Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.

5.3 Cataloging
the
Energy Resources Available
to the
ConsiHent Mechanism
of
Energy Conversion
145
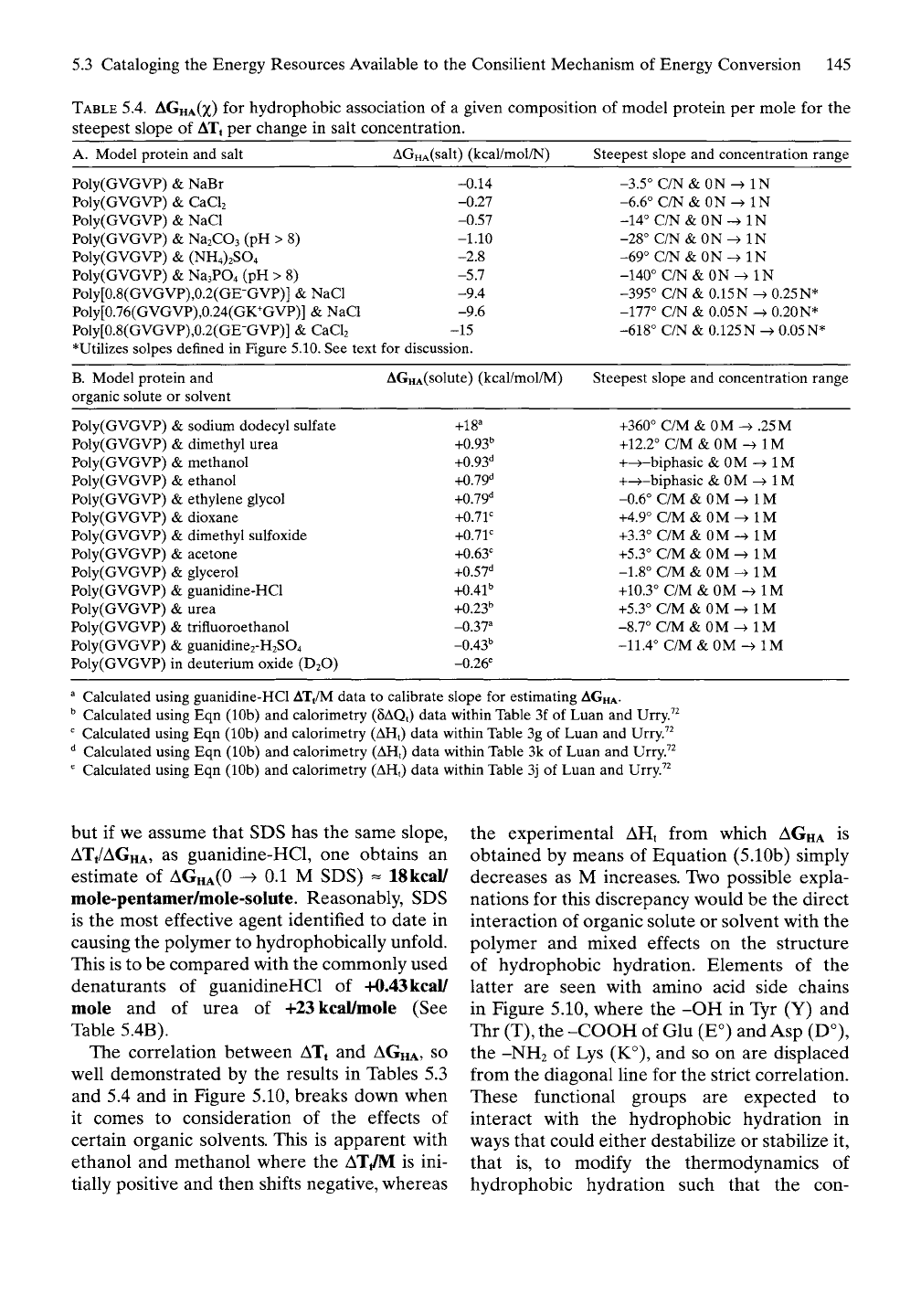
TABLE
5.4.
AGHA(X)
for
hydrophobic association
of a
given composition
of
model protein
per
mole
for the
steepest slope
of
ATt
per
change
in
salt concentration.
A. Model protein
and
salt
Poly(GVGVP)
&
NaBr
Poly(GVGVP)
&
CaCl2
Poly(GVGVP)
&
NaCl
Poly(GVGVP)
&
NazCOs
(pH
> 8)
Poly(GVGVP)
&
(NH4)2S04
Poly(GVGVP)
&
Na3P04
(pH
>
8)
Poly[0.8(GVGVP),0.2(GE-GVP)]
&
NaCl
Poly[0.76(GVGVP),0.24(GK^GVP)]
&
NaCl
Poly[0.8(GVGVP),0.2(GE-GVP)]
&
CaCl2
AGHA(salt) (kcal/mol/N)
-0.14
-0.27
-0.57
-1.10
-2.8
-5.7
-9.4
-9.6
-15
*Utilizes solpes defined
in
Figure 5.10.
See
text
for
discussion.
B.
Model protein
and
organic solute
or
solvent
Poly(GVGVP)
&
sodium dodecyl sulfate
Poly(GVGVP)
&
dimethyl urea
Poly(GVGVP)
&
methanol
Poly(GVGVP)
&
ethanol
Poly(GVGVP)
&
ethylene glycol
Poly(GVGVP)
&
dioxane
Poly(GVGVP)
&
dimethyl sulfoxide
Poly(GVGVP)
&
acetone
Poly(GVGVP)
&
glycerol
Poly(GVGVP)
&
guanidine-HCl
Poly(GVGVP)
&
urea
Poly(GVGVP)
&
trifluoroethanol
Poly(GVGVP)
&
guanidine2-H2S04
Poly(GVGVP)
in
deuterium oxide (D2O)
AGHA(solute) (kcal/mol/M)
+18^
+0.93"
+0.93^*
+0.79^^
+0.79^^
+0.7r
+ojr
+0.63^
+0.57'^
+0.41"
+0.23"
-0.37^
-0.43"
-0.26^
Steepest slope
and
concentration range
-3.5°C/N&0N^1N
-6.6°C/N&0N^1N
-14°C/N&0N-»1N
-28°C/N&0N^1N
-69°C/N&0N-^1N
-140°C/N&0N^1N
-395°
C/N
&
0.15 N
-^
0.25 N*
-177° C/N
&
0.05 N
-^
0.20
N*
-618° C/N
&
0.125 N
-^
0.05
N*
Steepest slope
and
concentration range
+360°C/M&0M-^.25M
+12.2°C/M&0M^1M
+^-biphasic
&
OM
-^ IM
+^-biphasic
&
OM
^
1M
-0.6°C/M&0M^1M
+4.9°C/M&0M^1M
+3.3°C/M&0M-^1M
+5.3°C/M&0M^1M
-1.8°C/M&0M->1M
+10.3°C/M&0M^1M
+5.3°C/M&0M-»1M
-8.7°C/M&0M^1M
-11.4°C/M&0M^1M
Calculated using guanidine-HCl AT/M data
to
calibrate slope
for
estimating
AGHA-
Calculated using Eqn (10b)
and
calorimetry (6AQt) data within Table
3f of
Luan
and
Urry.^^
Calculated using Eqn (10b)
and
calorimetry (AHt) data within Table
3g of
Luan
and
Urry.^^
Calculated using Eqn (10b)
and
calorimetry (AHt) data within Table
3k of
Luan
and
Urry.^^
Calculated using Eqn (10b)
and
calorimetry (AHt) data within Table 3j
of
Luan
and
Urry^^
but if we assume that SDS has the same slope,
ATt/AGHA, as guanidine-HCl, one obtains an
estimate of
AGHA(0
-^ 0.1 M SDS) - ISkcal/
mole-pentamer/mole-solute. Reasonably, SDS
is the most effective agent identified to date in
causing the polymer to hydrophobically unfold.
This is to be compared with the commonly used
denaturants of guanidineHCl of +0.43 kcal/
mole and of urea of +23 kcal/mole (See
Table 5.4B).
The correlation between ATt and
AGHA,
SO
well demonstrated by the results in Tables 5.3
and 5.4 and in Figure 5.10, breaks down when
it comes to consideration of the effects of
certain organic solvents. This is apparent with
ethanol and methanol where the AT/M is ini-
tially positive and then shifts negative, whereas
the experimental AHt from which
AGHA
is
obtained by means of Equation (5.10b) simply
decreases as M increases. Two possible expla-
nations for this discrepancy would be the direct
interaction of organic solute or solvent with the
polymer and mixed effects on the structure
of hydrophobic hydration. Elements of the
latter are seen with amino acid side chains
in Figure 5.10, where the -OH in Tyr (Y) and
Thr (T), the -COOH of Glu (E°) and Asp (D°),
the -NH2 of Lys (K°), and so on are displaced
from the diagonal line for the strict correlation.
These functional groups are expected to
interact with the hydrophobic hydration in
ways that could either destabilize or stabilize it,
that is, to modify the thermodynamics of
hydrophobic hydration such that the con-
146
5.
Consilient Mechanisms for Diverse Protein-based Machines
100
Poly[0,8(VPGVG), 0.2(VPGEG)]
100
o
I- 50-^ t
0.2 0.4 0.6 0.8
Salt Molar Concentration
Poly[0.8(IPGVG),0.2(IPGEG)]
o
•g 25
0.04
1
Ca^**----*
-T 1 1 1 1 T-
O 0.2 0.4 0.6 0.8 1
Salt Molar Concentration
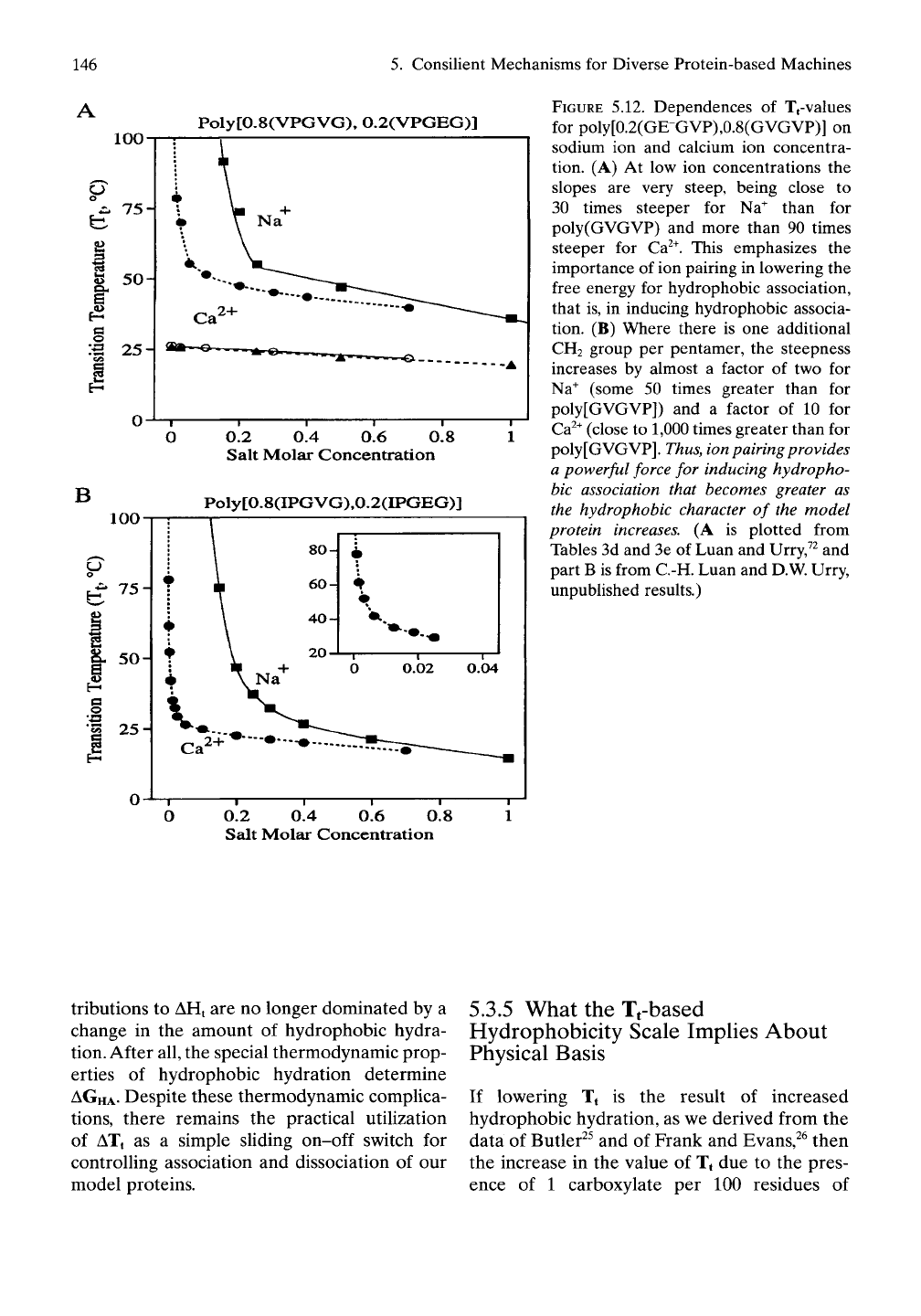
FIGURE 5.12. Dependences of Tfvalues
for poly[0.2(GE-GVP),0.8(GVGVP)] on
sodium ion and calcium ion concentra-
tion. (A) At low ion concentrations the
slopes are very steep, being close to
30 times steeper for Na^ than for
poly(GVGVP) and more than 90 times
steeper for Ca^^. This emphasizes the
importance of ion pairing in lowering the
free energy for hydrophobic association,
that is, in inducing hydrophobic associa-
tion. (B) Where there is one additional
CH2 group per pentamer, the steepness
increases by almost a factor of two for
Na^ (some 50 times greater than for
poly[GVGVP]) and a factor of 10 for
Ca^^ (close to 1,000 times greater than for
poly[GVGVP].
Thus,
ion
pairing provides
a powerful force for inducing hydropho-
bic association that becomes greater as
the hydrophobic character of the model
protein increases. (A is plotted from
Tables 3d and 3e of Luan and Urry,^^ and
part B is from C.-H. Luan and D.W. Urry,
unpublished results.)
tributions to AHt are no longer dominated by a
change in the amount of hydrophobic hydra-
tion. After all, the special thermodynamic prop-
erties of hydrophobic hydration determine
AGHA-
Despite these thermodynamic complica-
tions,
there remains the practical utilization
of ATt as a simple sliding on-off switch for
controlling association and dissociation of our
model proteins.
5.3.5 What the Trbased
Hydrophobicity Scale ImpHes About
Physical Basis
If lowering Tt is the result of increased
hydrophobic hydration, as we derived from the
data of Butler^^ and of Frank and Evans,^^ then
the increase in the value of Tt due to the pres-
ence of 1 carboxylate per 100 residues of

5.4 Visual Observation of Elastic Protein-based Machines at Work
147
poly(GVGVP) would seem to arise from the
loss of hydrophobic hydration; this suggests
that the negatively charged ion destructures
hydrophobic hydration in the process of achiev-
ing its own hydration shell. Such insight,
supported by a series of less direct arguments,
became confirmed by the direct observation of
hydrophobic hydration (see in section 5.7).
Before definitively addressing the physical
basis,
however, demonstrations are given of
many of the diverse energy conversion possible
by the consilient mechanism.
5.4 Visual Observation of
Elastic Protein-based Machines
at Work
5.4.1 Straightforward Development of
Elastic Protein-based Machines for
Pumping Iron
Development of our understanding of energy
conversion by the consilient mechanism of
hydrophobic association began with the
expected thermally driven contraction of cross-
linked bands composed of model protein^^'^^
Next, it was anticipated and found that proto-
nation of a carboxylate within the model
protein lowered the temperature of the transi-
tion and that protonation of the carboxylate
within the cross-linked elastic band drove con-
traction.^"^ This was immediately followed by
the predicted demonstration that addition of
salt could drive contraction by lowering the
temperature of the transition of the simple
cross-linked model protein, poly(GVGVP)7^
Subsequently and expectedly came pressure-
driven relaxation with aromatic residues^^ and
reduction-driven contraction using a chemi-
cally attached redox couple.^^ These energy
conversions with the energy output being
the mechanical work of lifting a weight were
visually demonstrated. Elastic bands, with the
appropriate model protein composition and a
suspended weight, contract and lift weights, that
is,
pump iron, when designed to be responsive
to different energy inputs. Despite many differ-
ent energy inputs, all of the contractions utilize
the common mechanism of hydrophobic
association to lift the weights. A video provides
visual recordings of these reversible contrac-
tions and relaxations.
5.4.1.1 Simplicity of the Fundamental
Observations
5.4.1.1.1 Onset of Aggregation on Raising the
Temperature of Model Proteins in Solution
Again, the initial experimental observations are
very simple. For the model proteins in solution,
a clear solution becomes cloudy as the temper-
ature is raised. The onset of cloudiness signals
aggregation, or association, of the model pro-
teins,
as shown in Figure 5.1. During this asso-
ciation, oil-Uke R-groups of the model protein
chains separate from water and become insol-
uble.
Specifically, the differential calorimetry
curve of Figure 5.1C provides graphic repre-
sentation of the "cusp of insolubility." Moving
the cusp to lower temperatures than the
working temperature gives insolubility, and
moving the "cusp" to higher temperatures than
the operating temperature gives solubility. The
temperature range over which aggregation
(insolubilization) occurs is called the tempera-
ture interval. How each of 20 different R-groups
changes the temperature interval for aggrega-
tion becomes the primary experimental data-
base (see Figures 5.5 and 5.10 and associated
discussion). The database, called a Tj-based
hydrophobicity scale, in a most practical form
reports the relative oil-like character of the 20
naturally occurring R-groups (see Table 5.1 and
associated discussions). Figure 5.10 provides
the relationship between Tt and the fundamen-
tal Gibbs free energy of hydrophobic associa-
tion, AGHA, required for a more complete
analysis of energy conversion by protein-based
machines, and relevant values are given in
Tables
5.2,5.3
and 5.4.
5.4.1.1.2 Temperature Dependence of
Solubility of Oil-like Groups and Formation
of Insoluble Structures
In relation to the above discussion of the
Butler^^ alcohol series, which considered solu-
bility of oil-like groups in terms of the equation
AG = AH - TAS, raising the temperature
148
5.
Consilient Mechanisms for Diverse Protein-based Machines
increases the magnitude of the (-TAS) term
until it dominates over the AH term and solu-
bility is lost. In the basic model protein of inter-
est here, poly(GVGVP), the aggregated state
still contains 60% water by weight.^^ Because
of this, the separated oil-like groups become
spatially localized hydrophobic patches on the
surface of helically folded and coiled model
protein. This may be thought of in two steps:
(1),
on folding of the helices (P-spirals) the
localized hydrophobic patches tend to form
helical ribbons of oil-like groups on the surface
of the P-spirals; and (2), the heUcally folded
structures associate by means of the oil-like
heUcal ribbons on the surface to form twisted
filaments (see Figure 5.6E,F). In reality the
hydrophobic folding of a single chain and the
association (coiling) of chains to form twisted
filaments are cooperative and generally
inseparable.
5,4.1.2 Constructing Macroscopic Elastic
Protein-based Machines
The simple process of constructing the contrac-
tile elastic bands follows. It involves forming
the phase-separated state comprised of inter-
penetrating twisted filaments as represented in
Figure 5.6F, shaping the phase-separated state
into thin sheets and cross-linking the sheets
into elastic matrices, most simply, by exposure
to y-irradiation.
5.4.1.2.1 Formation and Shaping of a
Glue-like Substance
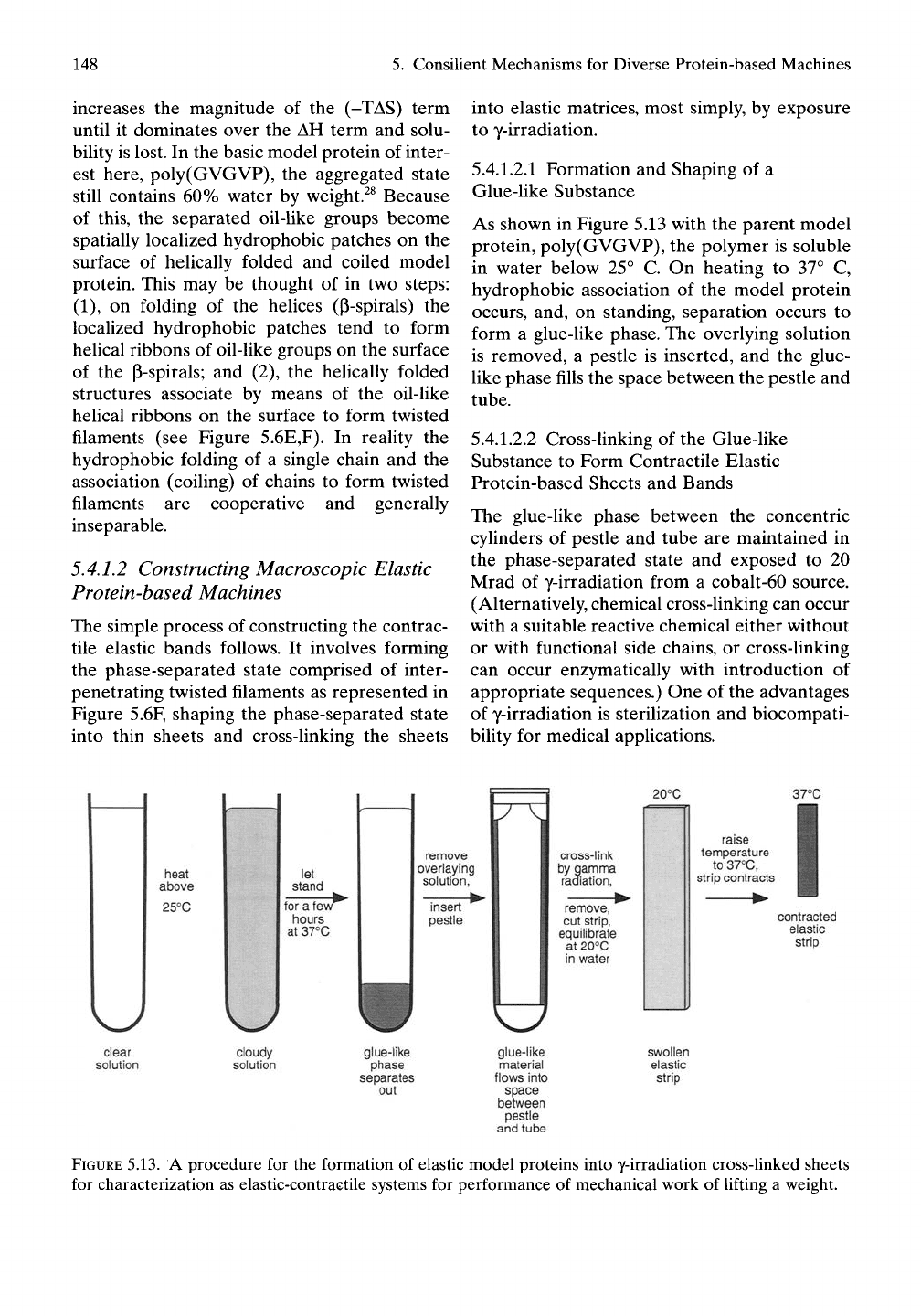
As shown in Figure 5.13 with the parent model
protein, poly(GVGVP), the polymer is soluble
in water below 25° C. On heating to 37° C,
hydrophobic association of the model protein
occurs, and, on standing, separation occurs to
form a glue-like phase. The overlying solution
is removed, a pestle is inserted, and the glue-
Uke phase fills the space between the pestle and
tube.
5.4.1.2.2 Cross-linking of the Glue-like
Substance to Form Contractile Elastic
Protein-based Sheets and Bands
The glue-like phase between the concentric
cylinders of pestle and tube are maintained in
the phase-separated state and exposed to 20
Mrad of y-irradiation from a cobalt-60 source.
(Alternatively, chemical cross-linking can occur
with a suitable reactive chemical either without
or with functional side chains, or cross-linking
can occur enzymatically with introduction of
appropriate sequences.) One of the advantages
of y-irradiation is sterilization and biocompati-
bility for medical applications.
u
heat
above
25°C
let
stand
for
a
few
hours
at 37°C
KJ
remove
overlaying
solution,
re-
insert
pestle
20°C
cross-link
by gamma
radiation,
W
remove,
cut strip,
equilibrate
at 20°C
in water
\y
raise
temperature
to
37°C,
strip contracts
37°C
I
contracted
elastic
strip
clear
solution
cloudy
solution
glue-like
phase
separates
out
glue-like
material
flows into
space
between
pestle
and tube
swollen
elastic
strip
FIGURE
5.13. A procedure for the formation of elastic model proteins into y-irradiation cross-linked sheets
for characterization as elastic-contractile systems for performance of mechanical work of lifting a weight.

5.4 Visual Observation of Elastic Protein-based Machines at Work 149
Informative anecdotes relate to effective
y-irradiation cross-linking. Adequate cross-
linking does not occur unless the temperature
has been raised through the temperature inter-
val to drive the hydrophobic folding and assem-
bly. Solutions of polymer at concentrations
lower than that of the usual phase-separated
state,
obtainable only by being at temperatures
lower than the temperature interval, do not
cross-link. Irreversible denaturation of poly
(GVGVP), as represented by the denaturation
peak of Figure 5.2, results in a phase-separated
state that is twice as concentrated, 70%
polymer and 30% water.^^ Even though at twice
the concentration, the denatured polymer does
not effectively cross-link by y-irradiation. In
each case the absence of the state of inter-
penetrating folded and regularly associated
chains interferes with formation of cross-links
between chains.
The elastic strip or sheet is removed from the
tubes,
and it is found to swell on lowering the
temperature and to contract on raising the tem-
perature through the temperature interval from
20° to 37° C, as depicted at the right in Figure
5.13.
A sheet of y-irradiation cross-linked
(GVGVP)25i is shown in Figure 5.14.
5.4,1.3 Hydrophobic Association in
Solution Becomes Contraction in the
Elastic Matrix
The same process of hydrophobic association,
observed in solution and characterized by the
phase diagram in Figure 5.3, occurs in the
elastic matrix where hydrophobic association
displays as a visible contraction. The parallel
process of cloudy formation of polymers in
solution transforms in the elastic matrix into a
contraction capable of performing mechanical
work.
Contraction of elastic matrices occurs as the
temperature is raised through the temperature
interval of Figure 5.5A; this constitutes the
input of thermal energy (TE), measurable as
the heat of the transition seen in Figure 5.1C.
Visible contraction also occurs as any of the
independent variables move through the tran-
sition zone in Figure 5.5B. Depending on the
composition of the model protein that consti-
FiGURE 5.14. Sheet of elastic model protein of y-
irradiation cross-linked (GVGVP)25i as demon-
strated in Figure 5.13 directly usable in thermally
driven contraction and in chemically driven
contraction.
tutes the elastic matrix, the independent vari-
able can be one of a number of energy inputs
such as chemical energy (CE), electrochemical
energy (ECE), pressure-volume energy (PVE),
and electromagnetic radiation energy (EMRE)
such as
Ught.
Thus, one can design elastic matri-
ces capable of contracting and performing the
mechanical work of lifting weights, and with
the unaided eye all can see that the design is
successful.
As discussed below, some designs of model
proteins require less of a given energy input to
perform a given amount of mechanical work.
The effectiveness of the given energy input in
performing mechanical work depends on the
change in the Gibbs free energy for hydropho-
bic association, AGHA, resulting from the energy
input acting on the particular model protein
composition.

150
5.
Consilient Mechanisms for Diverse Protein-based Machines
5.4,1.4 Solvent Entropy Changes in Elastic
Protein Band on Loading (Stretching)
5.4.1.4.1 Below Tt, Solvent Entropy Changes
due to Stretching of a Fully Swollen
Elastic Band
Carefully hanging a weight on the fully swollen
elastic band causes elastic extension sufficient
to support a light load. The elastic force, /,
within the band that supports the load is com-
prised of two components, an internal energy
component,
Z^,
and an entropy component,/s,
that is.
f
=
fE+fs
(5.12)
Stretching at a temperature below Tj of the
fully swollen elastic band results in no change
in hydrophobic hydration. Therefore, any elastic
force developed below Tt is not expected to arise
due to solvent entropy changes The entropy
component,/s, of the elastomeric force arises
from the decrease in the internal chain motion
such as rotation about bonds, while the internal
energy component,/^;, results from straining of
bond lengths and deforming bond angles that
could lead to chain rupture.
5.4.1.4.2 Above Tt, Solvent Entropy
Changes Due to Stretching of a Contracted
Elastic Band
The fully contracted elastic band is in a state of
maximized hydrophobic association. Because
of this, stretching of the contracted band
exposes hydrophobic groups to water. Recall
that when an oil-like group dissolves in water,
formation of hydrophobic hydration occurs
with the release of heat. Thus, hanging a weight
on the contracted band exposes hydrophobic
groups to water and releases heat as hydropho-
bic hydration forms. Associated with the exoth-
ermic hydrophobic hydration is a decrease in
entropy of the water that goes from bulk water
to hydrophobic hydration, such that the change
in the (-TAS) term of the Gibbs free energy is
positive. For elastic bands composed of
poly(GVGVP), 90% of the force developed on
stretching the hydrophobically associated state
is due to/5 (see Figure 5.7).^^
5.4.1.4.3 Effects on Solvent Entropy of
Raising the Temperature of Loaded Elastic
Band Through the Temperature Interval
for Contraction
Obviously, the effect of raising the temperature
of a loaded, cross-linked elastic band composed
of elastic protein-based polymers of the
poly(GVGVP) family is to drive hydrophobic
association with the consequence of lifting of
the attached weight. How does this combine
with the above understanding of elasticity to
perform mechanical work?
Hydrophobic association has the corollary
of a positive change in entropy of water as
hydrophobic hydration becomes bulk water.
This occurs whether the experiment is the
change in length at constant force (load) of
pumping iron and the work done is fAL or
whether the experiment is the increase in force
at constant length and the energy output is LAf.
In the latter case there is an increase in force
as thermally driven hydrophobic association
occurs, and the increase in force occurs when
the solvent entropy change is positive.
Thus, for the LAf case the increase in force
cannot arise from a decrease in solvent entropy,
because, in fact, the process is accompanied by
an increase in solvent entropy. This brings us to
the following perspective. In both cases, the
entropic elastic force is maintained or increased
by greater extension resulting in an increased
damping of internal chain dynamics within a
chain segment of fewer repeats as the chains
within a substantial part of the elastic band
undergo hydrophobic association, as repre-
sented in Figure
5.8.^^
5.4.2 Thermal Energy Produces
Mechanical Work of Lifting a Weight
(Heating Through the Temperature
Interval Drives Contraction)
5.4.2.1 Using Cross-linked Model Proteins
as Thermally Driven Contractile
Elastic Bands
For the performance of mechanical work by
model proteins cross-linked to form elastic
bands,
a weight is attached to the rubber-Uke

5.4 Visual Observation of Elastic Protein-based Machines at Work 151
band and the temperature raised. Over a par-
ticular temperature interval, the band contracts
and lifts the w^eight. Relatively little contraction
occurs below the temperature interval, and rel-
atively little contraction occurs above the tem-
perature interval for the particular composition
(see Figure 5.5
A).
The presence of more oil-like
R-groups low^ers the temperature interval over
which contraction occurs, and the presence of
less oil-like or more polar groups R-groups
raises the temperature interval over which con-
traction occurs. Thus, the temperature depen-
dence of contraction of the cross-Unked elastic
matrix follows the dependence of the Tt-divide
on hydrophobicity of the protein-based poly-
mers in solution. The relative hydrophobicity is
reconfirmed in this simplistic and visual obser-
vation of the conversion of thermal energy
(heat) into mechanical work (see Figures 2.4
and 2.5). An obvious elementary statement of
the experimental findings is given as Axiom 2.
Axiom 2: Heating to raise the temperature
from below to above the temperature interval for
hydrophobic association of cross-linked elastic
model protein chains drives contraction with the
performance of mechanical work.
5.4.2.2 Thermal Energy (TE), Measurable
as the Heat of the Transition of the Band
Under
Load,
AHi, Produces Mechanical
Work (MW) of Lifting a Weight, That Is,
TE^MW
At any temperature within the temperature
interval for the thermally driven transition,
there exists an equilibrium between hydropho-
bic association and dissociation. Obviously, at
the low temperature side of the temperature
interval, Umited hydrophobic association
occurs. At the high temperature side of the
temperature interval, hydrophobic association
is limited only by the extending load. The rela-
tionship is such that hydrophobic association is
inversely proportional to the load.
If we knew the heat of the transition for the
elastic band under load, AHi, we could calculate
the ratio of the mechanical work performed
to the heat input expended in lifting the load.
that is, the efficiency of the energy conversion.
Stretching of these hydrophobically associated
elastomers, however, is exothermic due to the
forced exposure and hydration of hydrophobic
groups. Accordingly, AHi is less than AHt,
but the experimental determination of AHi is
difficult due to the nature of the differential
scanning calorimeter required to determine the
heats of the transitions. Fortunately, the chem-
ical energy expended in moving the loaded
elastomer through the transition zone for the
performance of mechanical work can be deter-
mined, as discussed below.
5.4.3 Chemical Energy Produces
Mechanical Work of Lifting Weight
(Changing Concentration of Chemical
Through the Transition Zone
Drives Contraction)
5.4.3.1 Equivalence of Moving the
Temperature
Interval and Moving
Through the
Transition
Zone
5.4.3.1.1 Moving the Temperature Interval
The temperature interval for aggregation of
poly(GVGVP) begins just above room temper-
ature in water, that is, above 25° C, and ends at
about 37° C (see Figure 5.1C and Figure 5.5A).
Addition of salt (NaCl) at 25° C lowers the
temperature for aggregation and causes the
solution to go cloudy. As discussed below, salt
drives contraction by lowering the temperature
interval for heat-driven contraction.
5.4.3.1.2 Relationship Between Temperature
Interval and Transition Zone for Salt-driven
Contraction in the Absence of Functional
Side Chains
The decrease in temperature for the onset of
the transition due to the addition of salt is 14°
C for the addition of 58 grams in 1 liter, that is,
1 N NaCl.^^ Accordingly, the addition of 1 N
NaCl moves the temperature interval originally
25° to 37° C approximately one interval width
to 11° to 23° C and drives contraction of the
elastic matrix. Increasing the concentration of
salt from 1 N to 2 N, however, does little to
further the contraction. Thus the transition

152
5.
Consilient Mechanisms for Diverse Protein-based Machines
zone for the NaCl-cross-linked-poly(GVGVP)
system is 0 to 1 N NaCl (see Figure 5.5). When
observing the cross-Unked elastic band
attached to a w^eight, added salt causes the
rubber-like band to contract and perform the
work of lifting a weight. The cross-linked
elastic-contractile model protein transforms
chemical energy of increasing salt concentra-
tion into mechanical work of pumping iron.
5.4.3.1.3 Relationship Between Changing the
Temperature Interval and Moving Through
Transition Zone for Contraction by Changing
the State of Functional Side Chains
When the polymer contains vinegar-like func-
tional groups, the appropriate counter-ion of
the salt ion pairs with the functional group. Ion
pairing markedly lowers the temperature inter-
val for contraction. Ion pairing places the tran-
sition zone at a much lower salt concentration,
for example, between 0.01 and 0.15 N NaCl.
In particular, when the counter-ion for a car-
boxylate, COO", is the special case of a proton,
H^, the transition zone can occur at very low
concentrations, for example, between a concen-
tration-1 (Ci) of 0.000001 and concentration-2
(C2) of
0.00001
N HCl. Another way to write this
small concentration difference is to use powers
of 10, that is, Ci = 10"' N HCl and C2 = 10"^ N
HCl. Yet another way to write this difference is
to use logarithms to express the increase in con-
centration, for example, log(C2) - log(Ci) =
(-5) - (-6) =
1.
The change in chemical energy,
AE,
required to drive a process is the product of
the change in chemical potential,
A|LI,
times
the change in number of moles. An, that
is,
AE =
AjLiAn. For simpUcity, the present argument
focuses on chemical potential such that AE/An
= 2.3RT[log(C2) - log(Ci)] = 1420cal/mole,
where R = 1.987 cal/mole-deg and at physiolog-
ical temperature (e.g., 37° C such that T = 310
K).
Pumping iron, performing mechanical work,
by the mechanism of charge neutralization, for
example, ion pairing, requires less chemical
energy to do the same amount of mechanical
work than if the model protein had no charged
functional group or if the charged group of the
model protein were already neutralized. This
comparison is graphically demonstrated below.
5.4.3.2 Relationship Between Temperature
Interval and Transition Zone for Chemical
Energy Input
5.4.3.2.1 Relationship Between Changing the
Temperature Interval and Moving Through
the Transition Zone for Contraction Using
Carboxylate (-COO") Functional Side Chains
and Calcium ion (Ca^^)
The temperature interval for poly(GVGVP)
begins at 25° C and ends at 37° C. The same
temperature interval is considered for two
different microbially prepared analogues of
poly(GVGVP), namely. Model protein I:
(GVGIP GFGEP GEGFP GVGVP GFGFP
GFGIP)26 and Model Protein ii: (GVGVP
GVGFP GEGFP GVGVP GVGVP
GVGVP)4o of Table 5.5. Figure 5.15 contains
the dependence on the logarithm of the calcium
ion concentration, log[Ca^^], of the value of Tj
for both model proteins in water. For Model
Protein ii aggregation of the model protein in
solution and contraction of the cross-linked
band in the absence of a load begin at 37° C, as
the calcium chloride reaches a concentration of
0.034 mole/liter (log[Ca'T = -1.47), and
hydrophobic association is complete as the
calcium chloride concentration increases to
0.446 mole/Hter (log[Ca2^] = -0.351). Thus, the
temperature interval of 25° to 37° C corre-
sponds to a transition zone for the independent
variable of calcium ion concentration of 0.034
to 0.446 mole/liter. This is a change in concen-
tration of 0.412 mole/liter, but, more to the
point, on the log scale, the difference is
1.12.The
chemical energy divided by the number of
moles of calcium ion consumed in the process
of driving contraction of Model Protein ii in the
limit of zero load is AE/An == 2.3RT[1.12] «
1,580 cal/mole. For Model Protein I the transi-
tion zone is narrower with a difference on the
log scale of only 0.116. The chemical energy
per mole required to drive the contraction of
Model Protein I is AE/An - 2.3RT[0.116] - 164
cal/mole. The two carboxyls of Model Protein I
would reasonably bind a single calcium ion in a
bidentate manner, and this is less Ukely for
Model Protein ii where the carboxyls are sepa-
rated by 30 residues instead of two residues.
5.4 Visual Observation of Elastic Protein-based Machines at Work
153
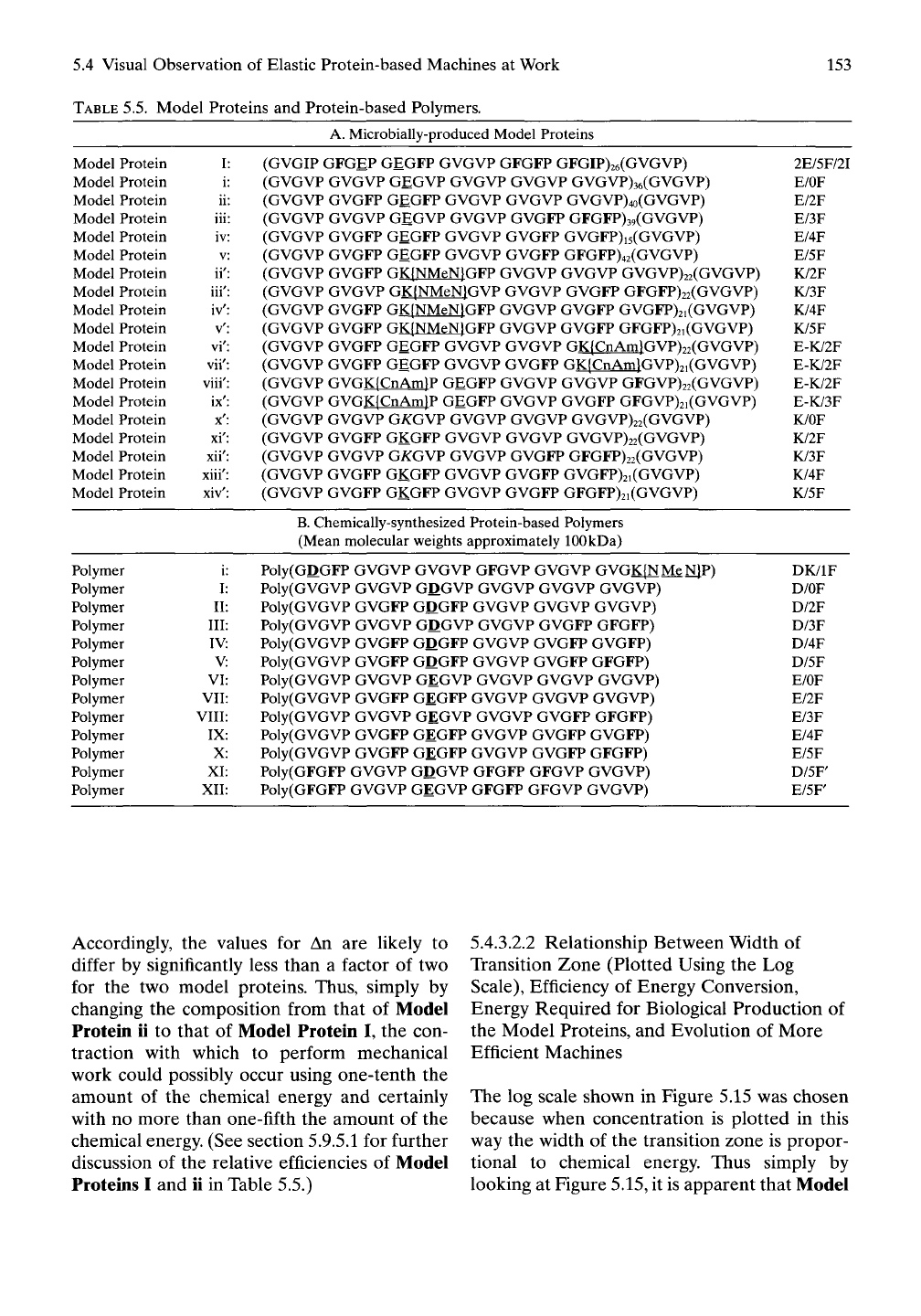
TABLE
5.5. Model Proteins and Protein-based Polymers.
A. Microbially-produced Model Proteins
(GVGIP GFGEP GEGFP GVGVP GFGFP GFGIP)26(GVGVP) 2E/5F/2I
(GVGVP GVGVP GEGVP GVGVP GVGVP GVGVP)36(GVGVP) E/OF
(GVGVP GVGFP GEGFP GVGVP GVGVP GVGVP)4o(GVGVP) E/2F
(GVGVP GVGVP GEGVP GVGVP GVGFP GFGFP)39(GVGVP) E/3F
(GVGVP GVGFP GEGFP GVGVP GVGFP GVGFP)i5(GVGVP) E/4F
(GVGVP GVGFP GEGFP GVGVP GVGFP GFGFP)42(GVGVP) E/5F
(GVGVP GVGFP GK(NMeN}GFP GVGVP GVGVP GVGVP)22(GVGVP) K/2F
(GVGVP GVGVP GK(NMeN}GVP GVGVP GVGFP GFGFP)22(GVGVP) K/3F
(GVGVP GVGFP GK{NMeN}GFP GVGVP GVGFP GVGFP)2i(GVGVP) K/4F
(GVGVP GVGFP GK(NMeN)GFP GVGVP GVGFP GFGFP)2i(GVGVP) K/5F
(GVGVP GVGFP GEGFP GVGVP GVGVP GK(CnAm}GVpi.(GVGVP^ E-K/2F
(GVGVP GVGFP GEGFP GVGVP GVGFP GK{CnAinlGVP)2i(GVGVP) E-K/2F
(GVGVP GVGK{CnAm}P GEGFP GVGVP GVGVP GFGVP)22(GVGVP) E-K/2F
(GVGVP GVGK{CnAm}P GEGFP GVGVP GVGFP GFGVP)2i(GVGVP) E-K/3F
(GVGVP GVGVP GKGWF GVGVP GVGVP GVGVP)22(GVGVP) K/OF
(GVGVP GVGFP GKGFP GVGVP GVGVP GVGVP)22(GVGVP) K/2F
(GVGVP GVGVP
GA:GVP
GVGVP GVGFP GFGFP)22(GVGVP) K/3F
(GVGVP GVGFP GKGFP GVGVP GVGFP GVGFP)2i(GVGVP) K/4F
(GVGVP GVGFP GKGFP GVGVP GVGFP GFGFP)2i (GVGVP) K/5F
Model Protein
Model Protein
Model Protein
Model Protein
Model Protein
Model Protein
Model Protein
Model Protein
Model Protein
Model Protein
Model Protein
Model Protein
Model Protein
Model Protein
Model Protein
Model Protein
Model Protein
Model Protein
Model Protein
I
i
ii
iii
iv
V
ii'
iii'
iv'
v'
vi'
vii'-
viii':
ix':
x':
xi':
xii':
xiii':
xiv':
B.
Chemically-synthesized Protein-based Polymers
(Mean molecular weights approximately 100 kDa)
Poly(GDGFP GVGVP GVGVP GFGVP GVGVP GVGK{NMeN|P^ DK/IF
Poly(GVGVP GVGVP GDGVP GVGVP GVGVP GVGVP) D/OF
Poly(GVGVP GVGFP GDGFP GVGVP GVGVP GVGVP) D/2F
Poly(GVGVP GVGVP GDGVP GVGVP GVGFP GFGFP) D/3F
Poly(GVGVP GVGFP GDGFP GVGVP GVGFP GVGFP) D/4F
Poly(GVGVP GVGFP GDGFP GVGVP GVGFP GFGFP) D/5F
Poly(GVGVP GVGVP GEGVP GVGVP GVGVP GVGVP) E/OF
Poly(GVGVP GVGFP GEGFP GVGVP GVGVP GVGVP) E/2F
Poly(GVGVP GVGVP GEGVP GVGVP GVGFP GFGFP) E/3F
Poly(GVGVP GVGFP GEGFP GVGVP GVGFP GVGFP) E/4F
Poly(GVGVP GVGFP GEGFP GVGVP GVGFP GFGFP) E/5F
Poly(GFGFP GVGVP GDGVP GFGFP GFGVP GVGVP) D/5F
Poly(GFGFP GVGVP GEGVP GFGFP GFGVP GVGVP) E/5F
Polymer
Polymer
Polymer
Polymer
Polymer
Polymer
Polymer
Polymer
Polymer
Polymer
Polymer
Polymer
Polymer
I:
II:
III:
IV:
V:
VI:
VII:
VIII:
IX:
X:
XI:
XII:
Accordingly, the values for An are likely to
differ by significantly less than a factor of tv^o
for the two model proteins. Thus, simply by
changing the composition from that of Model
Protein ii to that of Model Protein I, the con-
traction v^ith which to perform mechanical
work could possibly occur using one-tenth the
amount of the chemical energy and certainly
with no more than one-fifth the amount of the
chemical energy. (See section
5.9.5.1
for further
discussion of the relative efficiencies of Model
Proteins I and ii in Table 5.5.)
5.4.3.2.2 Relationship Between Width of
Transition Zone (Plotted Using the Log
Scale),
Efficiency of Energy Conversion,
Energy Required for Biological Production of
the Model Proteins, and Evolution of More
Efficient Machines
The log scale shown in Figure 5.15 was chosen
because when concentration is plotted in this
way the width of the transition zone is propor-
tional to chemical energy. Thus simply by
looking at Figure 5.15, it is apparent that Model
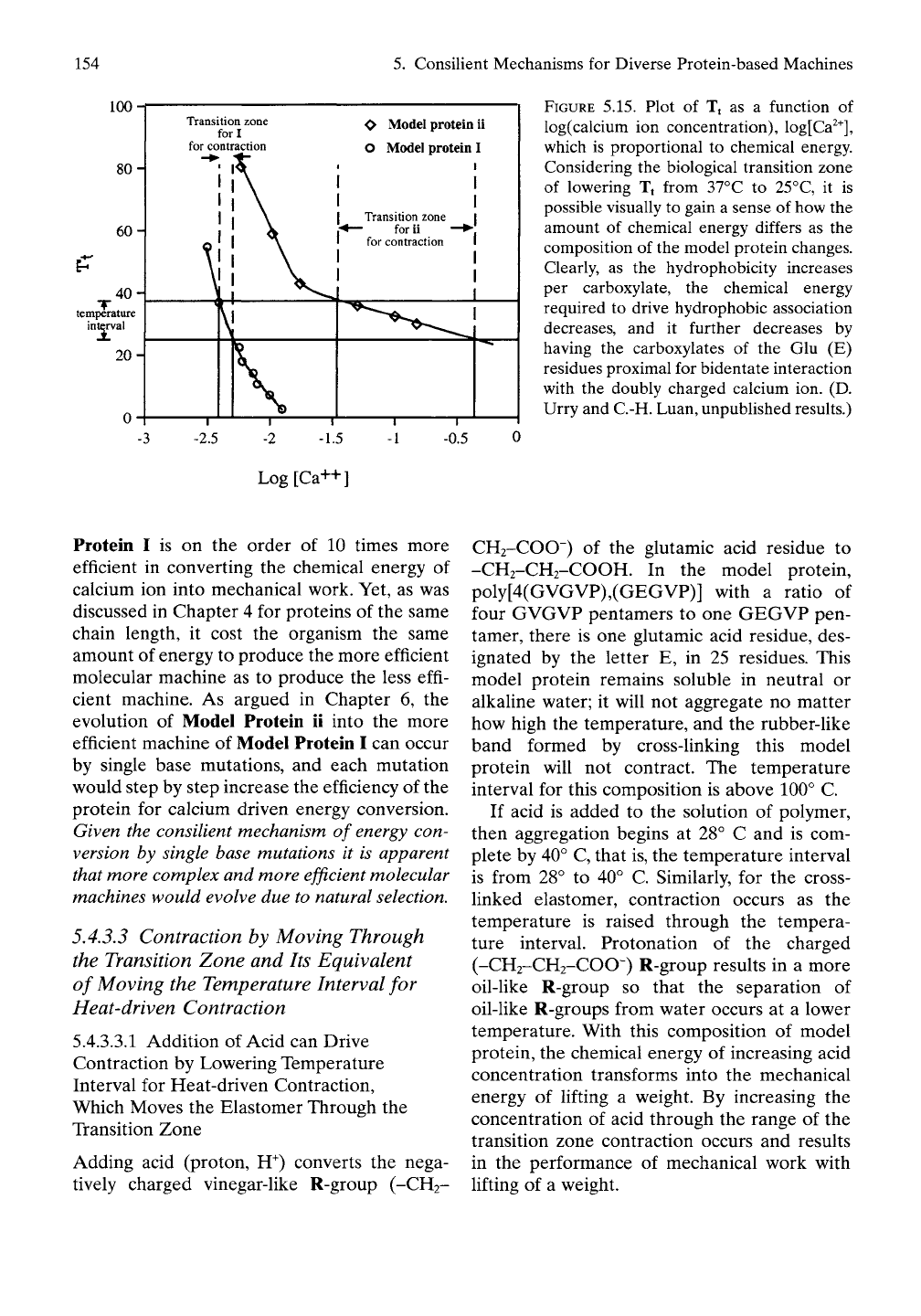
154
5.
Consilient Mechanisms for Diverse Protein-based Machines
FIGURE 5.15. Plot of Tt as a function of
log(calcium ion concentration), log[Ca^^],
which is proportional to chemical energy.
Considering the biological transition zone
of lowering Tt from 37°C to 25°C, it is
possible visually to gain a sense of how the
amount of chemical energy differs as the
composition of the model protein changes.
Clearly, as the hydrophobicity increases
per carboxylate, the chemical energy
required to drive hydrophobic association
decreases, and it further decreases by
having the carboxylates of the Glu (E)
residues proximal for bidentate interaction
with the doubly charged calcium ion. (D.
Urry and C.-H. Luan, unpublished results.)
Log[Ca++]
Protein I is on the order of 10 times more
efficient in converting the chemical energy of
calcium ion into mechanical work. Yet, as was
discussed in Chapter 4 for proteins of the same
chain length, it cost the organism the same
amount of energy to produce the more efficient
molecular machine as to produce the less effi-
cient machine. As argued in Chapter 6, the
evolution of Model Protein ii into the more
efficient machine of Model Protein I can occur
by single base mutations, and each mutation
would step by step increase the efficiency of the
protein for calcium driven energy conversion.
Given the consilient mechanism of energy con-
version by single base mutations it is apparent
that more complex and more efficient molecular
machines would evolve due to natural selection.
5.4.3.3 Contraction by Moving Through
the Transition Zone and Its Equivalent
of Moving the Temperature Interval for
Heat-driven Contraction
5.4.3.3.1 Addition of Acid can Drive
Contraction by Lowering Temperature
Interval for Heat-driven Contraction,
Which Moves the Elastomer Through the
Transition Zone
Adding acid (proton, H^) converts the nega-
tively charged vinegar-like R-group (-CH2-
CH2-COO ) of the glutamic acid residue to
-CH2-CH2-COOH. In the model protein,
poly[4(GVGVP),(GEGVP)] with a ratio of
four GVGVP pentamers to one GEGVP pen-
tamer, there is one glutamic acid residue, des-
ignated by the letter E, in 25 residues. This
model protein remains soluble in neutral or
alkaline water; it will not aggregate no matter
how high the temperature, and the rubber-like
band formed by cross-linking this model
protein will not contract. The temperature
interval for this composition is above 100° C.
If acid is added to the solution of polymer,
then aggregation begins at 28° C and is com-
plete by 40° C, that is, the temperature interval
is from 28° to 40° C. Similarly, for the cross-
linked elastomer, contraction occurs as the
temperature is raised through the tempera-
ture interval. Protonation of the charged
(-CH2-CH2-COO~) R-group results in a more
oil-like R-group so that the separation of
oil-like R-groups from water occurs at a lower
temperature. With this composition of model
protein, the chemical energy of increasing acid
concentration transforms into the mechanical
energy of lifting a weight. By increasing the
concentration of acid through the range of the
transition zone contraction occurs and results
in the performance of mechanical work with
lifting of a weight.
