pairing markedly restores
hydrophobic hydration,
Nhh,
and, once too much
hydrophobic hydration has formed, the result is
hydrophobic association as the
(-TAS)
term of the
Gibbs free energy for solubihty becomes too positive
and dominates the free energy and insolubility of
hydrophobic association results! (C.-H. Luan and
D.W. Urry, unpublished results.)
an estimate of the number of molecules of
hydrophobic hydration,
A^^^,
for the two model
proteins. The model proteins both contain the
vinegar-Hke group in its carboxylate state,
-COO",
and in both the Val residues have been
replaced with more hydrophobic
residues.
Their
dependence of Tt on concentration of calcium
ion, however, differs remarkably.
The same difference in Tt exhibited by the
two different polymer compositions appears in
184
5.
Consilient Mechanisms for Diverse Protein-based Machines
the inversely related number of water mole-
cules of hydrophobic hydration,
NHH-
This strik-
ing mirroring of Nhh and Tt occurs for each
polymer composition, even though the calcium
ion dependence is itself strikingly different
between the less hydrophobic and the more
hydrophobic polymers. Clearly Tt, the temper-
ature at which the onset of hydrophobic asso-
ciation occurs, depends on the number of
waters of hydrophobic hydration. As the
amount of hydrophobic hydration increases, Tt
decreases in an exactly mirrored manner.
Accordingly, the amount of hydrophobic hydra-
tion determines the value of Tt.
5.7.8 Earlier Studies Indicated the
Fundamental Process to be
Competition for Hydration
Above, the special structural organization of
these model proteins provided the unique
opportunity to identify and observe the behav-
ior of hydrophobic hydration. Under relevant
circumstances hydrophobic hydration was
more prevalent than bulk water. Key variables
showed that the amount of hydrophobic hydra-
tion,
Nhh,
determined Tt. Numerous systematic
studies, however, preceded the direct observa-
tion of hydrophobic hydration by microwave
dielectric relaxation. The earUer studies built
the foundation on which direct observation of
Nhh became the capstone. Below, interpreta-
tions,
implications, and/or conclusions follow
brief descriptions that lay bare the power of the
inverse temperature transition and add to the
panoply of data constituting the comprehensive
hydrophobic effect.
5.7.8,1
Dependence of Heat of Transition,
AHt, on Degree of Ionization
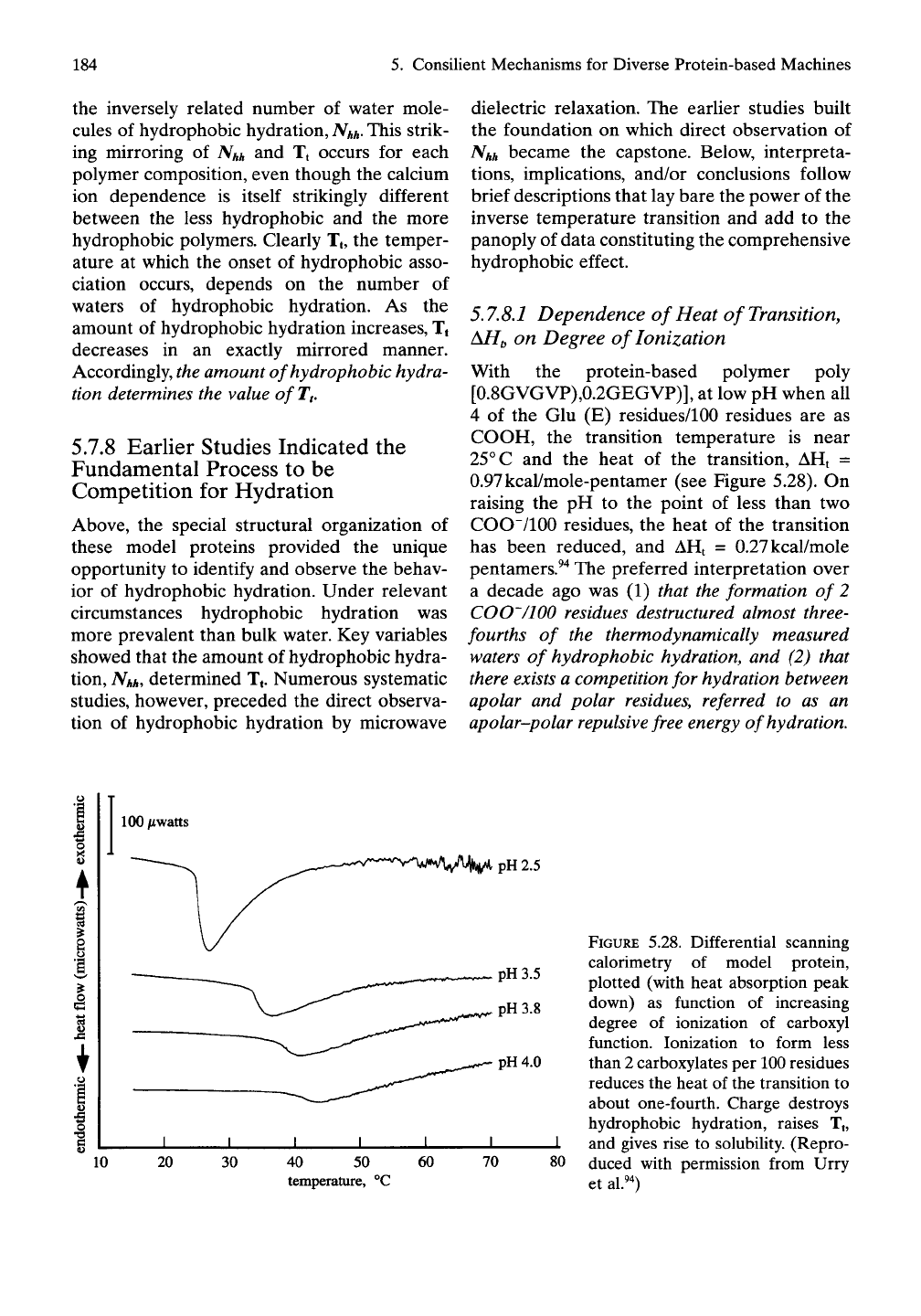
With the protein-based polymer poly
[0.8GVGVP),0.2GEGVP)], at low pH when all
4 of the Glu (E) residues/100 residues are as
COOH, the transition temperature is near
25° C and the heat of the transition, AHt =
0.97kcal/mole-pentamer (see Figure 5.28). On
raising the pH to the point of less than two
COO"/100 residues, the heat of the transition
has been reduced, and AHt = 0.27kcal/mole
pentamers.^"^ The preferred interpretation over
a decade ago was (1) that the formation of 2
COO'/lOO residues destructured almost three-
fourths of the thermodynamically measured
waters of hydrophobic hydration, and (2) that
there exists a competition for hydration between
apolar and polar residues, referred to as an
apolar-polar repulsive free energy of hydration.
I
I
I
I
I
100
/iwatts
pH2.5
pH3.5
pH3.8
pH4.0
I
10 20 30 40 50 60
temperature, °C
70
80
FIGURE 5.28. Differential scanning
calorimetry of model protein,
plotted (with heat absorption peak
down) as function of increasing
degree of ionization of carboxyl
function. Ionization to form less
than 2 carboxylates per 100 residues
reduces the heat of the transition to
about one-fourth. Charge destroys
hydrophobic hydration, raises Tt,
and gives rise to solubility. (Repro-
duced with permission from Urry
et al.^0
