Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.

5.5 Energy Conversions
Due to
Coupling
of
Functional Groups
165
experience
for the
many ways
in
which
the
energy
of the
hydrophobically associated state
can
be
changed
and the
effectiveness
of
each
way
to do so.
Specifically, section
5.3.4.4
pro-
vides estimates of the
AGHA
due to the
reduction
of
oxidized N-methyl nicotina-
mide (NMeN^)
in
poly(GVGVP), about
-9.0kcal/mole,
and
section 5.3.4.3, estimates
AGHA(G1U-COOH
-^
Glu-COO)
to be
about
+5.22kcal/mole.
In
this section, experimental
results demonstrate
the
coupling
of
these
two
different functional groups.
5.5.3.1 Change
in the
Hydrophobicity
(Extent
of
Oil-Uke Character)
Due to
Action
on One
Functional Group,
for
Example, Reduction
of
Oxidized N-methyl
Nicotinamide, Moves
the
Transition Zone
for
a
Second Functional Group,
for
Example,
the
Carboxyl/Carboxylate
Chemical Couple: Electro ^-^ Chemical
Transduction
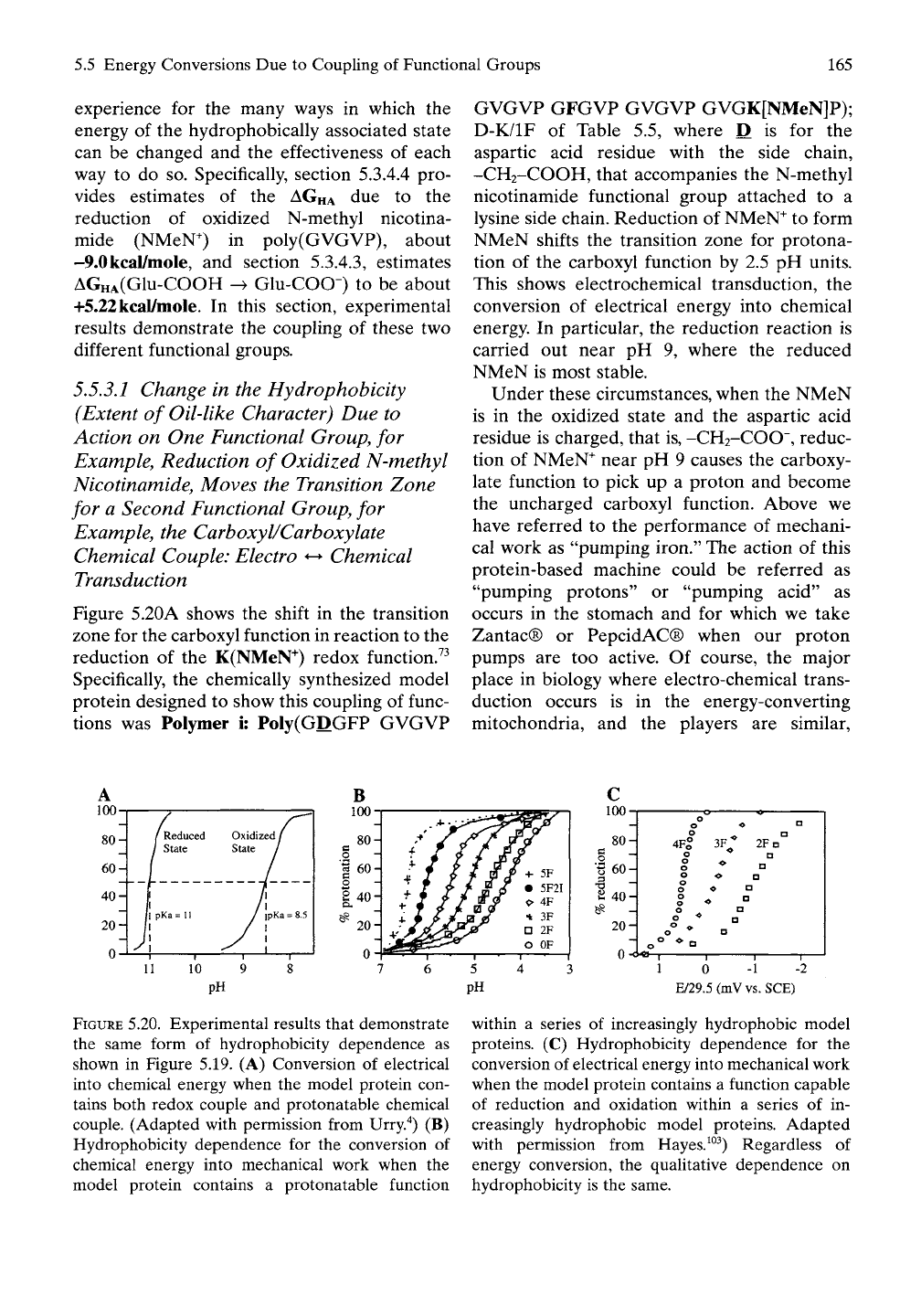
Figure 5.20A shows
the
shift
in the
transition
zone
for
the carboxyl function
in
reaction
to the
reduction
of the
K(NMeN"^) redox function.^^
Specifically,
the
chemically synthesized model
protein designed
to
show this coupling
of
func-
tions
was
Polymer
i:
PoIy(GDGFP GVGVP
GVGVP GFGVP GVGVP GVGK[NMeN]P);
D-K/IF
of
Table
5.5,
where
D is for the
aspartic acid residue with
the
side chain,
-CH2-COOH, that accompanies
the
N-methyl
nicotinamide functional group attached
to a
lysine side chain. Reduction
of
NMeN^
to
form
NMeN shifts
the
transition zone
for
protona-
tion
of the
carboxyl function
by 2.5 pH
units.
This shows electrochemical transduction,
the
conversion
of
electrical energy into chemical
energy.
In
particular,
the
reduction reaction
is
carried
out
near
pH 9,
where
the
reduced
NMeN
is
most stable.
Under these circumstances, when
the
NMeN
is
in the
oxidized state
and the
aspartic acid
residue
is
charged, that is, -CH2-COO~, reduc-
tion
of
NMeN^ near
pH 9
causes
the
carboxy-
late function
to
pick
up a
proton
and
become
the uncharged carboxyl function. Above
we
have referred
to the
performance
of
mechani-
cal work
as
"pumping iron." The action
of
this
protein-based machine could
be
referred
as
"pumping protons"
or
"pumping acid"
as
occurs
in the
stomach
and for
which
we
take
Zantac®
or
PepcidAC® when
our
proton
pumps
are too
active.
Of
course,
the
major
place
in
biology where electro-chemical trans-
duction occurs
is in the
energy-converting
mitochondria,
and the
players
are
similar.
A
80-
60-
40-
20-
/
Reduced
/
State
ll
/| pKa=
11
Jl
1
1
11
10
Oxidized
/
State
/
/ IpKa
1
9
= 8.5
1
8
B
100
0
1
^^
g
40
0
pH
FIGURE
5.20. Experimental results that demonstrate
the same form
of
hydrophobicity dependence
as
shown
in
Figure
5.19. (A)
Conversion
of
electrical
into chemical energy when
the
model protein con-
tains both redox couple
and
protonatable chemical
couple. (Adapted with permission from Urry.'*)
(B)
Hydrophobicity dependence
for the
conversion
of
chemical energy into mechanical work when
the
model protein contains
a
protonatable function
10-1-2
E/29.5
(mV vs. SCE)
within
a
series
of
increasingly hydrophobic model
proteins.
(C)
Hydrophobicity dependence
for the
conversion
of
electrical energy into mechanical work
when
the
model protein contains
a
function capable
of reduction
and
oxidation within
a
series
of in-
creasingly hydrophobic model proteins. Adapted
with permission from Hayes.^°^) Regardless
of
energy conversion,
the
qualitative dependence
on
hydrophobicity
is the
same.

166
5.
Consilient Mechanisms for Diverse Protein-based Machines
nicotinamide redox couples and carboxyl/
carboxylate chemical couples (see Chapter 8).
On reduction of the nicotinamide, the transi-
tion zone in Figure 5.20A for the carboxyl/car-
boxylate chemical couple shifts to lower acid
concentrations and becomes narrower, just as is
demonstrated by data for the series of model
proteins in Figure 5.20B (and schematically
represented in Figure 5.19B) on increasing the
oil-like character by replacing V by
F.
The series
of model proteins for Figure 5.20B is the set of
Model Proteins i, ii, Hi, iv, and v of Table 5.5.
Clearly, the increase in oil-like character due to
systematic replacement of V by F for Model
Proteins i through v shifts the pKa, the pH at
50%
protonation, to higher pH
values.
The pKa
defines the center of the transition zone. This
unambiguous stepwise increase in hydrophobic-
ity stepwise lowers proton concentrations and
narrows the width of the transition zone, that is,
it decreases the chemical energy required to
drive the hydrophobic association. Figure 5.20B
shows the experimental data on which the
schematic representation in Figure 5.19B is
based. The important point to note is that the
reduction of nicotinamide has exactly the same
effect on the carboxyl/carboxylate chemical
couple as increasing the oil-Uke character by
replacing less oil-like V by more oil-Uke F
In an exactly analogous manner, equivalent
behavior is seen with the series of Model
Proteins ii', iii', and iv' of Table 5.5 containing
the nicotinamide functional group attached to
a lysine (Lys, K) residue by an amide linkage
(See Figure 5.20C).The systematic replacement
of V by F shifts the reduction potential of the
nicotinamide, that is, shifts the transition zone
to lower electron concentrations, and results in
a narrower transition zone. Figure 5.20C repre-
sents an explicit experimental example of the
schematic representation in Figure 5.19C.
In conclusion, coupling of different func-
tional groups occurs when both functional
groups affect and are affected by the oil-like
domain of which they are a part. When one
group brings about a change in the oil-like char-
acter of a common protein domain, that change
affects the second functional group. This cou-
pling of functions through the oil-like character
of the protein-based machine occurs by means
of the mutual dependence of the Gibbs free
energy of hydrophobic association,
AGHA,
on
the state of the functional groups.
5.5.3.2 Change in the Hydrophobicity
(Extent of Oil-like Character) Due to
Action on One Functional Group, for
Example, Absorption of Light by the
Cinnamide Chromophore, Moves the
Transition Zone for a Second Functional
Group, for Example, the
Carboxyl/Carboxylate Chemical Couple:
Photo ^ Chemical Transduction
In this demonstration of photo-mechanical and
photo-chemical transduction, cinnamic acid,
C6H5-CH=CH-COOH, was attached by amide
linkage to the side chain of a lysine (Lys, K)
residue, abbreviated as K{CnAm}. The studies
utilized the designed Model Proteins vi', vii',
viii' and ix' of Table 5.5. Light of
300
nm drove
the trans geometrical isomer of cinnamide into
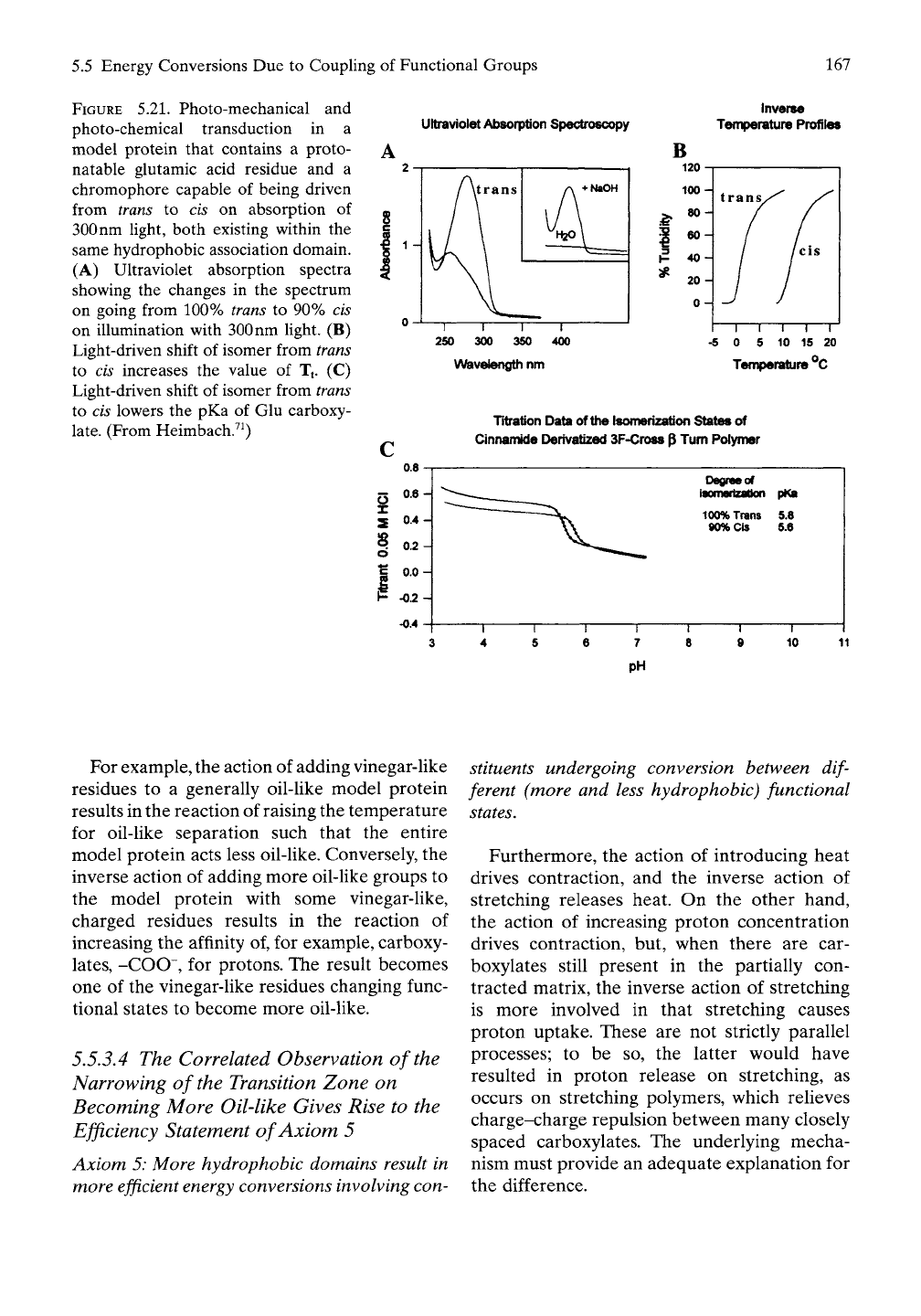
the cis isomer and raised the value of Tt by
about 10° C, which effects a small increase in
AGHA, that is, a shift to the hydrophobically
dissociated state (see Figure 5.21
A).
When the
acid-base titrations were carried out a small
decrease of a few tenths of a pH unit was
reported as shown in Figure 5.21C.^^ The pH
shift is in line with what is expected from the
small ATt in Figure 5.21B with the resulting
small increase in
AGHA-
5.5.3.3 The Statement for Energy
Conversion by the Coupling of Functions
with the Consilient Mechanism Becomes
Axiom 4
Axiom 4: Two or more different functional
groups of a m,odel protein, each of which can be
acted upon by a different energy input that
changes the temperature interval for oil-like
folding and assembly, become coupled one to
the other by being part of the same oil-like
folding and assembly domain, that
is,
the energy
input acting on one functional constituent alters
the oil-like character of the model protein and
by doing so acts as an energy output on the other
functional constituent through its dependency
on the oil-like character of the model protein.
5.5 Energy Conversions Due to Coupling of Functional Groups 167
FIGURE 5.21. Photo-mechanical and
photo-chemical transduction in a
model protein that contains a proto-
natable glutamic acid residue and a
chromophore capable of being driven
from trans to cis on absorption of
300 nm light, both existing within the
same hydrophobic association domain.
(A) Ultraviolet absorption spectra
showing the changes in the spectrum
on going from 100% trans to 90% cis
on illumination with 300 nm light. (B)
Light-driven shift of isomer from trans
to cis increases the value of Tf (C)
Light-driven shift of isomer from trans
to cis lowers the pKa of Glu carboxy-
late.
(From Heimbach.^^)
Ultraviolet Absorption Spectroscopy
Inverse
Temperature Profiles
1 -
/ \trans
1 1
/\ +NaOH
1
250 300 350 400
Wavelength nm
1 I r
10 15 20
Temperature C
0.8
Titration Data of
the
Isomerization States of
Cinnamide Derivatized 3F-Cross P Turn Polymer
Degree of
laomerizatton pKa
100%
Trans
90%
Cis
5.8
5.6
"1—
10
pH
For example, the action of adding vinegar-like
residues to a generally oil-like model protein
results in the reaction of raising the temperature
for oil-like separation such that the entire
model protein acts less oil-like. Conversely, the
inverse action of adding more oil-like groups to
the model protein with some vinegar-like,
charged residues results in the reaction of
increasing the affinity of, for example, carboxy-
lates,
-COO", for protons. The result becomes
one of the vinegar-like residues changing func-
tional states to become more oil-like.
5.5,3.4 The Correlated Observation of the
Narrowing of the Transition Zone on
Becoming More Oil-like Gives Rise to the
Efficiency Statement of Axiom 5
Axiom 5: More hydrophobic domains result in
more efficient energy conversions involving con-
stituents undergoing conversion between
dif-
ferent (more and less hydrophobic) functional
states.
Furthermore, the action of introducing heat
drives contraction, and the inverse action of
stretching releases heat. On the other hand,
the action of increasing proton concentration
drives contraction, but, w^hen there are car-
boxylates still present in the partially con-
tracted matrix, the inverse action of stretching
is more involved in that stretching causes
proton uptake. These are not strictly parallel
processes; to be so, the latter would have
resulted in proton release on stretching, as
occurs on stretching polymers, which relieves
charge-charge repulsion between many closely
spaced carboxylates. The underlying mecha-
nism must provide an adequate explanation for
the difference.

168
5.
Consilient Mechanisms for Diverse Protein-based Machines
5.5.4 Coupling of Reactions:
A Key Step Toward More
Complex Biological
Interactions
With the above sense of action and reaction,
vinegar-like groups become coupled in model
proteins that are sufficiently oil-like. For
example, if a sufficiently oil-like model protein
contains two different changeable (ionizable
and reducible) R-groups, then the conversion of
one to a more oil-like group by eUminating its
charge causes the second vinegar-like R-group
to adjust its property such that it too can
become more
oil-like.
This is quite analogous to
the principle of Le Chatelier; the stress caused
by the increase in oil-like character on reduc-
tion of a positively charged group becomes
relieved by the protonation of a negatively
charged carboxylate, as discussed above in
section
5.5.3.1.
5.6 Systematic Classification of
Energy Conversions by
Consilient Protein-based
Machines
The change in Gibbs free energy of hydropho-
bic association,
AGHA,
affects and is affected by
virtually every energy accessible to, and con-
vertible by, living organisms. It is at the
foundation of the consilient mechanism; it is
the result of inverse temperature transi-
tions,
and it is a representation of the compre-
hensive hydrophobic effect. In general, it would
seem to provide for the most efficient mecha-
nism whereby protein-based machines can
perform various energy conversions in aqueous
systems.
This section categorizes consiUent protein-
based machines. In preparation for doing so,
however, we seek out appropriate definitions
with which to deUneate the several different
kinds of machines possible by means of inverse
temperature transitions.
5.6.1 Clarification of the Terminology
for Energy Converting Machines
5.6.1.1 Machine Is the More General Term
for a Device That Performs Energy
Conversion
Based on Webster's Third New International
Dictionary, a machine is "an assemblage of
parts that transmit forces, motion, and energy
one to another in some predetermined manner
and to some desired end." Also drawing on the
definition in the McGraw-Hill Concise Ency-
clopedia of Science and Technology, 2nd
Edition, 1989, a machine is "a combination of
rigid or resistant bodies having definite motions
and capable of performing useful work." The
protein-based machines of focus here are
elastic bodies capable of contraction/relaxation
by the motions arising out of hydrophobic asso-
ciation/dissociation.
5.6.1.2 Protein-based Machines Convert
Mumerous Sources of Energy from One
Form or Location to Another
The above definitions of machines do not Umit
the kind of useful work that is the output of the
machine, nor do they limit the kind of energies
that the machine interconverts, that is, that it
changes one into another. In particular, protein-
based machines are amphiphilic polymers, con-
taining oil-like and vinegar-like substituents
arranged along the chain molecule, that are
capable of converting many different sources of
energy from one form or location to another.
The consideration of relocating an energy
source from one site to another is to include, for
example, the relocation of the chemical energy
represented by the oxygen molecule from the
lungs to the tissues.
5.6.1.3 Engines and Motors Are Machines
That Produce Motion
According to the McGraw-Hill Concise Ency-
clopedia of Science and Technology, Second
Edition, 1989, an engine is "a machine designed
for the conversion of energy into useful mechan-
ical motion." According to Webster's Third New
International Dictionary, an engine is "a

5.6 Systematic Classification of Energy Conversions by Consilient Protein-based Machines
169
machine for converting energy (in such forms as
heat, chemical energy, nuclear energy, radiation
energy, and the potential energy of elevated
water) into mechanical force and motion."
On the other hand, a motor, as defined by
Webster's, is "one that imparts motion: a source
of mechanical power." In addition, McGraw-
Hill Concise Encyclopedia of Science and Tech-
nology, Second Edition, gives a somewhat more
restricting definition of a motor as "an electric
rotating machine that converts electric energy
into mechanical energy." The term mechanical
energy includes both the kinetic energy of
producing motion whereby an applied mechani-
cal force, f, moves an object through a distance,
AL,
and the potential energy in terms of the
development of mechanical force at fixed
length, LAf. Furthermore, there may be recog-
nized both linear and rotary motors, and these
may be driven by energies other than electrical.
5.6.1.4 Protein-based Engines and
Motors Convert Numerous Sources of
Energy into the Performance of Useful
Mechanical Energy
Consistent with use in biology, we will not
dif-
ferentiate between engines and motors and will
use the terms interchangeably to refer to
machines that produce mechanical energy. To
be more specific, designed protein-based
engines and motors convert a number of ener-
gies,
namely, thermal, pressure-volume, chemi-
cal,
electrical, and electromagnetic, into motion.
Also,
as certain protein-based machines exhibit
nearly ideal elastic behavior, the resulting
elastic-contractile protein-based motors can be
reversible. These elastic protein-based
machines can be stretched, allowing that this
mechanical energy input can, with the proper
model protein composition, result in outputs of
thermal, pressure-volume, chemical, electrical,
and lower frequency electromagnetic radiation
energies. The one clear exception to simple
reversibility is photo-mechanical transduction
because the hght energy input is on the order
of 70kcal/mole, whereas the changes in Gibbs
free energy of hydrophobic association would
generally be no larger than ±10kcal/mole. This
means that in a single step of stretching there
would not develop sufficient energy for the
emission of visible or ultraviolet light, although
built-up chromophores could conceivably exist
within associated hydrophobic domains that
would emit light on stretch-effected exposure
of their hydrophobic domain to more polar
water.
5.6.2 Summary Hexagon of Energy
Conversions by the Consilient
Mechanism of
AGHA
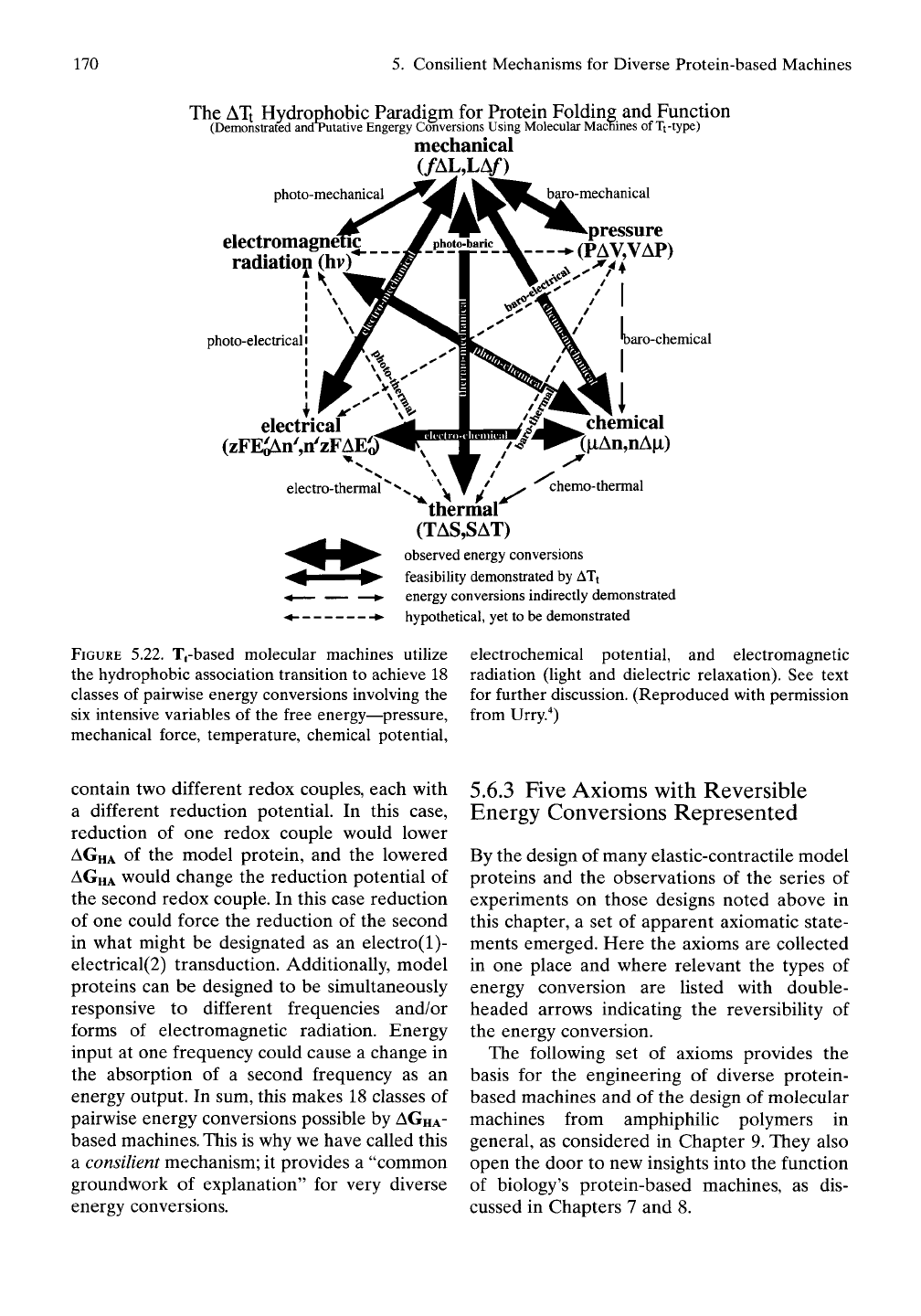
Figure 5.22 summarizes the set of different
forms of energy that can be interconverted by
the consilient mechanism of changes in the
Gibbs free energy of hydrophobic association,
AGHA- AS
evident in Equation (5.9), the quan-
tity, ATt, is central to the definition of
AGHA-
Because of this, we have referred to this emerg-
ing understanding as the ATt hydrophobic par-
adigm for protein folding and function.
Macromolecules that exhibit the phenomenon
of hydrophobic association on raising the tem-
perature, that
is,
the property of an inverse tem-
perature transition of increase in polymer order
on increasing the temperature, function as mol-
ecular machines by the consilient mechanism.
Accordingly, we speak of molecular machines
of the Tt-type.
Figure 5.22 represents the pairwise intercon-
version of six different energies. This gives rise
to 15 classes of pairwise energy conversions.
Furthermore, because the chemical couples of
phosphorylated/dephosphorylated and car-
boxyl/carboxylate each independently change
the free energy for hydrophobic association,
they can participate in a chemo-chemical trans-
duction. Consider, for example, a model protein
capable of hydrophobic association/dissocia-
tion that contained both a carboxyl/carboxylate
chemical couple and a phosphorylation site. In
this case the model protein could be designed
such that dephosphorylation would cause pro-
tonation of the carboxylate. This would be
proton pumping by means of phosphoryla-
tion/dephosphorylation. Alternatively, protona-
tion of carboxylates could raise the free energy
of (i.e., activate) a bound phosphate.
Similarly, the model protein capable of
hydrophobic association/dissociation could
170
5.
Consilient Mechanisms for Diverse Protein-based Machines
The ATt Hydrophobic Paradigm for Protein Folding and Function
(Demonstrated and Putative Engergy Conversions Using Molecular Machines of Tt-type)
mechanical
(/AL,LA/) ^
baro-mechanical
photo-mechanical
electromagnetic_
radiation
(hv)^*
pressure
— (PAV,VAP)
photo-electrical
elecfical
(zFE^n',n'zFAE'J
electro-thermal
>aro-chemical
chemical
"dixAn^nAiLi)
^ ^^
^ \ • y ^ chemo-thermal
thermal
(TAS,SAT)
observed energy conversions
feasibility demonstrated
by ATt
—• energy conversions indirectly demonstrated
-• hypothetical, yet
to be
demonstrated
FIGURE
5.22. Tj-based molecular machines utilize
the hydrophobic association transition to achieve 18
classes of pairwise energy conversions involving the
six intensive variables of the free energy—pressure,
mechanical force, temperature, chemical potential.
electrochemical potential, and electromagnetic
radiation (light and dielectric relaxation). See text
for further discussion. (Reproduced with permission
from Urry."^)
contain tw^o different redox couples, each with
a different reduction potential. In this case,
reduction of one redox couple would lower
AGHA
of the model protein, and the lowered
AGHA
would change the reduction potential of
the second redox couple. In this case reduction
of one could force the reduction of the second
in what might be designated as an electro(l)-
electrical(2) transduction. Additionally, model
proteins can be designed to be simultaneously
responsive to different frequencies and/or
forms of electromagnetic radiation. Energy
input at one frequency could cause a change in
the absorption of a second frequency as an
energy output. In sum, this makes 18 classes of
pairwise energy conversions possible by
AGHA-
based machines. This is why we have called this
a consilient mechanism; it provides a "common
groundwork of explanation" for very diverse
energy conversions.
5.6.3 Five Axioms with Reversible
Energy Conversions Represented
By the design of many elastic-contractile model
proteins and the observations of the series of
experiments on those designs noted above in
this chapter, a set of apparent axiomatic state-
ments emerged. Here the axioms are collected
in one place and where relevant the types of
energy conversion are listed with double-
headed arrows indicating the reversibility of
the energy conversion.
The following set of axioms provides the
basis for the engineering of diverse protein-
based machines and of the design of molecular
machines from amphiphilic polymers in
general, as considered in Chapter 9. They also
open the door to new insights into the function
of biology's protein-based machines, as dis-
cussed in Chapters 7 and 8.

5.6 Systematic Classification of Energy Conversions by Consilient Protein-based Machines
171
Axiom 1: The change in temperature interval^
over which occurs the oil-like folding and
assembly transition of a host model protein on
introduction of different guest substituents,
becomes
a
functional measure of relative oil-like
character of the substituents, that
is,
of their rel-
ative hydrophobicity, and it provides a measure
of the change in free energy of the resulting
hydrophobically associated state.
This axiom provides the database and insight
for the design and function of protein-based
molecular machines.
Axiom 2: Heating to raise the temperature
from below to above the temperature interval for
hydrophobic association of cross-linked elastic
model protein chains drives contraction with the
performance of mechanical work.
This is the thermally driven contraction
inherent in inverse temperature transitions.
Example: Thermo -^ mechanical transduction.
Axiom 3: At constant temperature, an energy
input that changes the temperature interval for
thermally driven hydrophobic association in a
model protein can drive contraction, that is,
oil-like folding and assembly, with the perfor-
mance of mechanical work; in other words, the
energy input moves the system through the tran-
sition zone for contraction due to hydrophobic
association.
This requires macromolecules that can
achieve equilibrium as the functional states of
constituents fractionally vary; it provides a uni-
fying basis not only for different means of pro-
ducing motion of living organisms, but for all
types of biological energy conversions. The
double-headed arrows indicate the reversibil-
ity of the energy conversion in the following
examples:
Chemo -^ mechanical transduction
Electro
*-^
mechanical transduction
Baro ^ mechanical transduction
Photo -> mechanical transduction
Axiom 4: Two or more different functional
groups of an amphiphilic macromolecule, each
of which can be acted upon by a different energy
input that changes the temperature interval for
oil-like folding and assembly, become coupled
one to the other by being part of the same oil-
like folding and assembly domain, that is, the
energy input acting on one functional con-
stituent by altering the oil-like character acts as
an energy output on the other functional con-
stituent by the latter's dependency on oil-like
character.
This axiom is the fundamental energy cou-
pling axiom; it utilizes the hydrophobic asso-
ciation transition common to all energy
conversions by the consilient mechanism.
Importantly, in doing so, it involves the perfor-
mance of kinds of work in addition to mechan-
ical work. Examples include the following:
Electro
*-^
chemical transduction
Electro -^ thermal transduction
Baro ^ electrical transduction
Photo -^ voltaic transduction
Thermo ^-^ chemical transduction
Photo -^ thermal transduction
Baro <^ thermal transduction
Baro ^ chemical transduction
Photo -^ baric transduction
Photo -^ chemical transduction
Chemo -^ chemical transduction
Electro ^ electrical transduction
Electromagnetic radiation(l) ->
netic radiation(2) transduction
electromag-
Axiom 5: More hydrophobic domains make
more efficient the energy conversions involving
polar constituents undergoing conversion
between more and less hydrophobic functional
states.
This is the efficiency axiom (poising or
biasing).
5.6.4 Protein-(Tt)-based Molecular
Machines of the First Kind (Molecular
Engines and Motors for the
Performance of Mechanical Work)
Protein-based molecular machines of the first
kind directly use the change in free energy of
hydrophobic association,
AGHA,
as a contrac-
tion for the performance of mechanical work.

172
5.
Consilient Mechanisms for Diverse Protein-based Machines
As shown in the hexagonal array in Figure 5.22,
five different energy inputs can perform
mechanical work by the consilient mechanism.
The set of elastic-contractile model proteins
capable of direct utilization of hydrophobic
association for contraction are called protein-
based molecular machines of the first
kind.
These are enumerated below with brief con-
sideration of the reversibiUty of these machines.
5.6.4,1 Thermo
Transduction
Mechanical
5.6.4.1.1 Thermo
—>
Mechanical Transduction
(Heat-Driven Contraction)
The input of thermal energy over the tempera-
ture interval for hydrophobic association (see
Figure 5.5) drives contraction, as represented in
Figures 5.18B and 5.19A.^^'^^ This was first
demonstrated with elastic protein-based poly-
mers dissolved in water in which the tempera-
ture ranges for heat-induced aggregation of the
polymers (GVGVP)^, (GVGIP)n, and (GGVP)n
correlated with the temperature range for con-
traction of the cross-linked matrices.
5.6.4.1.2 Mechano ^ Thermal Transduction
(Stretch-Induced Release of Heat)
As is expected for a reversible process, the
reverse process of stretching, applying a
mechanical force to extend the elastic protein
band, is an exothermic process, primarily due
to hydration of oil-like groups that become
exposed on stretching.^^'^^ As emphasized pre-
viously, Butler^^ in 1937 was the first to appre-
ciate that hydration of oil-like groups was
exothermic.
5.6.4.2 Chemo
Transduction
Mechanical
5.6.4.2.1 Chemo -^ Mechanical Transduction
(Proton-Driven and Salt-Driven Contraction)
Chemo -^ mechanical transduction can be
divided into two types: one (polymer based)
utilizes a functional group of the polymer, and
the other (solvent based) involves changing the
activity of water or directly altering the
hydrophobic hydration. The most apparent
examples of polymer-based chemo
—>
mechan-
ical transduction involve changing the degree
of ionization of a side chain by protonation/
deprotonation^"^ or the extent of ion pairing to
the charged functional group. Examples of
solvent-based chemo -> mechanical transduc-
tion are best given with a polymer having no
functional side chains, such as is the case with
poly(GVGVP) and poly(GVGIP). The data in
Figure 5.11 provide many examples of changes
in solvent that change the value of Tt, and
thereby those chemical energy sources can be
used to drive contraction. Changes in the chem-
ical energy (concentration) of NaCl has been
explicitly demonstrated to drive contraction
and relaxation.^^
5.6.4.2.2 Mechano -^ Chemical Transduction
(Stretch-Induced Proton Uptake)
The conversion of the mechanical energy of
stretching into chemical energy recognized as a
change in the concentration of a chemical
species, mechano -^ chemical transduction, has
been explicitly demonstrated by means of
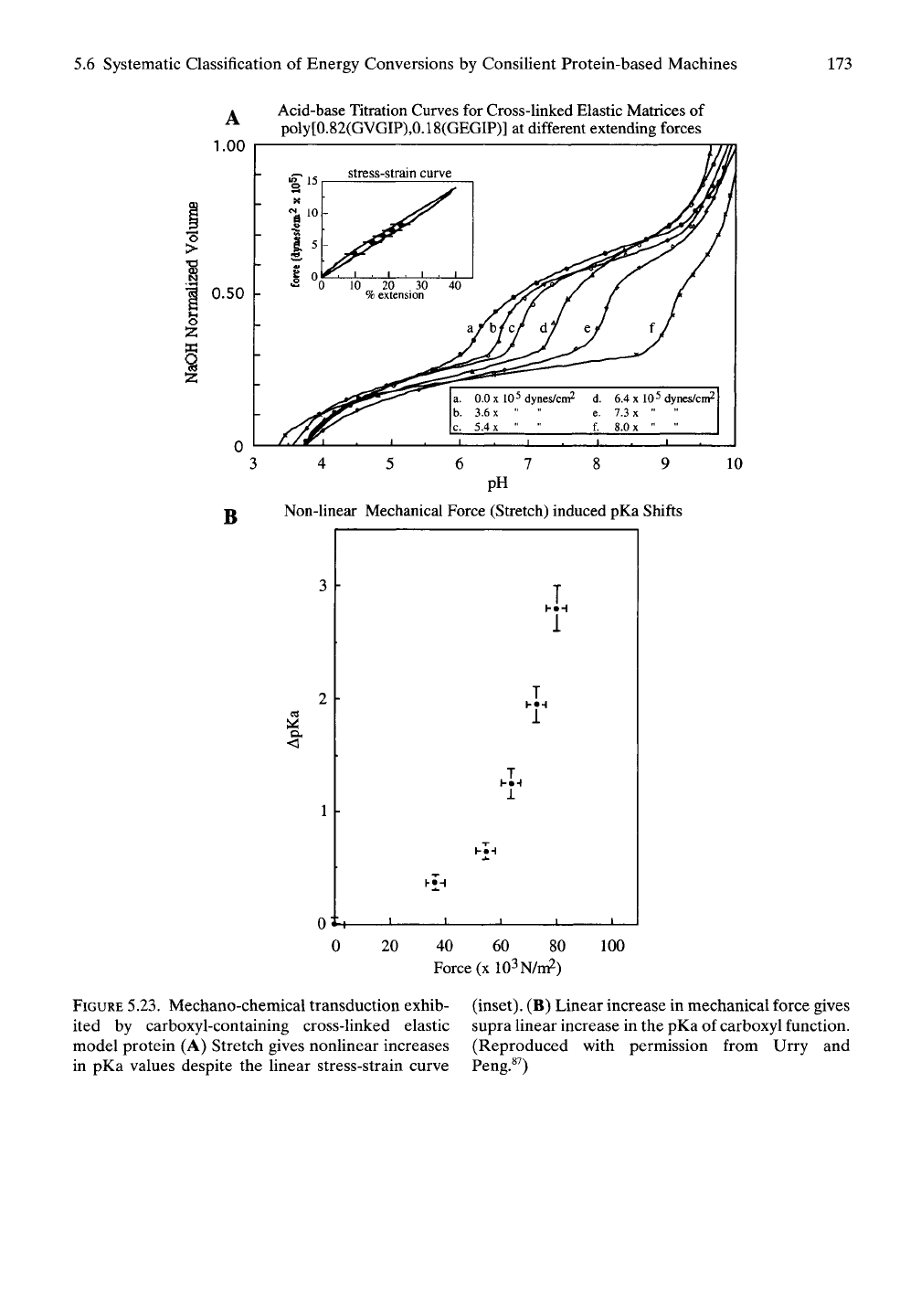
stretch-induced pKa shifts (see Figure 5.23)
exhibited by the carboxyl function of a glutamic
acid residue. As shown in Figure 5.23, stretch-
ing increases the affinity of the carboxyl of glu-
tamic acid for its proton. In this case stretching
induces proton uptake, and it could similarly
increase affinity for ion pairing and effect for-
mation of an ion pair.^^'^^
In the absence of a functional group,
however, stretching can be used to effect a
change in the concentration of a chemical in
the surrounding solution. In particular,
these protein-based machines functioning as
mechano -> chemical transducers could be
used in desalination. On stretching there occurs
an exothermic hydration of hydrophobic
groups, that is, an uptake of pure water and for-
mation of an environment where ions would be
at high energy. Relaxation after removal from
the overlying saline solution results in release
of partially desaUnated water.
5.6 Systematic Classification of Energy Conversions by Consilient Protein-based Machines 173
A
1.00
Acid-base Titration Curves for Cross-linked Elastic Matrices of
poly[0.82(GVGIP),0.18(GEGIP)] at different extending forces
o
>
o
9
0.50
B
Stress-Strain curve
•^ 0 10 20 30 40
% extension
a. 0.0
X
10 5 dynes/cm2 d. 6.4 x 10^ dynes/cm^
b.
3.6
X
" " e.
7.3
x
"
c. 5.4 x " " f. 8.0 X " "
4 5 6 7 8 9
pH
Non-linear Mechanical Force (Stretch) induced pKa Shifts
10
<
I
h
4H
^ 1—
T
l-»H
1
T
i
T
1
1 1 1
20 40 60 80
Force (xlO^N/n^)
100
FIGURE
5.23. Mechano-chemical transduction exhib-
ited by carboxyl-containing cross-linked elastic
model protein (A) Stretch gives nonlinear increases
in pKa values despite the linear stress-strain curve
(inset).
(B) Linear increase in mechanical force gives
supra linear increase in the pKa of carboxyl function.
(Reproduced with permission from Urry and
Peng.«^)

174
5.
Consilient Mechanisms for Diverse Protein-based Machines
5.6.4.3 Electro
Transduction
Mechanical
5.6.4.3.1 Electro -^ Mechanical Transduction
(Reduction-Driven Contraction)
Synthesis of poly[0.73(GVGVP),0.27(GK
{NMeNjGVF)] allowed determination of the
dependence of the temperature interval for
heat-induced hydrophobic association on
reduction of the redox couple N-methyl nicoti-
namide (NMeN), which resulted in inclusion in
the Tfbased hydrophobicity scale with the Tf
values given in Table 5.2.^^ On cross-linking to
form the elastic matrix, reduction was found to
drive contraction, and oxidation resulted in
relaxation.^^
5.6.4.3.2 Mechano -^ Electrochemical
(Electrical) Transduction (Stretch-Induced
Electron Uptake)
This experiment has yet to be done, but the
expectation is clear and substantiated experi-
mentally by hydrophobic-induced shifts in
reduction potential as shown in Figure 5.20C.
Stretching causes exposure of hydrophobic
groups that would result in predictable stretch-
induced changes in reduction potential.
5.6.4.4 Baro
<-^
Mechanical
Transduction
5.6.4.4.1 Baro -^ Mechanical Transduction
(Pressure Release Drives Contraction)
Using the series of protein-based polymers
poly[fx(GXGVP),fv(GVGVP)], where X may
be tryptophan (Trp,W), phenylalanine (Phe,F),
tyrosine (Tyr, Y), methionine (Met, M), or
valine (Val, V) and where fx values ranged from
0.14 to 1, the application of pressure was found
to increase the value of Tt. The application of
pressure leads to hydrophobic dissociation with
an increase in the formation of hydrophobic
hydration. Plots of ATt versus log(P), with the
pressure, P, ranging from 1.29 to 115 atmos-
pheres, gave linear slopes from which decreases
in volume due to appUcation of pressure could
be calculated.^"^ Cross-linked elastic matrices,
suspending a weight, relax and lower the weight
on increasing pressure and contract to lift the
weight on release of pressure.^^
5.6.4.4.2 Mechano -^ Baric Transduction
Stretching is expected to decrease volume
without change in composition. This would be
due to the increase in water of hydrophobic
hydration, which yields a state of lesser volume.
Under conditions of fixed volume, stretching is
expected to yield a decrease in pressure. With
the perspective that the inverse temperature
transition occurs when AH, = T,ASt, an adequate
explanation of the pressure effect requires a
separate determination of the pressure depen-
dence of AH.
5.6.4.5 Electromagneto -«-• Mechanical
Transduction
In our studies and characterization of the
elastic protein-based polymers, they have been
found and/or designed to be responsive to
energy inputs over a very wide frequency range
of the electromagnetic spectrum from absorp-
tion of energy in the ultraviolet range of greater
than
10^^
Hz to the lowest dielectric relaxation
for which a prominent absorption maximum
has been found near
10^
Hz. Additional impor-
tant dielectric relaxations occur about
5
MHz
(radio frequency region)^^'^^ and 5 GHz
(microwave frequency region).^^ Thus, input of
energy into the polymer at specific frequencies
over this electromagnetic spectrum could be
expected to drive contraction/relaxation.
Accordingly, energy inputs over the electro-
magnetic frequency range from the high-energy
side of greater than
10^^
Hz to the low-energy
side of
10^
Hz should be able to effect what
might, for want of a better term, be called elec-
tromagneto-mechanical transduction.
The absorption in the microwave region at
5 GHz, as will be discussed below, is due to
hydrophobic hydration. Putting in energy of
this frequency ought to be the most effective
way to heat the elastic protein-based polymer
and drive contraction. Another intense absorp-
tion occurs near
5
MHz that is due to the inter-
nal chain motions of the protein backbone. It is
the damping of these motions on stretching that
is the source of the entropic component of the
