Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


5.3 Cataloging the Energy Resources Available to the Consilient Mechanism of Energy Conversion 135
aggregation as the result of increasing the free
energy of the hydrophobically associated state!
Therefore, within the practical approach of
the Tfbased hydrophobicity scale, we argue
that any R-group capable of increasing the tem-
perature for aggregation is more vinegar-like
than the R-group it replaced. Therefore, we
evaluate each of the naturally occurring R-
groups by these criteria. Recall that oil-like
groups lower the temperature interval for
aggregation, in our view, due to increasing the
amount of hydrophobic hydration surrounding
them. (Refer to the discussion in sections
5.1.3.3,
5.1.3.4, and
5.7.7)
In our interpretation
below, charged groups raise the temperature
interval by destructuring the pentagonally
arranged hydrophobic hydration surrounding
oil-Hke groups in the unfolded and disassoci-
ated model protein, that is, by decreasing the
total number of molecules of hydrophobic
hydration possible in the disassociated model
protein.
53.2,7 Systematic Comparison of All 20
R-groups of Proteins
Meaningful comparison of the relative oil-like
character of all of the 20 naturally occurring R-
groups of Table 5.1, including the different
states of functional R-groups, requires adher-
ence to stringent criteria and conditions. These
experimental conditions, discussed above, are
given by footnote here.^^ The criteria and
conditions are remarkably met by the elastic
repeating peptide sequence that originated in
the mammaUan elastic fiber, (GVGVP)n.
Axiom 1, given above and the first of five
axioms Hsted below in section 5.6.3, becomes
the basis for consideration of protein function
and for the design of protein-based materials
for the future (see Chapter 9). As simply inter-
preted below, when there are more water mol-
ecules surrounding oil-like groups, the phase
separation of the oil-like groups from water
occurs at a lower temperature.
5.3.2,8 The Continuous Nature of the
Tt-based Hydrophobicity Scale
The terms oil-like and vinegar-like provide
good insight into the basic issues, but they are
limited when addressing particular issues. To
overcome preconceptions and to limit the need
for clarifications of what is oil-like and what is
vinegar-like when discussing the practical Tt-
based hydrophobicity scale, more general
terms prove helpful. Thus, the term apolar
often replaces oil-like, and the term polar often
replaces vinegar-like. Although the Tt-based
hydrophobicity scale warrants much discussion,
only a few issues are noted here.
5.3.2.8.1 A Scale from Apolar to Polar
As shown in Table
5.1,
tryptophan (Trp, W) has
the most apolar R-group, and glutamic acid
(Glu, E), when ionized, has the most polar
R-group. Between these two extremes exists
an essentially continuous set of values. The
side chain of glutamine, -CH2-CH2-CO-NH2,
though uncharged is quite polar, the most polar
uncharged side chain. This indicates that the
peptide moiety
itself,
-CO-NH-, is polar and
suggests that some half-dozen peptide moieties
could sum to be as effective as a single car-
boxylate. It would seem that the hydrogen
bonding of peptide moieties with water could
limit the amount of hydrophobic hydration and
raise the value of Tt. Also when the oil-like
residues dominate, even though the CO or NH
may be at the interface with water, they would
be at a higher free energy due to limited hydro-
gen bonding with water. This would lower the
free energy for formation of secondary struc-
ture,
that is, intramolecular and intermolecular
hydrogen bonding becomes favored by the
presence of more hydrophobic residues. In our
view, this factor contributes to the growth of
amyloid deposits of Alzheimer's disease and
assists in driving prions into insoluble aggre-
gates (see Chapter 7).
5.3.2.8.2 Comparison of Negative and
Positive Ions
The Tt-based hydrophobicity scale shows the
side chain of aspartic acid, -CH2-COO~, to be
significantly less polar than that of glutamic
acid, -CH2-CH2-COO~. This is not as expected
from the number of CH2 groups in the side
chains and likely arises from steric-dependent
hydrogen bonding interactions with nearby

136
5.
Consilient Mechanisms for Diverse Protein-based Machines
peptide groups. Significant also is the difference
between the effectiveness of -NHs^, and
-COO",
in raising the value of Tf These
delineations are relevant to protein function
and dysfunction in Chapters 7 and 8 and
are explored further below once estimates
for AGHA(E-) and AGHACK-") have been
obtained.
5.3.3 The Tt-based Hydrophobicity
Scale for Additional Biologically
Relevant Chemical Groups
5.3.3.1 The Special
Position
of
the
Phosphate Group^-OPOs^
Many chemical groups in addition to the amino
acid side chains are important in biology
because of their attachment to proteins. Most
notable is the phosphate group, -OPO3", the
most important chemical entity in biological
energy conversion. The phosphate group is also
the most important chemical entity in the con-
silient mechanism for the function of protein-
based machines. The most polar chemical entity
of all those examined for inclusion in the Tj-
based hydrophobicity scale is the phosphate
group. As shown in Table 5.2, phosphate
attached to a Ser OH constitutes the most
polar, the most supra-vinegar-like chemical
group attachable to our model proteins; it is the
least oil-like. The removal of a phosphate most
dramatically makes the model protein more oil-
Uke and most emphatically drives hydrophobic
association.
5.3.3.2 Effects on Tt of Oxidation and
Reduction of Redox Functional Groups
Nicotinamide plays a central role in both pho-
tosynthesis and respiration, as introduced in
Chapter 2. In photosynthesis, light effectively
splits water molecules into electrons, protons,
and oxygen, O2. The protons, H^, drive the
rotary ATP synthase motor to produce ATP
from ADP and Pi. The electrons reduce nicoti-
namide, which then provides for reduction of
CO2 to result in glucose. In respiration, nicoti-
namides and flavins become reduced in the
process of glucose oxidation to form CO2. This
occurs by the reactions of glycolysis, the transi-
tion reaction, and the Krebs cycle of inter-
mediary metabolism. Subsequent oxidation of
nicotinamides and flavins in the inner mito-
chondrial membrane results in the pumping of
protons across the inner mitochondrial mem-
brane to the intermembrane space between the
inner and outer mitochondrial membranes (see
Chapter 8 and specifically section 8.3). In this
process oxidation of nicotinamide results in
50%
more protons pumped across the mem-
brane than the oxidation of flavin. The higher
concentration of protons in the inner mem-
brane space then drives the ATP synthase that
combines ADP and Pi to produce 32 of the 36
ATP molecules for each molecule of glucose
oxidized.
The change of the glutamic acid side chain
from -COO" to -COOH resuhs in the largest
change in Tt (ATt = -220) of all of the vinegar-
like side chains of the natural amino acid
residues (see Table 5.1). As reported in Table
5.2, the model nicotinamide, N-methyl nicoti-
namide (NMeN) used for its relative stability,
exhibits a ATt of -250° C on reduction to the
dihydro-NMeN. The ATt decreases to -105° C
on subsequent reaction that adds a water mol-
ecule to give the more polar 6-OH tetrahydro-
NMeN. Reduction of nicotinamide adenine
dinucleotide (NAD^ -> NADH) results in a ATt
of -150° C, and that of flavin adenine dinu-
cleotide (FAD -^ FADH2) results in a ATt of
-95° C. The relative ATt values of -150° C for
NAD^
and -95° C for FAD are consistent with
their relative contributions to the pumping of
protons across the inner mitochondrial
membrane.
5.3.3.3 Nitration and Sulfation of the
Aromatic Side Chain of Tyrosine
From Table 5.1 the Tt of tyrosine is -55° C and
in Table 5.2 that of sulfated tyrosine is 140° C.
This gives a large ATt of -195° C. The ATt due
to nitration of tyrosine is an even larger 275° C.
These are large effects, but only one-third to
one-fourth of the magnitude due to phospho-
rylation. It is no surprise, therefore, that
phosphorylation/dephosphorylation became
biology's primary process for energy conver-
sion, biology's universal energy currency.
5.3 Cataloging the Energy Resources Available to the Consilient Mechanism of Energy Conversion 137
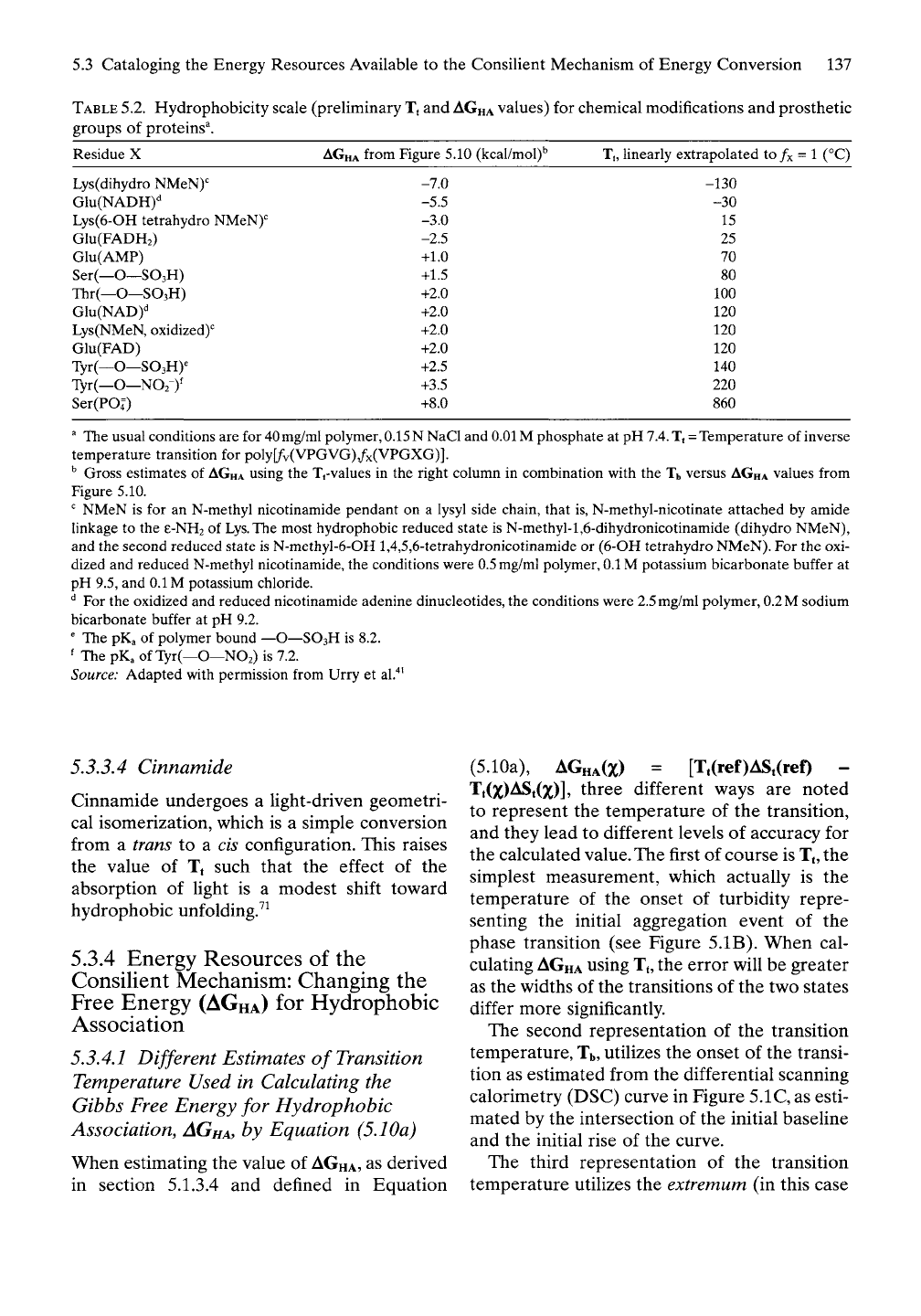
TABLE
5.2. Hydrophobicity scale (preliminary Tt and
AGHA
values) for chemical modifications and prosthetic
groups of proteins^.
Residue X
AGHA
from Figure 5.10 (kcal/mol)^ T,, linearly extrapolated to /x = 1 (°C)
Lys(dihydro NMeN)'=
Glu(NADH)''
Lys(6-OH tetrahydro NMeN)'=
Glu(FADH2)
Glu(AMP)
Ser(—O—SO3H)
Thr(—O—SO3H)
Glu(NAD)'^
Lys(NMeN, oxidized)''
Glu(FAD)
Tyr(—O—SOsH)^
Tyr(—O—NO2-)'
Ser(POl)
-7.0
-5.5
-3.0
-2.5
+1.0
+1.5
+2.0
+2.0
+2.0
+2.0
+2.5
+3.5
+8.0
-130
-30
15
25
70
80
100
120
120
120
140
220
860
^ The usual conditions are for 40mg/ml polymer, 0.15N NaCl and 0.01 M phosphate at pH 7.4. T, = Temperature of inverse
temperature transition for poly[/v(VPGVG),/x(VPGXG)].
'' Gross estimates of
AGHA
using the Tt-values in the right column in combination with the Tb versus
AGHA
values from
Figure 5.10.
""
NMeN is for an N-methyl nicotinamide pendant on a lysyl side chain, that is, N-methyl-nicotinate attached by amide
linkage to the e-NH2 of Lys.The most hydrophobic reduced state is N-methyl-l,6-dihydronicotinamide (dihydro NMeN),
and the second reduced state is N-methyl-6-OH 1,4,5,6-tetrahydronicotinamide or (6-OH tetrahydro NMeN). For the oxi-
dized and reduced N-methyl nicotinamide, the conditions were 0.5mg/ml polymer, 0.1 M potassium bicarbonate buffer at
pH 9.5, and 0.1 M potassium chloride.
'^ For the oxidized and reduced nicotinamide adenine dinucleotides, the conditions were 2.5mg/ml polymer, 0.2 M sodium
bicarbonate buffer at pH 9.2.
' The pKa of polymer bound —O—SO3H is 8.2.
' The pKa of Tyr(—O—NO2) is 7.2.
Source: Adapted with permission from Urry et al."*^
5.3.3,4
Cinnamide
Cinnamide undergoes a light-driven geometri-
cal isomerization, which is a simple conversion
from a trans to a cis configuration. This raises
the value of Tt such that the effect of the
absorption of light is a modest shift toward
hydrophobic unfolding/^
5.3.4 Energy Resources of the
Consilient Mechanism: Changing the
Free Energy (AGHA) for Hydrophobic
Association
5.3.4.1
Different Estimates of Transition
Temperature Used in Calculating the
Gibbs Free Energy for Hydrophobic
Association, AGHA, by Equation (5.10a)
When estimating the value of AGHA, as derived
in section
5.1.3.4
and defined in Equation
(5.10a), AGHA(X) = [Tt(ref)ASt(ref) -
Tt(x)ASt(x)], three different ways are noted
to represent the temperature of the transition,
and they lead to different levels of accuracy for
the calculated value. The first of course is Tt, the
simplest measurement, which actually is the
temperature of the onset of turbidity repre-
senting the initial aggregation event of the
phase transition (see Figure 5.IB). When cal-
culating AGHA using Tt, the error will be greater
as the widths of the transitions of the two states
differ more significantly.
The second representation of the transition
temperature,
Tb,
utilizes the onset of the transi-
tion as estimated from the differential scanning
calorimetry (DSC) curve in Figure 5.1C, as esti-
mated by the intersection of the initial baseline
and the initial rise of the curve.
The third representation of the transition
temperature utilizes the extremum (in this case

138
5.
Consilient Mechanisms
for
Diverse Protein-based Machines
the maximal value)
of
the DSC curve
in
Figure
5.1C. This estimate will
be
designated
as T^.
Use
of Tm
would normally result
in a
more
accurate AGnA-This, however, requires accurate
DSC data
on
both states. Such data
are not as
easily obtained because
of the
small heats
of
the transitions,
the
long time required
for
com-
pleting the association
of
the transition, and
the
higher value
of T^.
5.3.4.2 AGHA'
The
Change
in
Gibbs Free
Energy
for
Hydrophobic Association
Due
to Change
in
Amino Acid Composition
With AG„A(Gly)
= 0
5.3.4.2.1 Calculation
of
AGHA
and
Choice
of
Reference State
Equation (5.10b),
AGHA(%)
=
[AHt(ref)
-
AHt(x)],
utilizes
the AHt
values
for the
phase
transition. Because
for the
inverse temperature
transition,
AHt =
TtASt,
the
heat
of
this transi-
tion
to the
hydrophobically associated state
becomes
a
measure
of
the change
in
Gibbs free
energy
for
the transition,
AGHA-
It
now becomes
necessary
to
define
an
appropriate reference
state
to
achieve meaningful comparisons. This
involves both
the
fraction
of
composition
as
well
as the
specific amino acid composition
to
be referenced.
For the
former,
the AHt
values
reported
in
Table
5.1 for fx = 0.2 are
extrapo-
lated
to fx = 1 and for the
latter
the Gly (G)
residue
is
taken
as the
amino acid
of
reference,
because
its
side chain,
a
single hydrogen atom,
is neither oil-like
nor
vinegar-like. This change
in Gibbs free energy
for
hydrophobic associa-
tion
on
substitution
of Gly (G) in
GGGVP
by
the guest amino acid residue
X
may
be
written
as
AGHA(GGGVP
-^
GXGVP), but is indicated
as
a
reference value
by the
superscript.
5.3.4.2.2 Estimated Values
for
AGHA(GGGVP
-^ GXGVP)
For
the
following estimates,
Tb
values
are
available
for
guest amino acid residues without
functional groups.^^ With Equation (5.10b),
the calculated values
for the
residues more
hydrophobic than glycine
(Gly, G),
that
is,
AGHA(GGGVP
-^
GXGVP) substitutions,
in
units
of
kcal/mol-pentamer become
AGHA(GGGVP
-^
GWGVP)
=
-7.00,
AGHA(GGGVP
-^
GYGVP)
=
-5.85,
AGHA(GGGVP
-^
GFGVP)
=
-6.15,
AGHA(GGGVP
^
GLGVP)
=
-4.05,
AGHA(GGGVP
-^
GIGVP)
=
-3.65,
AGHA(GGGVP
^
GVGVP)
=
-2.50,
AGHA(GGGVP
-^
GMGVP)
=
-1.50,
AGHA(GGGVP
->
GEGVP)
=
-1.30,
AGHA(GGGVP
-^
GPGVP)
=
-1.10,
AGHA(GGGVP
^
GAGVP)
=
-0.75,
AGHA(GGGVP
^
GTGVP)
=
-0.60,
AGHA(GGGVP
-^
GD°GVP)
=
-0.40,
AGHA(GGGVP
->
GK°GVP)
=
-0.05,
AGHA(GGGVP
^
GNGVP)
=
-0.05,
and AGHA(GGGVP
-^
GGGVP)
=
0.00, Thus,
we have
a
measure
of
the favorable decrease
in
free energy
of the
hydrophobically associated
state
for
those residues more hydrophobic
than
G
For residues less hydrophobic,
or
more polar,
than glycine, AGHA(GGGVP
-^
GSGVP)
=
+0.55,
AGHA(GGGVP
-^
GQGVP)
=
+0.75,
AGHA(GGGVP
^
GYGVP)
=
+1.95,
AGHA(GGGVP
^ GDGVP) - +2.6,
AGHA(GGGVP
->
GK^GVP)
=
+2.94,
and
AGHA(GGGVP
-^
GEGVP)
=
+3.72.
A
positive
AGHA
disfavors
the
hydrophobically
associated state,
and a
negative
AGHA
favors
hydrophobic association. The values
are
plotted
in Figure
5.10 and
listed
in
Table
5.3.
In Figure
5.10 a
sigmoid curve
is
drawn
through
the
data points, which
on the
hydro-
phobic side follows closely
the
hydrophobic
residues W, F,
L, I,
V, P,
and A to the
neutral
G
residue. Those residues with mixed,
but
uncharged, hydroxyl, carboxyl, amino,
and so
forth, functional substituents fall
off of the
sigmoid.
On the
polar side
the
sigmoid curves
around until
it
takes
the
slope defined
by
the
K^
-
E"
data points. Interestingly, continuing
the
K^
- E"
slope
to
860° C,
the
TfValue resulting
from
a
phosphate attached
to a
serine (Ser,
S)
residue, gives
a
value
for
AGHA(P04")
of
approximately
8
kcal/mole-phosphate.
Another interesting point about Figure
5.10
is provided
by the
diagonal straight line though
the data points. This line illustrates
the
approx-
imation
of the
Tb(Tt)-based hydrophobicity
scale, which provides
the
most directly usable
5.3 Cataloging the Energy Resources Available to the Consilient Mechanism of Energy Conversion 139
^ 40 ^
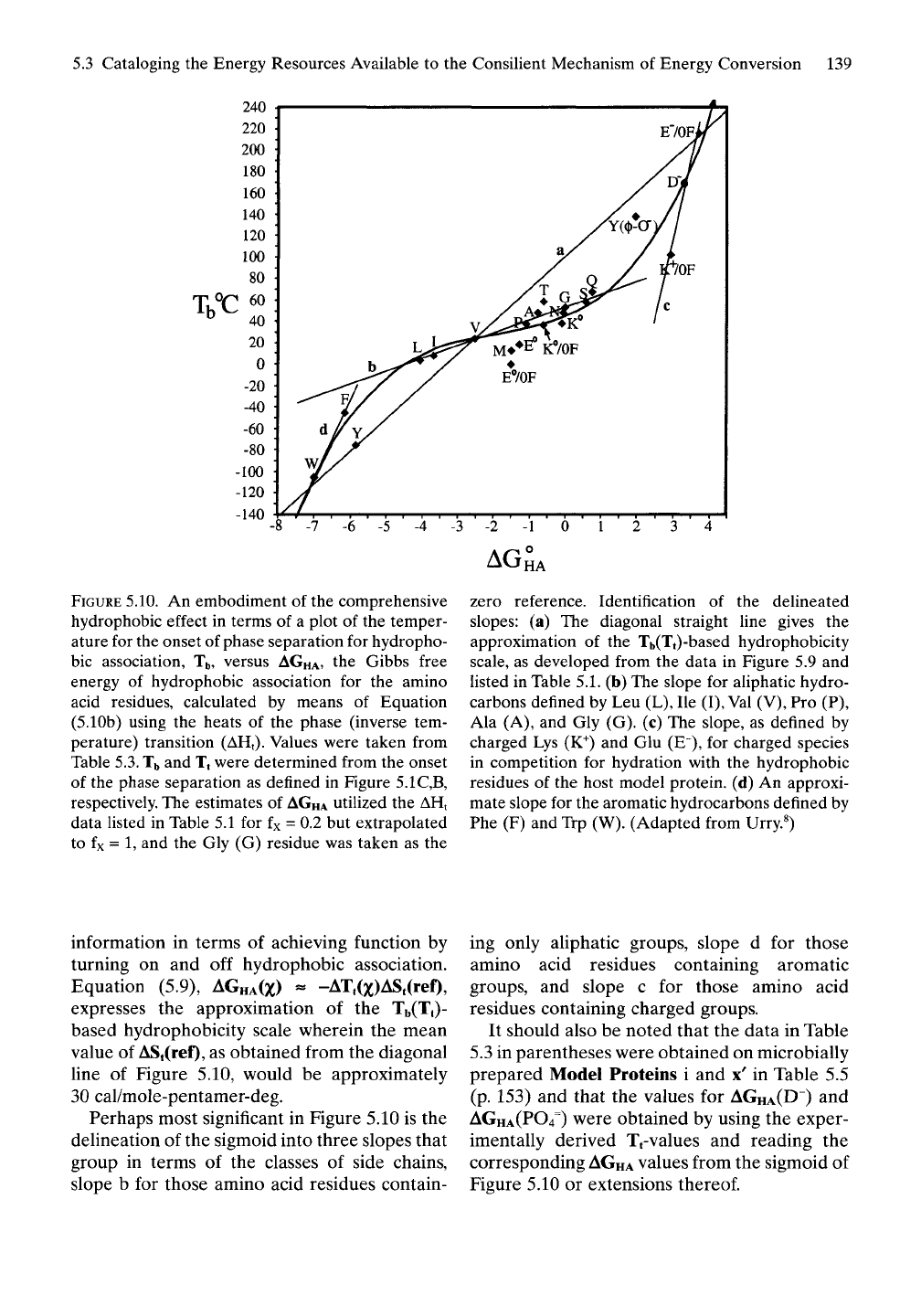
FIGURE 5.10. An embodiment of the comprehensive
hydrophobic effect in terms of a plot of the temper-
ature for the onset of phase separation for hydropho-
bic association, Tb, versus AGHA, the Gibbs free
energy of hydrophobic association for the amino
acid residues, calculated by means of Equation
(5.10b) using the heats of the phase (inverse tem-
perature) transition (AHt). Values were taken from
Table
5.3.
Tb
and Tt were determined from the onset
of the phase separation as defined in Figure 5.1C,B,
respectively. The estimates of
AGHA
utilized the AHt
data listed in Table 5.1 for fx = 0.2 but extrapolated
to fx = 1, and the Gly (G) residue was taken as the
AG
HA
zero reference. Identification of the delineated
slopes: (a) The diagonal straight line gives the
approximation of the Tb(Tt)-based hydrophobicity
scale, as developed from the data in Figure 5.9 and
Usted in Table
5.1.
(b) The slope for aliphatic hydro-
carbons defined by Leu (L), He
(I),
Val
(V), Pro (P),
Ala (A), and Gly (G). (c) The slope, as defined by
charged Lys (K^) and Glu (E"), for charged species
in competition for hydration with the hydrophobic
residues of the host model protein, (d) An approxi-
mate slope for the aromatic hydrocarbons defined by
Phe (F) and Trp (W). (Adapted from Urry.«)
information in terms of achieving function by
turning on and off hydrophobic association.
Equation (5.9), AGHA(X) « -AT,(x)ASt(ref),
expresses the approximation of the Tb(T|)-
based hydrophobicity scale wherein the mean
value of ASt(ref), as obtained from the diagonal
line of Figure 5.10, would be approximately
30 cal/mole-pentamer-deg.
Perhaps most significant in Figure 5.10 is the
delineation of the sigmoid into three slopes that
group in terms of the classes of side chains,
slope b for those amino acid residues contain-
ing only aliphatic groups, slope d for those
amino acid residues containing aromatic
groups, and slope c for those amino acid
residues containing charged groups.
It should also be noted that the data in Table
5.3 in parentheses were obtained on microbially
prepared Model Proteins i and x' in Table 5.5
(p.
153) and that the values for AGHA(D~) and
AGHA(P04") were obtained by using the exper-
imentally derived Tt-values and reading the
corresponding AGHA values from the sigmoid of
Figure 5.10 or extensions
thereof.
140
5.
Consilient Mechanisms
for
Diverse Protein-based Machines
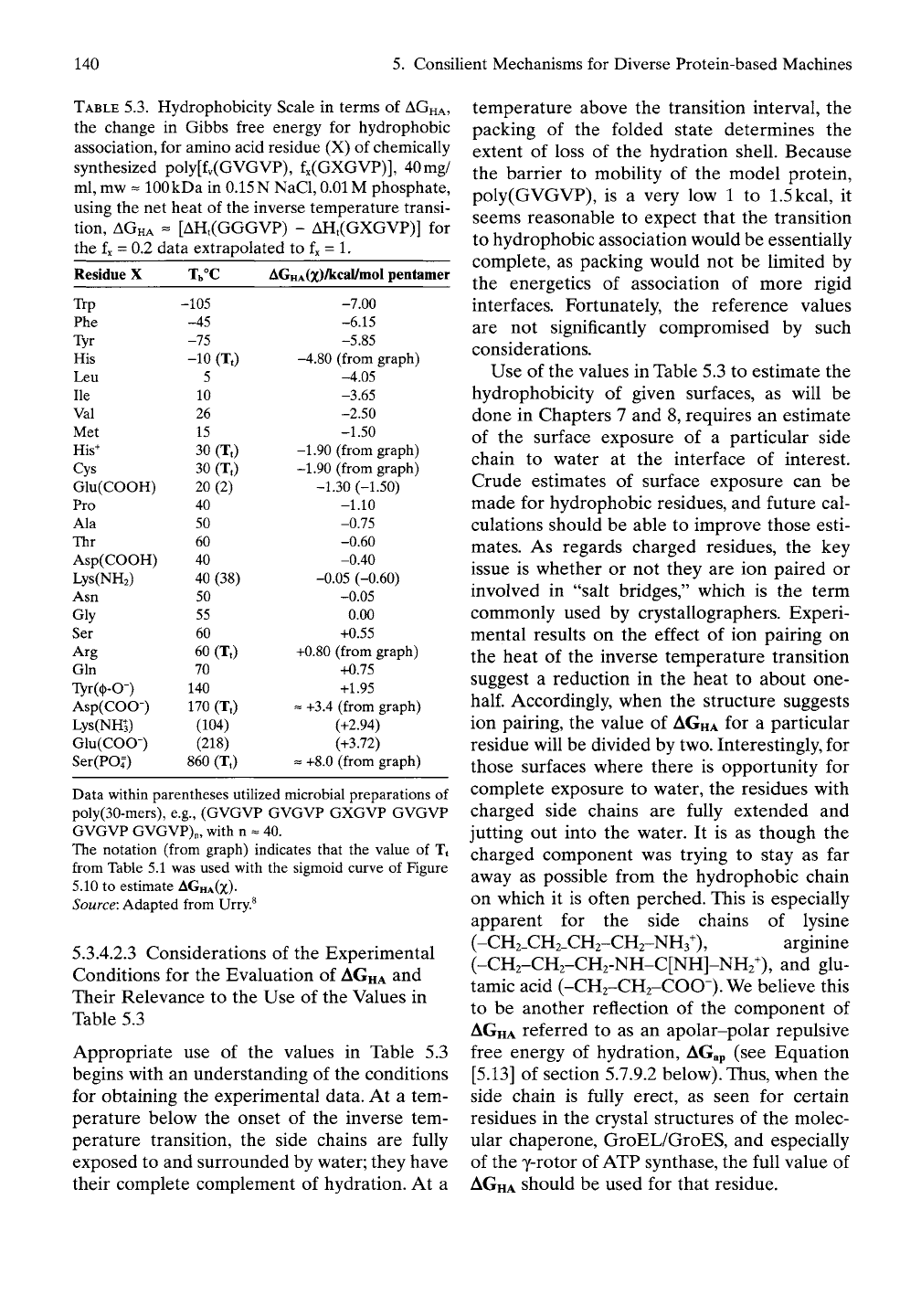
TABLE
5.3. Hydrophobicity Scale in terms of
AGHA,
the change
in
Gibbs free energy
for
hydrophobic
association, for amino acid residue (X)
of
chemically
synthesized poly[fv(GVGVP), fx(GXGVP)], 40mg/
ml,
mw
==
100 kDa
in
0.15 N
NaCl,
0.01 M
phosphate,
using the
net
heat
of
the inverse temperature transi-
tion,
AGHA
-
[AHt(GGGVP)
-
AHt(GXGVP)]
for
the fx = 0.2 data extrapolated
to
fx =
1.
Residue
X
Trp
Phe
Tyr
His
Leu
He
Val
Met
His^
Cys
Glu(COOH)
Pro
Ala
Thr
Asp(COOH)
Lys(NH2)
Asm
Gly
Ser
Arg
Gin
Tyr((t)-0-)
Asp(COO-)
Lys(NH^)
Glu(COO-)
Ser(P04=)
Tb°C
-105
-45
-75
-10 (TO
5
10
26
15
30 (TO
30 (TO
20(2)
40
50
60
40
40 (38)
50
55
60
60 (TO
70
140
170 (Tt)
(104)
(218)
860 (TO
AGHA(X)/l^<^^l/niol
pentamer
-7.00
-6.15
-5.85
-4.80 (from graph)
-4.05
-3.65
-2.50
-1.50
-1.90 (from graph)
-1.90 (from graph)
-1.30 (-1.50)
-1.10
-0.75
-0.60
-0.40
-0.05 (-0.60)
-0.05
0.00
+0.55
+0.80 (from graph)
+0.75
+1.95
~ +3.4 (from graph)
(+2.94)
(+3.72)
= +8.0 (from graph)
Data within parentheses utilized microbial preparations
of
poly(30-mers),
e.g.,
(GVGVP GVGVP GXGVP GVGVP
GVGVP
GVGVP)n,
with
n «
40.
The notation (from graph) indicates that
the
value
of Tt
from Table
5.1 was
used with
the
sigmoid curve
of
Figure
5.10 to
estimate
AGHA(X).
Source: Adapted from Urry.^
5.3.4.2.3 Considerations
of the
Experimental
Conditions for the Evaluation of
AGHA
and
Their Relevance
to the Use of the
Values
in
Table
5.3
Appropriate
use of the
values
in
Table
5.3
begins with
an
understanding
of the
conditions
for obtaining
the
experimental data.
At a
tem-
perature below
the
onset
of the
inverse tem-
perature transition,
the
side chains
are
fully
exposed
to and
surrounded
by
water; they have
their complete complement
of
hydration.
At a
temperature above
the
transition interval,
the
packing
of the
folded state determines
the
extent
of
loss
of the
hydration shell. Because
the barrier
to
mobility
of the
model protein,
poly(GVGVP),
is a
very
low 1 to
1.5kcal,
it
seems reasonable
to
expect that
the
transition
to hydrophobic association would
be
essentially
complete,
as
packing would
not be
limited
by
the energetics
of
association
of
more rigid
interfaces. Fortunately,
the
reference values
are
not
significantly compromised
by
such
considerations.
Use
of
the values
in
Table
5.3 to
estimate
the
hydrophobicity
of
given surfaces,
as
will
be
done
in
Chapters
7 and 8,
requires
an
estimate
of
the
surface exposure
of a
particular side
chain
to
water
at the
interface
of
interest.
Crude estimates
of
surface exposure
can be
made
for
hydrophobic residues,
and
future cal-
culations should
be
able
to
improve those esti-
mates.
As
regards charged residues,
the key
issue
is
whether
or not
they
are ion
paired
or
involved
in
"salt bridges," which
is the
term
commonly used
by
crystallographers. Experi-
mental results
on the
effect
of ion
pairing
on
the heat
of the
inverse temperature transition
suggest
a
reduction
in the
heat
to
about
one-
half.
Accordingly, when
the
structure suggests
ion pairing,
the
value
of
AGHA
for a
particular
residue will
be
divided
by
two. Interestingly,
for
those surfaces where there
is
opportunity
for
complete exposure
to
water,
the
residues with
charged side chains
are
fully extended
and
jutting
out
into
the
water.
It is as
though
the
charged component
was
trying
to
stay
as far
away
as
possible from
the
hydrophobic chain
on which
it is
often perched. This
is
especially
apparent
for the
side chains
of
lysine
(-CH2_CH2_CH2-CH2-NH3^), arginine
(-CH2-CH2-CH2-NH-C[NH]-NH2^),
and
glu-
tamic acid (-CH2-CH2-COO-). We believe this
to
be
another reflection
of the
component
of
AGHA
referred
to as an
apolar-polar repulsive
free energy
of
hydration, AGap
(see
Equation
[5.13]
of
section
5.7.9.2
below). TTius, when
the
side chain
is
fully erect,
as
seen
for
certain
residues
in the
crystal structures
of the
molec-
ular chaperone, GroEL/GroES,
and
especially
of the y-rotor
of
ATP synthase,
the
full value
of
AGHA should
be
used
for
that residue.

5.3 Cataloging the Energy Resources Available to the Consilient Mechanism of Energy Conversion 141
5.3.4.3 Determination of AG
HA
for a
Change in Functional State of Naturally
Occurring Vinegar-like Side Chain
5.3.4.3.1 The AG^ArOlu-COOH->
GhkCOO)
Using Equation (5.10b) and differential
calorimetry data on Model protein i of Table
5.5 as listed in Table 5.3 with a Tb(Glu-COOH)
of
2° C,
a Tb(Glu-COO-) of
218° C,
a AGHA(G1U-
COOH) of -1.50kcal/mole, and a AGHA(G1U-
COO") of +3.72kcal/mole, the calculated
increase in Gibbs free energy of hydrophobic
association on ionization of the carboxyl func-
tion, AGHA(G1U-COOH -> Glu-COO) = [+
3.72 - (-1.50)] = +5.22 kcal/mole-pentamer. This
quantity is of interest in connection with that
calculated below for AGHA(Lys-NH2 -^ Lys-
NHs^) for the purpose of estimating effects of
ion pairing between model proteins.
Additional questions can be addressed. For
example, how does AGHA(G1U-COOH -^ Glu-
COO") compare with the AGHA of other func-
tional groups in the same model protein, for
example, functional groups that are coupled
with proton pumping in oxidative phosphoryla-
tion of the mitochondria, such as nicoti-
namides? This question is answered in section
5.3.4.4. Another question is, how does the mag-
nitude of AGHA(G1U-COOH -^ Glu-COO)
compare with that of an opposite signed
vinegar-like functional group, for example, the
quantity AGHA(Lys-NH2 -^ Lys-NHg^)? The
latter is given immediately below.
5.3.4.3.2 The AGHA(Lys-NH2 -^ Lys-NHj^)
Using Equation (5.10b) and differential
calorimetry data at pH 7.5 on Model Protein x'
of Table 5.5 with a Tb(Lys-NH2) of 38° C, a
Tb(Lys-NH3^) of 104° C, a AGHA(Lys-NH2) of
-0.60kcal/mole-(GKGVP), and a AGHA(Lys-
NHs^) of
-h2.94
kcal/mole-(GK^GVP), the
calculated increase in Gibbs free energy for
hydrophobic association on protonation of the
amine function, AGHA(Lys-NH2 -^ Lys-NHs^)
= +3.54 kcal/mole-pentamer. The effect on
AGHA of forming the charged Lys-NHs^ is less
than on forming the charged Glu-COO", that is,
AGHA(G1U-COOH -^ Glu-COO) of +5.22
kcal/mole-pentamer. Thus, one might under-
stand when using ion pairing to lower Tt and
AGHA that a thermodynamic equivalence could
require more positively charged groups than
negatively charged groups. In this regard, data
on the allosteric protein hemoglobin are rele-
vant. The allosteric binding, of biphosphoglyc-
erate with five negative charges to eight
positively charged groups at the diad axis of
hemoglobin, enhances oxygen release. This ion
pairing favors the more hydrophobically asso-
ciated deoxyhemoglobin state, with the charge
stoichiometry such that there are to be antici-
pated more positive charges than negative
charges (see Chapter 7).
5.3.4.3.3 Calculation of the Effect of Ion
Pairing Between Chains, AGHA[(G1U-COO-;
Lys-NHs^) -^ (COO- NHs^)]
Using Equation (5.10b) and differential
calorimetry data on the COO" state of Model
Protein i of Table 5.5 with a Tb(Glu-COO-) of
218° C yields a value for AGHA(GIU-COO-) of
3.72kcal/mole-(GE-GVP). Similarly for Model
Protein x' of Table 5.5 with a Tb(Lys-NH3^) of
104° C yields a value for AGHA(Lys-NH3^) of
+2.94kcal/mole-(GK^GVP). The AGHA(COO-
NHs^) value, obtained for a pH 7.5 solution con-
taining equimolar (Glu-COO") and (Lys-NHs"^)
functional groups, is 0.36 kcal/mole. This means
that the decrease in Gibbs free energy for
hydrophobic association due to the ion-pair
formation between Model proteins i and x' is
approximately 3 kcal/mole-pentamer, that is,
AGHA[(G1U-COO-;
Lys-NHs^)
-^ (COO"
NHs^)],
becomes [0.36 - (3.72 + 2.94)/2] - -3.0
kcal/mole-pentamer. This exemplifies the
power of ion pairing in lowering the Gibbs free
energy for hydrophobic association, which
increases with increasing hydrophobicity.
5.3.4.4 Calculation of
AGHA
for a Change
in the State of Bound Biologically
Relevant Functional Groups
5.3.4.4.1 The
AGHA
Due to the Reduction of
Oxidized N-methyl Nicotinamide (NMeN^)
AS
Tt is equivalent to Tb, a direct means of esti-
mating values of AGHA[GK(NMeN^)GVP],
of AGHA[GK(dihydroNMeN)GVP], and of

142
5.
Consilient Mechanisms for Diverse Protein-based Machines
AGHA[GK(NMeN)GVP] is to use the values of
Tt in Table 5.2 and the plot of Tb versus AGHA
of Figure 5.10. The Gibbs free energy of
hydrophobic hydration, obtained in this way, is
given in Table 5.2 to be +2.0, -3.0, and -7.0,
respectively. Therefore, an estimate of the
change in Gibbs free energy for hydrophobic
association on reduction of the N-methyl nico-
tinamide, AGHAINMCN"^ -> NMeN] becomes
[-7.0 - [+2.0]] = -9.0kcal/mole-pentamer.
Comparison of the change in free energy for
hydrophobic association on reduction of this
nicotinamide, AGHA[NMeN^ -^ NMeN], to the
decrease in free energy of hydrophobic asso-
ciation due to protonation of the carboxylate
of Glu,
AGHA[G1U-COO-
-^
Glu-COOH]
of
-5.22kcal/mole, gives insight into the maximal
efficiency for the coupling of these functional
groups. Accordingly, the coupling of the two
reactions, wherein reduction drives the uptake
of a proton by a Glu-COO", could be expected
to occur in the particular model protein with a
60%
efficiency of energy conversion. A similar
number for efficiency is obtainable on calculat-
ing the carboxyl/carboxylate pKa shift due to
reduction in the same polymer containing both
functional groups by comparing the free energy
change on reduction with the observed pKa
shift (see section 5.9, below).^^
5.3.4.4.2 The
AGHA
Due to the Reduction
of Oxidized Nicotinamide Adenine
Dinucleotide (NAD)
Following the same process as used above for
N-methyl nicotinamide, involving the data in
Table 5.2 and the plot in Figure 5.10, an esti-
mate of the change in Gibbs free energy for
hydrophobic association on reduction of nicoti-
namide adenine dinucleotide, AGHA(NAD -^
NADH) becomes [-5.5 - (+2.0)] = -7.5kcal/
mole-pentamer.
5.3.4.4.3 The
AGHA
Due to the Reduction of
Oxidized Flavin Adenine Dinucleotide (FAD)
Continuing as immediately above using the
data in Table 5.2 and in Figure 5.10, an estimate
of the change in Gibbs free energy for
hydrophobic association on reduction of flavin
adenine dinucleotide, AGHA(FAD -^ FADH2)
becomes [-2.5 - (+2.0)] = ^.Skcal/mole-
pentamer. The qualitative relationship between
AGHA(NAD -> NADH) of -7.5kcal/mole-
pentamer and AGHA(FAD -^ FADH2) of
-4.5kcal/mole-pentamer is consistent with the
resulting relative proton translocation due to
these molecules on oxidation in the electron
transport chain of the inner mitochondrial
membrane where the proton transport ratio for
FADH2:NADHis2:3.
5.3.4.4.4 The
AGHA
Due to Phosphorylation
Phosphorylation by the cardiac cyclic AMP-
dependent protein kinase at the serine (Ser, S)
residue of the polymer poly[30(GVGIP),
(RGYSLG)] resulted in an estimated Tt-value
in Table 5.2 of 860° C.^'*'^^ The equilibrium con-
stant for the reaction
ATP + poly [30(G
VGIP),
(RGYSLG)] =
ADP + poly(30[GVGIP],[RGYS]
{-OPOjJLG])
(5.11)
was essentially 1. In particular, the average
phosphorylation from many phosphorylation
attempts was
47%.^'^^
This means that the
change in free energy of the phosphate on
going from the terminal position of ATP
to poly(30[GVGIP],[RGYS{-OPO3=}LG]) is
effectively
zero.
The conclusion is that the phos-
phate in poly(30[GVGIP],[RGYS{-OPO3=}
LG]) is at the same high free energy state as it
is in the y-position of ATP.
Because hydrophobic association does not
occur until the temperature is above Tt for the
phosphorylated state, the phosphate cannot be
folded into a low dielectric constant site, but
instead would be fully exposed to the solvent.
It is fundamental to the consilient mechanism
of protein-based machines to understand how
this can be! As discussed in section 5.7, the
high-energy state of the phosphate is due to
competition for hydration between the phos-
phate and the hydrophobic groups, as reflected
in the large change in Tt.
Following the above approach of combining
the data in Table 5.2 with the relationship

5.3 Cataloging
the
Energy Resources Available
to the
Consilient Mechanism
of
Energy Conversion
143
between
Tb and
AGHA
of
Figure
5.10 and the
realization that
Tb ~ Tt, it
becomes possible
to
achieve
an
estimate
of
AGHAC-OPOS").
The
charged species,
K^ and E",
determine
a
slope
with which
to
extrapolate from
100° C to the
value
of
860°
C
determined
for the
phosphory-
lation discussed above. This gross means
of
estimation results
in the
remarkable value
of
AGHA(-0P03=)
= +8kcal/mole.
This free energy
of
interaction occurs
in the
unfolded state
and
serves
to
raise
the
free
energy
for
formation
of the
hydrophobically
associated state. Because
the
high-energy state
is
the
unfolded polymer,
it
cannot
be due to
charge-charge repulsion; there
is
only
1
phos-
phate
in
about
300
residues.
The
high-energy
state cannot
be due to the
phosphate being
in
the
low
dielectric region
of a
folded protein.
In
our view,
it
represents
a
clear measure
of the
apolar-polar repulsive free energy
of
hydration
arising
out of the
competition
for
hydration
between
the
oil-like side chains
of the
isoleucine
(He, I) and
valine (Val,
V)
residues
and
the
phosphate
in the
unfolded model
protein.
Phosphate removal drives hydrophobic asso-
ciation, that
is,
contraction, more effectively
than
any
other chemical change measured thus
far.
The
binding
of ATP,
adenosine triphos-
phate, the universal energy currency
of
biology,
could even more effectively increase
the
value
of
Tt, but
this
can be
modified
by the
associa-
tions
of the ATP at the
binding site. Also,
as in
Table
5.2, the
estimate
of
AGHA[G1U(AMP)]
is
a
modest -i-l kcal/mole,
and the
addition
of
each phosphate would
add
approximately
8kcal/mole. Furthermore,
we
expect that
the
state
of
greatest apolar-polar repulsion would
result from
the
presence
of
ADP plus
Pi.
5.3.4.5 Using
ATf Due to
Addition
of
Salts
to Calculate Change
in
Free Energy
(AGHA)
for
Hydrophobic Association
of
Neutral Poly(GVGVP)
The dependence
of
Tt
for
poly(GVGVP)
on the
addition
of a
series
of
salts
(the
Hoffmeister
series)
is
shown
in
Figure 5.11A. With
the
exceptions
of
I"
and
SCN",
the
addition
of
salts
to poly(GVGVP) lowers
the
value
of T^
With
the
AHt
data from Table
I of
Luan
et
al.^^
for
use
in
Equation (5.10b), calculation
of
AGHA
(0
N ^ 1.0 N
NaCl) yields
a
value
of -0.57
kcal/mole. Calorimetry
(AHt)
data
are not yet
available
for the
other salts.
If we
assume
the
values
to be
proportional
to
ATt with
the
NaCl
data providing
the
proportionality constant,
however, estimates
of
AGHA(0
N -^ 1.0 N
salt)
can
be
obtained
for a
series
of
salts. These
are
listed below
and in
Table 5.4A. The decrease
in
Tt(GVGVP)
due to the
addition
of 1
mole
of
salt
is
found
in
Table
3c of
Luan
and
Urry.^^
In
what follows, after identification
of
the polymer
composition
and
salt, listed
are the
numbers
for
ATt
on
increasing
the
amount
of
salt from
0 to
1.0 N and the
calculated value
of
AGHA
in
kcal/mole based
on a
proportionality
to the
NaCl data: NasPO^pH
> 8), -140° C, -5.7;
(NH4)250,, -69° C, -2.8; Na2C05(pH
>
8),
-28°
C, -1.1; NaCl, -13.9° C, -0.57; CaCls, -6.6° C,
-0.27;
NaBr, -3.5° C, -0.14;
Nal and
NaSCN,
+3.5° C,+0.14.
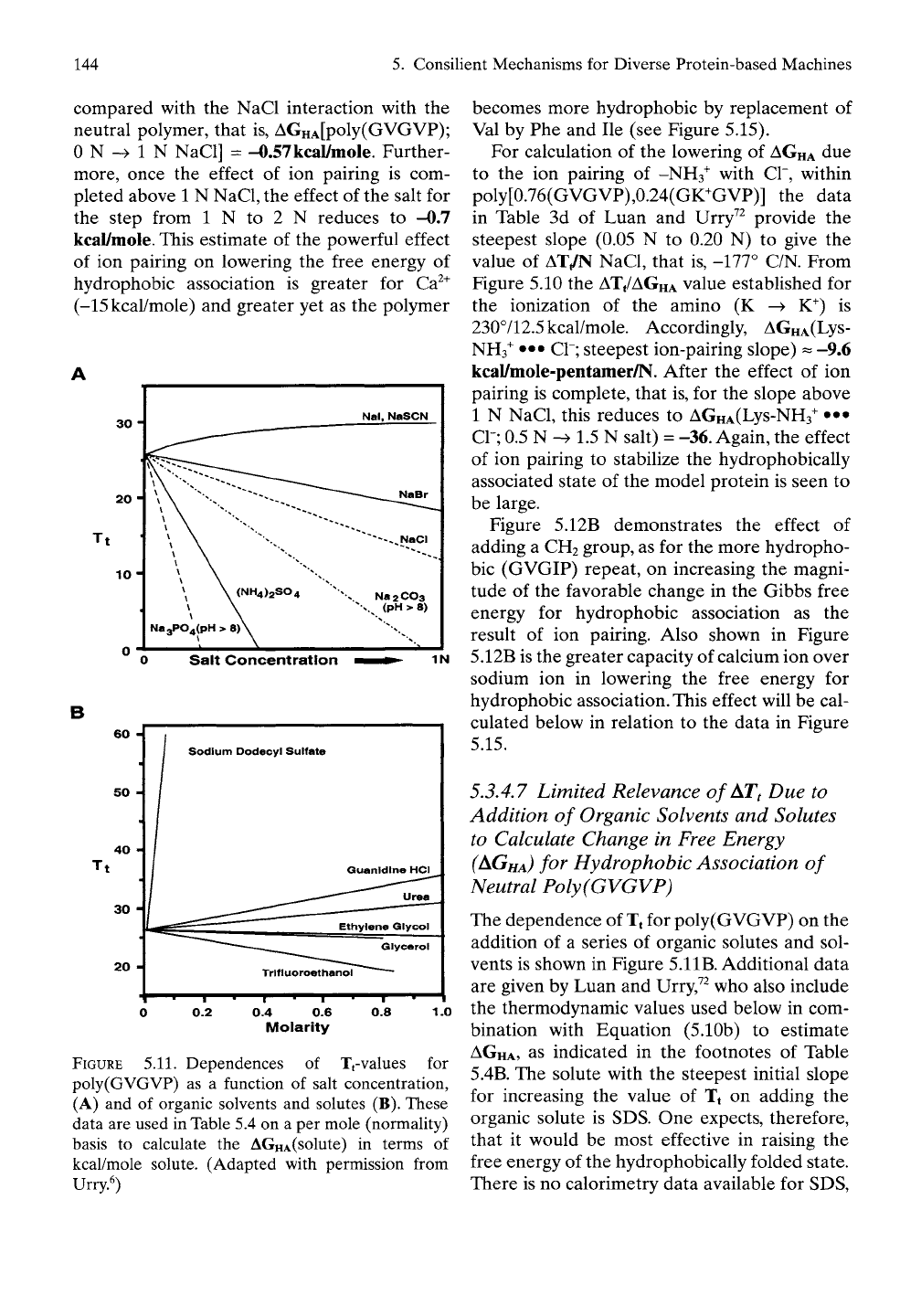
5.3.4.6 Using
ATt Due to
Ion-Pairing
of
Salts with Functional Groups
to
Estimate
Change
in
Free Energy (AGHA)
for
Hydrophobic Association
As shown
by
comparison
of the
data
in
Figure
5.12A with those
in
Figure 5.11A,
the
effect
of salts
in
lowering
the
free energy
of the
hydrophobically associated state
of
the protein-
based polymer
is
much greater when
the
polymer contains
a
charged side chain.
An
esti-
mate
of
AGHA(G1U-COO"
•••
Na"^; steepest
ion-pair slope), that is, the change
in
Gibbs free
energy
for ion
pairing over
the
change
in
salt
concentration with
the
steepest slope,
may be
obtained using the
slopes,
ATJ/AGHA established
in Figure 5.10
for the
ionization
of
the carboxyl
(E
-^ E") to be
380°/9 kcal/mole. Table
3d
Luan
and Urry^^ provides
the
data points used
in
Figure
5.12A
from which
the
steepest slope
(0.15
N to
0.25
N) is
obtained
to
give
the
value
of ATt/N NaCl
to be
-395°
C/N. The
result
is
AGHA(G1U-COO"
•••
Na^; steepest ion-pair
slope)
=
-9.4kcal/mole-pentamer/N. The effect
of
ion
pairing
of Na^
with
COO"
lowers
the
free energy
of the
hydrophobically associated
state
by
more than
an
order
of
magnitude when
144
5.
Consilient Mechanisms for Diverse Protein-based Machines
compared with the NaCl interaction with the
neutral polymer, that is, AGHA[poly(GVGVP);
0 N -> 1 N NaCl] = -0.57kcal/mole. Further-
more, once the effect of ion pairing is com-
pleted above 1 N NaCl, the effect of the salt for
the step from 1 N to 2 N reduces to -0.7
kcal/mole. This estimate of the powerful effect
of ion pairing on lowering the free energy of
hydrophobic association is greater for Ca^^
(-15 kcal/mole) and greater yet as the polymer
30 H
20 i
10 -I
Nal,
NaSCN
60 -
50 -
40 -
30 -
20 -
j
1 Sodium Dodecyl Sulfate 1
1
1
/
/ Guanldlne HClJ
/ ^ Urea J
1—^^=^^
Ethylene Glycol |
-^ .._____^^ Glycerol j
Trifluoroethanoi "—'
i~" • 1 ' r • 1 • I " 1
0.2
0.4 0.6
Molarity
0.8 1.0
FIGURE 5.11. Dependences of Tj-values for
poly(GVGVP) as a function of salt concentration,
(A) and of organic solvents and solutes (B). These
data are used in Table 5.4 on a per mole (normality)
basis to calculate the AGHA(solute) in terms of
kcal/mole solute. (Adapted with permission from
Urry^)
becomes more hydrophobic by replacement of
Val by Phe and He (see Figure 5.15).
For calculation of the lowering of
AGHA
due
to the ion pairing of -NHs^ with CI", within
poly[0.76(GVGVP),0.24(GK^GVP)] the data
in Table 3d of Luan and Urry^^ provide the
steepest slope (0.05 N to 0.20 N) to give the
value of ATt/N NaCl, that is, -177° C/N. From
Figure 5.10 the ATI/AGHA value established for
the ionization of the amino (K -^ K^) is
230°/12.5 kcal/mole. Accordingly, AGHA(Lys-
NHs^
••• CI"; steepest ion-pairing slope) ~ -9.6
kcal/mole-pentamer/N. After the effect of ion
pairing is complete, that is, for the slope above
1 N NaCl, this reduces to AGHA(Lys-NH3^ •••
CI";
0.5 N -^ 1.5 N salt) = -36. Again, the effect
of ion pairing to stabilize the hydrophobically
associated state of the model protein is seen to
be large.
Figure 5.12B demonstrates the effect of
adding a CH2 group, as for the more hydropho-
bic (GVGIP) repeat, on increasing the magni-
tude of the favorable change in the Gibbs free
energy for hydrophobic association as the
result of ion pairing. Also shown in Figure
5.12B is the greater capacity of calcium ion over
sodium ion in lowering the free energy for
hydrophobic association. This effect will be cal-
culated below in relation to the data in Figure
5.15.
5.3.4.7 Limited Relevance of AT^ Due to
Addition of Organic Solvents and Solutes
to Calculate Change in Free Energy
(AGHA)
for Hydrophobic Association of
Neutral Poly(GVGVP)
The dependence of Tt for poly(GVGVP) on the
addition of a series of organic solutes and sol-
vents is shown in Figure 5.1
IB.
Additional data
are given by Luan and Urry,^^ who also include
the thermodynamic values used below in com-
bination with Equation (5.10b) to estimate
AGHA,
as indicated in the footnotes of Table
5.4B. The solute with the steepest initial slope
for increasing the value of Tt on adding the
organic solute is SDS. One expects, therefore,
that it would be most effective in raising the
free energy of the hydrophobically folded state.
There is no calorimetry data available for SDS,
