Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.

5.4 Visual Observation of Elastic Protein-based Machines at Work
155
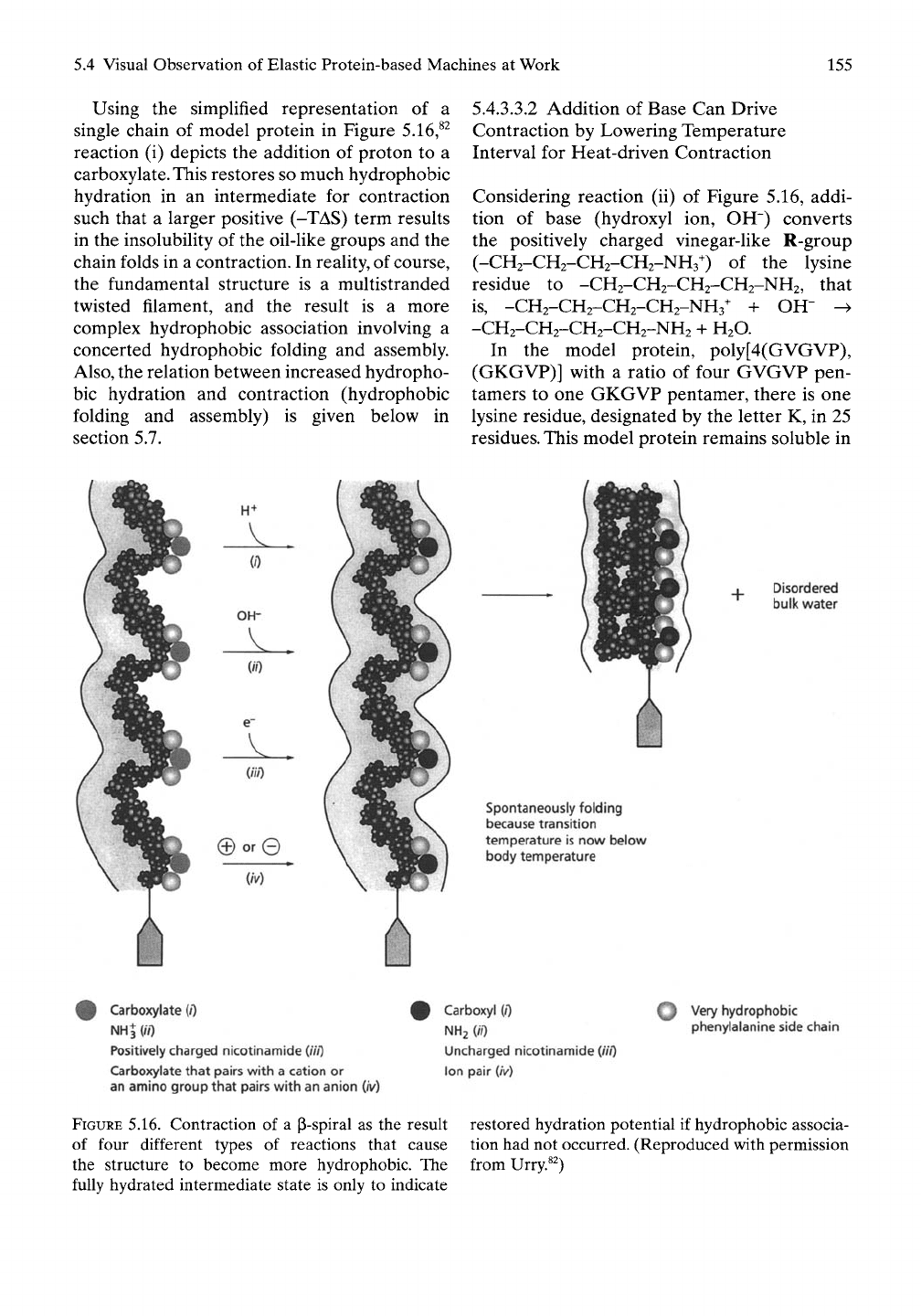
Using the simplified representation of a
single chain of model protein in Figure 5.16,^^
reaction (i) depicts the addition of proton to a
carboxylate.This restores so much hydrophobic
hydration in an intermediate for contraction
such that a larger positive (-TAS) term results
in the insolubility of the oil-like groups and the
chain folds in a contraction. In reality, of course,
the fundamental structure is a multistranded
twisted filament, and the result is a more
complex hydrophobic association involving a
concerted hydrophobic folding and assembly.
Also,
the relation between increased hydropho-
bic hydration and contraction (hydrophobic
folding and assembly) is given below in
section 5.7.
5.4.3.3.2 Addition of Base Can Drive
Contraction by Lowering Temperature
Interval for Heat-driven Contraction
Considering reaction (ii) of Figure 5.16, addi-
tion of base (hydroxyl ion, OH") converts
the positively charged vinegar-like R-group
(-CH2-CH2-CH2-CH2-NH3^) of the lysine
residue to -CH2-CH2-CH2-CH2-NH2, that
is,
-CH2-CH2-CH2-CH2-NH3^ + OH" ->
-CH2-CH2-CH2-CH2-NH2 + H2O.
In the model protein, poly[4(GVGVP),
(GKGVP)] with a ratio of four GVGVP pen-
tamers to one GKGVP pentamer, there is one
lysine residue, designated by the letter K, in 25
residues. This model protein remains soluble in
A^
(/)
OH-
(ii)
^
(///)
©or0
(/V)
Disordered
bulk water
Spontaneously folding
because transition
temperature is now below
body temperature
Carboxylate (/)
NH|(//)
Positively charged nicotinamide (///)
Carboxylate that pairs with a cation or
an amino group that pairs with an anion (/V)
Carboxyl (/)
NH2 (//)
Uncharged nicotinamide
{Hi)
Ion pair (/V)
Q Very hydrophobic
phenylalanine side chain
FIGURE 5.16. Contraction of a p-spiral as the result
of four different types of reactions that cause
the structure to become more hydrophobic. The
fully hydrated intermediate state is only to indicate
restored hydration potential if hydrophobic associa-
tion had not occurred. (Reproduced with permission
from Urry.^^)

156
5.
Consilient Mechanisms for Diverse Protein-based Machines
neutral or acidic water; it will not begin aggre-
gation in physiological saline until the temper-
ature is greater than 45° C, and the rubber-like
band formed by cross-linking this model protein
will not begin to contract unless the tempera-
ture is greater than 45° C. The temperature
interval begins just above 45° C. If hydroxyl
ion is added to the polymer solution sufficient
to convert all side chains to -CH2-CH2-
CH2-CH2-NH2, then aggregation begins at 28°
C and is complete by 45° C, that is, the temper-
ature interval is now from 28° to 45° C. Simi-
larly, for the cross-linked elastomer, contraction
occurs over the temperature interval from 28°
C to 45° C. Removal of the charged proton to
form the -CH2-CH2-CH2-CH2-NH2 results in
a more oil-like R-group so that the separation
of oil-like R-groups from water occurs at a
lower temperature. With this composition of
model protein, the chemical energy of increas-
ing hydroxyl ion concentration transforms into
the mechanical energy of pumping iron.
Again using the simplified representation of
a single chain of model protein in Figure 5.16,
reaction (ii) depicts the removal of a proton
from an ammonium, NHs^. Forming the
uncharged NH2 restores so much hydrophobic
hydration that a more positive (-TAS) term
causes AG(solubility) to become positive,
resulting in insolubility of the oil-like groups,
and the chain folds in a contraction due to
hydrophobic association.
5.4.3.3.3 Ion Pairing Drives Contraction
by Lowering Temperature Interval for
Heat-driven Contraction
Whether using a model protein with a negatively
charged functional group or one with a posi-
tively charged functional group, pairing with an
oppositely charged ion lowers the temperature
interval for heat-driven hydrophobic associa-
tion. Correspondingly, ion pairing in the cross-
linked elastic matrix moves the system through
the transition zone and drives contraction.
Once more using the simplified representa-
tion of a single chain of the model protein in
Figure 5.16, reaction (iv) depicts the addition
of a counter-ion to the oppositely charged
vinegar-like group. Partial neutralization of the
charged functional group provides yet another
means of restoring sufficient hydrophobic
hydration that the (-TAS) term becomes even
more positive. This results in AG(solubility)
becoming positive and the oil-like groups
becoming insolubile, and again the chain
hydrophobically folds in a contraction.
5.4.4 Electrical Energy Produces
Mechanical Work of Lifting Weight
(Increasing the Electrochemical
Potential Through the Transition Zone
to Reduce Oxidized Redox Groups
Drives Contraction)
5,4.4.1 Reduction (Adding Electrons)
Drives Contraction by Lowering
Temperature Interval for
Heat-driven Contraction
Reduction of the oxidized nicotinamide
(N-methyl nicotinamide, NMeN^) in poly[0.73
(GVGVP),0.27(GK{NMeN}GVP)] lowers the
temperature for the onset of the temperature
interval from 49° to 9° C, for a 60% reduction.^^
When forming the cross-Unked elastic matrix,
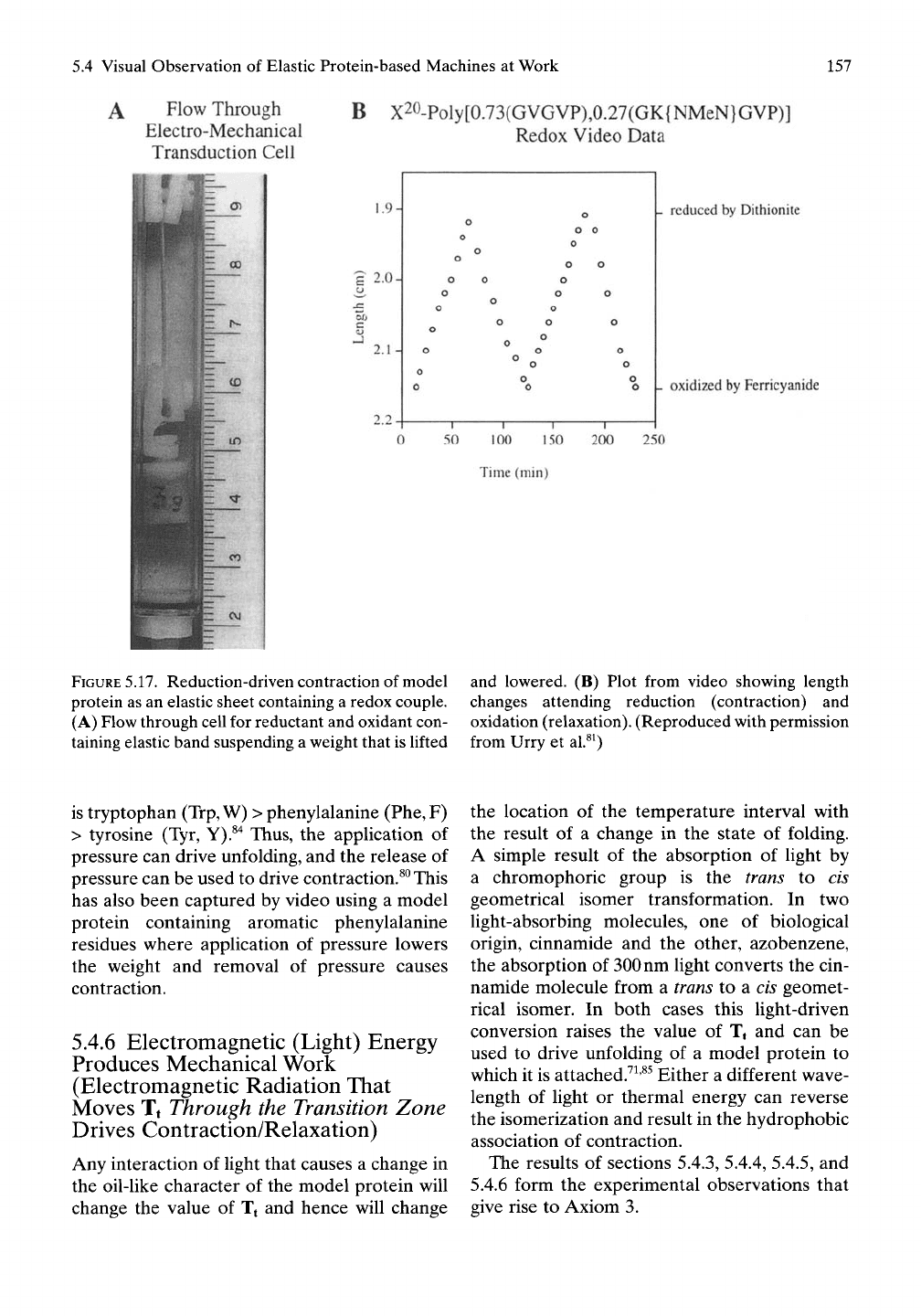
as shown in Figure 5.17 at room temperature
and as available in video, reduction drives con-
traction and oxidation drives relaxation.^^
Again with the simplified representation of a
single chain of model protein in Figure 5.16,
reaction (iii) depicts the addition of a negative
electron to a positively charged component
of a redox couple. Forming the reduced,
uncharged component of the redox couple,
again, restores so much hydrophobic hydration
that a positive (-TAS) term causes AG(solubil-
ity) to become positive, resulting in insolubility
of the oil-like groups, and the chain folds due
to hydrophobic association with the result of a
contraction.
5.4.5 Pressure-Volume Energy
Produces Mechanical Work (MW)
(Lowering Pressure Through the
Transition Zone Drives Contraction)
When aromatic groups are present, the appH-
cation of pressure increases the value of Tt.
Pressure increases the temperature range over
which the temperature interval occurs. The
order of responsiveness to increases in pressure
5.4 Visual Observation of Elastic Protein-based Machines at Work 157
A Flow Through B X20.poly[0.73(GVGVP),0.27(GK{NMeN}GVP)]
Electro-Mechanical Redox Video Data
Transduction Cell
L reduced
by
Dithionite
L oxidized
by
Ferricyanide
50 100
Time (min)
250
FIGURE
5.17.
Reduction-driven contraction of model
protein as an elastic sheet containing a redox couple.
(A) Flow through cell for reductant and oxidant con-
taining elastic band suspending a weight that is lifted
and lowered. (B) Plot from video showing length
changes attending reduction (contraction) and
oxidation (relaxation). (Reproduced with permission
from Urry et al.^^)
is tryptophan
(Trp,
W) > phenylalanine (Phe, F)
> tyrosine (Tyr,
Y).^"^
Thus, the apphcation of
pressure can drive unfolding, and the release of
pressure can be used to drive contraction.^^ This
has also been captured by video using a model
protein containing aromatic phenylalanine
residues where application of pressure lowers
the weight and removal of pressure causes
contraction.
5.4.6 Electromagnetic (Light) Energy
Produces Mechanical Work
(Electromagnetic Radiation That
Moves Tt Through the Transition Zone
Drives Contraction/Relaxation)
Any interaction of light that causes a change in
the oil-like character of the model protein will
change the value of Tt and hence will change
the location of the temperature interval with
the result of a change in the state of folding.
A simple result of the absorption of hght by
a chromophoric group is the trans to cis
geometrical isomer transformation. In two
light-absorbing molecules, one of biological
origin, cinnamide and the other, azobenzene,
the absorption of 300 nm light converts the cin-
namide molecule from a trans to a cis geomet-
rical isomer. In both cases this light-driven
conversion raises the value of Tt and can be
used to drive unfolding of a model protein to
which it is attached.^^'^^ Either a different wave-
length of light or thermal energy can reverse
the isomerization and result in the hydrophobic
association of contraction.
The results of sections 5.4.3, 5.4.4, 5.4.5, and
5.4.6 form the experimental observations that
give rise to Axiom 3.

158
5.
Consilient Mechanisms for Diverse Protein-based Machines
Axiom
3:
At constant
temperaturCy
an energy
input that changes the temperature interval for
thermally driven hydrophobic association in a
model protein can drive contraction^ that
is,
oil-
like folding and assembly, with the performance
of mechanical work; in other words, the energy
input moves the system through the transition
zone for contraction due to hydrophobic
association.
5.4.7 Summary of Consilient
Protein-based Machines (Engines and
Motors) That Pump Iron, That
Is,
That
Produce Motion
The term machine is a general designation for
any device that converts energy from one form
to another. The particular type of machine that
performs mechanical work or produces motion
is commonly called either a motor or an engine.
Here, we summarize the capacity of protein-
based machines to perform mechanical work
(pump iron) by the consilient mechanism of
hydrophobic association by utilizing a number
of different energy inputs.
5.4,7.1 Pumping Iron by Raising and
Lowering the Temperature Through
the Temperature Interval
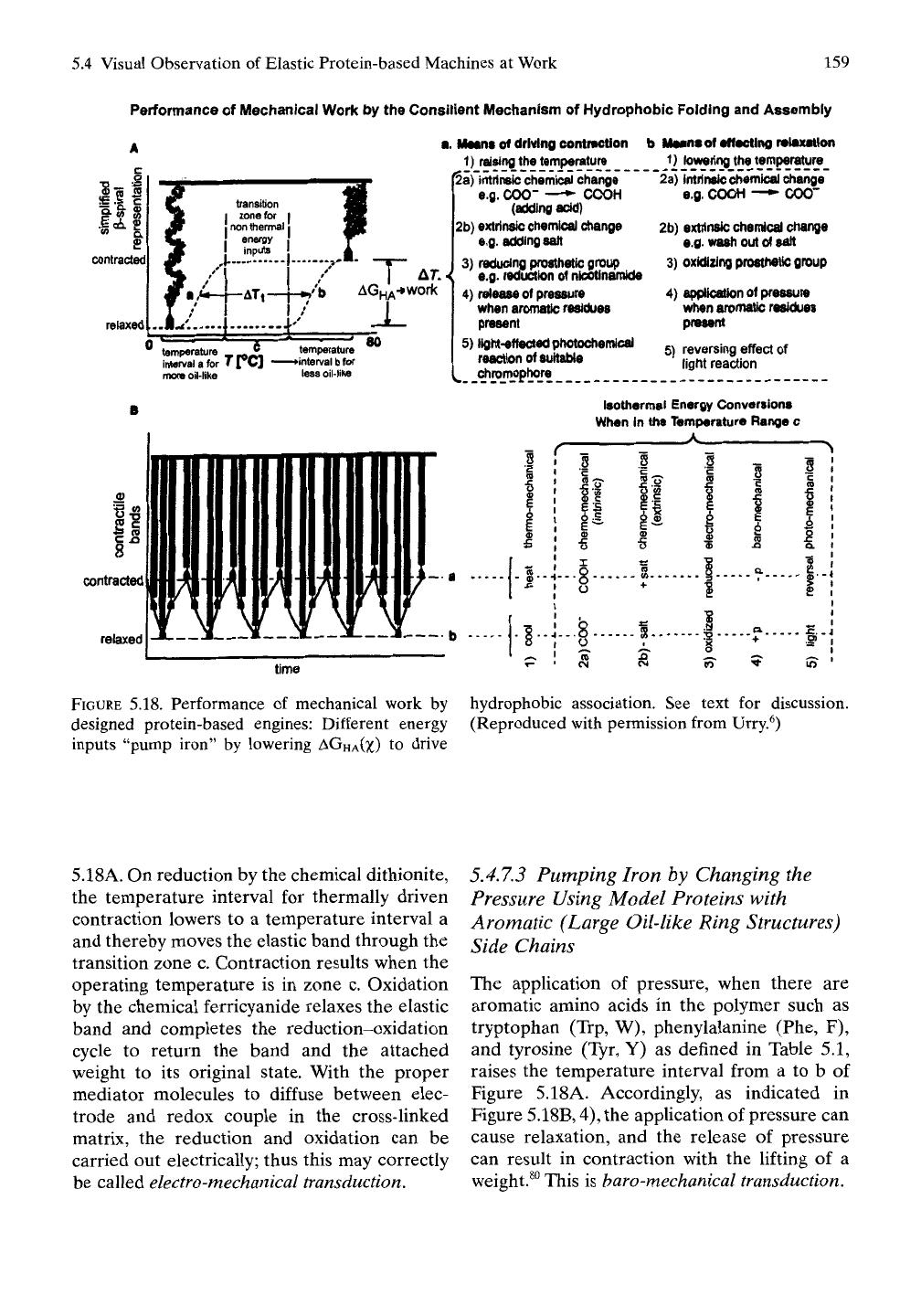
Figure 5.18A uses the representation of a single
p-spiral in the extended and contracted states.
The sigmoid curve at a represents the folding
of, and defines the temperature interval for, a
more oil-like model protein chain and that at
b represents the folding of, and defines the
temperature interval for, a less oil-Hke model
protein. Any energy input that makes more
oil-like the model protein with the temperature
interval at b lowers the temperature interval of
b toward that of a and defines the transition
zone c for that energy input. Figure 5.18B, 1),
represents the contraction of a band of elastic
model protein when heating through the tem-
perature interval for hydrophobic folding and
assembly of the model protein.
5.4.7.1.1 Pumping Iron by Protonation of a
Carboxylate (COO" -^ COOH) to Make the
Model Protein More Oil-like
Increasing the concentration of acid, H^, con-
stitutes a particular chemical energy input. The
addition of acid (H^), over the concentration
range that protonates the carboxylate, lowers
the temperature interval for the model protein,
drives contraction, and defines the transition
zone for this energy input indicated as c in
Figure 5.ISA. Figure 5.18B, 2a), represents the
contraction and relaxation by reversible proto-
nation and deprotonation of the carboxyl
functional group, i.e., chemo-mechanical
transduction.
5.4.7.1.2 Pumping Iron by Adding Salt to a
Neutral Model Protein or to a Model Protein
with a Charged Functional Group
Simply by the addition of NaCl, for example, to
a model protein with no ionized functional
groups can lower the temperature interval,
define a transition zone, and drive contraction.
The change in salt concentration required to
drive contraction of an uncharged model
protein can be 10 times more than if a charged
functional group is present. Driving contraction
when there is a charged group to which a
counter-ion from the salt can ion pair requires
a much smaller change in concentration of salt,
that is, a smaller chemical energy input. The
energy conversion achieved by ion pairing is
much more efficient. Both uses of salts are rep-
resented in Figure 5.18B, 2b).
5.4.7.2 Pumping Iron by Reduction of an
Oxidized Functional Group Attached to
the Model Protein
Figure 5.17 shows direct experimental results,
which give rise to the generalized representa-
tion in Figure 5.18,3). In the case of Figure 5.17,
for every 100 residues of model protein, there
are 5.4 lysine residues to each of which is
attached an N-methyl nicotinamide (NMeN)
group. When oxidized, the circumstance is as
indicated by temperature interval b of Figure
5.4 Visual Observation of Elastic Protein-based Machines at Work
159
Performance of Mechanical Work by the Consilient l\/lechanism of Hydrophobic Folding and Assembly
A
contracted!
relaxed
a. Means of driving contraction b Means of effecting relaxation
p raising the temperature 1) lowering the temperature
transition
I zone for i
j non themnal -
energy
j inputs
J--—------
•/*—i—AT,-
I
tenf>perature —-^^
interval a for » PCj
more oil-like
temperature
•^interval b for
less oil-like
80
2a) intrinsic chemical change
e.g.
COO' —^ COOH
(adding add)
2b) extrinsic chemical change
e.g.
adding salt
3) reducing prosthetic group
e.g.
reduction of nicotinamide
4) release of pressure
when aromatic residues
present
5) light-effected photochemical
reaction of suitable
2a) intrinsic chemical change
e.g.
COOH —^ COO"
2b) extrinsic chemical change
e.g.
wash out of salt
3) oxidizing prosthetic group
4) application of pressure
when aromatic residues
present
5) reversing effect of
_(*p.rnophore
^fl'!?!!!".
contracted
relaxed
r~i
Isothermal Energy Conversions
When in the Temperature Range c
A
.8
?T^
«1
5
c
(0
6
2
.8.
o
.1
time
FIGURE
5.18. Performance of mechanical work by hydrophobic association. See text for discussion,
designed protein-based engines: Different energy (Reproduced with permission from Urry.^)
inputs "pump iron" by lowering
AGHA(X)
to drive
5.18A.
On reduction by the chemical dithionite,
the temperature interval for thermally driven
contraction lowers to a temperature interval a
and thereby moves the elastic band through the
transition zone c. Contraction results when the
operating temperature is in zone c. Oxidation
by the chemical ferricyanide relaxes the elastic
band and completes the reduction-oxidation
cycle to return the band and the attached
weight to its original state. With the proper
mediator molecules to diffuse between elec-
trode and redox couple in the cross-linked
matrix, the reduction and oxidation can be
carried out electrically; thus this may correctly
be called electro-mechanical transduction.
5.4.7.3
Pumping Iron by Changing the
Pressure Using Model Proteins with
Aromatic (Large Oil-like Ring Structures)
Side Chains
The appUcation of pressure, when there are
aromatic amino acids in the polymer such as
tryptophan (Trp, W), phenylalanine (Phe, F),
and tyrosine (Tyr, Y) as defined in Table 5.1,
raises the temperature interval from a to b of
Figure
5.18A.
Accordingly, as indicated in
Figure 5.18B, 4), the application of pressure can
cause relaxation, and the release of pressure
can result in contraction with the lifting of a
weight.^^ This is baro-mechanical transduction.

160
5.
Consilient Mechanisms for Diverse Protein-based Machines
5.4.7,4 Pumping Iron by Using Light to
Change the Oil-like Character of a
Chromophore Attached to the
Model Protein
To obtain a change in hydrophobic association
in response to Ught, the natural product cin-
namic acid was attached to a lysine (Lys, K)
residue of the polymer to form the cinnamide.
With a protein-based polymer having IK
residue per 30 residues and generally 21
30-mers forming the high molecular weight
protein-based polymer, the structure can be
represented as (H5C6-CH=CH-CO-NH-
Lys)-polymer. The orientation about the
-CH=CH- bond can be either trans or cis. To
begin with, the configuration is 100% trans, and,
when it absorbs 300 nm ultraviolet light, it con-
verts to about 90% cis. Conversion of cis back
to trans occurs with
270
nm ultraviolet
Ught.
The
temperature interval for the cis isomer occurs
at a higher temperature, like interval b of
Figure 5.18A, while that of the trans isomer
occurs over the lower temperature interval, a.
The trans isomer is more oil-like than the cis
isomer. The change in Tt on an fx = 1 basis
is about 60° C.^^ Figure 5.18B, 5) represents
photo-mechanical transduction.
5.4.7.5 The Set of Energies and Intensive
Variables (Bold Faced) Involved in Using
Model Proteins as Molecular Engines
and Motors
Mechanical work = mechanical force x
distance lifted = fAL = mgh
Pressure-volume = pressure x volume =
PxV
Chemical energy = chemical potential x
number of moles used =
A)X
x An
Thermal energy = heat of the transition =
AHt = temperature x entropy = TASt
Electrical energy = electrochemical potential x
number of electrons used = zFAE x An
Electromagnetic radiation = photon energy(hv)
X
number of photons used = hv N
5.5 Energy Conversions Due to
Coupling of Functional Groups
Here we discuss the coupling of functional
groups as part of the general phenomena of
action and reaction and most specifically as
an integral part of the consilient mechanism
of energy conversion. The argument may be
given using two statements that derive from
experimental observations to be presented
below.
Statement 1: Within the context of the
hydrophobic consilient mechanism, a func-
tional group is a chemical entity that can exist
in one of two or more interconvertible states,
each with a different hydrophobicity, as mea-
sured by the Tt-based and AGnA-based hydro-
phobicity scales (see Tables 5.1, 5.2 and 5.3).
Examples of functional groups, each with a
strong dependence on hydrophobicity, are:
1) lonizable functional groups that are a
function of pH (the change in chemical poten-
tial of proton,
A|XH)
and exhibit hydrophobicity
dependent pKa values.
2) Redox functional groups with hydro-
phobicity dependent reduction potentials for
conversion between oxidized and reduced
states.
3) Groups that change their state on absorp-
tion of light, e.g., convert from trans to cis.
4) A functional group such as a hydroxyl
that can be reversibly, e.g., enzymatically, phos-
phorylated and dephosphorylated).
Statement
2:
Because the uncharged states of
ionizable functional groups and the reduced
states of oxidized functional groups are each
more oil-like or hydrophobic than the charged
and oxidized states, the formation of the more
hydrophobic state of one functional group can
cause the more polar state of a second kind of
attached and responsive functional group to
convert to its more hydrophobic state. This is
the coupling of functions by the consilient
mechanism!
In particular, the reduction of NMeN^
approximates the replacement of several valine
residues by phenylalanine residues. Just as

5.5 Energy Conversions Due to Coupling of Functional Groups
161
increasing the hydrophobicity of a polymer
causes the protonation of a carboxylate to
occur at a lower acid concentration, it is to be
expected, therefore, that the reduction of
NMeN^ would lower the acid concentration
required to protonate a carboxylate attached to
the same hydrophobic domain.
By the consilient mechanism, energy conver-
sions due to coupling of functional groups result
from a common dependence of the different
functional groups on hydrophobicity of the
model protein. This common dependence of
the essential property of different functional
groups (e.g., pKa, reduction potential) on
hydrophobicity makes possible energy conver-
sion by the coupling of functions using the con-
silient mechanism as described in more detail
below.
5.5.1 Action Begets Reaction
5.5.1.1 For Protein-based Machines Input
Energy Constitutes the Action and Output
Energy Constitutes the Reaction
In physics and chemistry a number of laws, rela-
tions,
and principles are statements that some
action (commonly expressible as an energy
input) causes a reaction (commonly expressible
as an energy output). Examples are Guldberg
and Waage's law of mass action for chemical
reactions, Onsager's reciprocal relations for
transport processes, and Newton's third law of
motion where a force acting on an object results
in a matching reaction force.
Another example is the Principle of Le
ChateUer, which may be stated as follows: For
any system at rest (at equilibrium) the intro-
duction of a stress (in our case an input energy)
causes the system to react in such a way as to
relieve the stress (in our case by an output
energy). This principle reasonably describes
protein-catalyzed energy conversion, that
is,
the
function of protein-based machines. Under
prescribed conditions, properly designed model
protein-based machines exhibit a behavior
where for each action there is a reaction. In
section 5.4, regardless of the action, which was
any one of several different input energies, the
performance of mechanical work was the reac-
tion (there was a common output energy). In
this section the energy inputs, which individu-
ally caused contraction, convert one into the
other by their common dependence on the
change in free energy of the hydrophobically
associated (contracted)
state.
This provides
def-
inition of the subset of protein-based machines
that, despite utilizing the hydrophobic con-
silient mechanism of hydrophobic association,
are not characterized as having mechanical
work as their output energy.
5.5.1.2 For Protein-based Machines, the
Input Energy Must Occur Through the
Transition Zone
Energy input must occur through the transition
zone,
which is determined by the design of
polymer composition, to operate under the
conditions in which the machine is to function.
The change in temperature, in concentration of
solute, in availability of electrons, and so forth,
must be such that the change in the indepen-
dent (intensive) variable traverses the transi-
tion zone of the model protein. When the
variable is temperature, that
is,
when heat is the
energy input, the transition zone would be the
temperature interval (see Figures 5.1 and 5.5A)
and the energy output can be contraction.
When the variable is an increase in the amount
of acid, H^, in solution, that is, an increase in
chemical energy per mole of proton, the transi-
tion zone (see Figure 5.5B) defines the range
for the change in concentration of acid (chem-
ical potential) that converts, for example,
-COO"
to -COOH, and contraction again can
be the energy output. When the variable is the
availabihty of electrons, that
is,
electrical energy
is the input, the transition zone defines the
change in electrochemical potential required to
change an oxidized component of a redox
couple to the reduced component, and again
contraction can be the energy output.
5.5.1.3 A Functional Group Can Occur
in Either One of at Least Two
Different States
Recall that a functional group, for example, a
vinegar-like group, can occur in either one of

162
5.
Consilient Mechanisms for Diverse Protein-based Machines
two or more different states. In general, one
state of a functional group is more oil-like,
and another is more vinegar-like. To say one is
less polar and the other is more polar is an eq-
uivalent statement. The carboxyl/carboxylate,
COOH/COO", pair represents one example of a
functional group, where COOH is more oil-like
(less polar) and COO" is less oil-like (more
polar).
The organic phosphate group can occur
in three different states,-OPOs^,-OPO3H-, and
-OPO3H2, in order of decreasing polarity and
increasing oil-like character. In these cases, the
proton, H^, is the chemical entity that is added
or released. Another functional group is the
redox couple, NMeN"^ and NMeN, where the net
addition of two electron, 2e", and a proton con-
verts the more polar NMeN^ to the more oil-like
(less polar) NMeNH.
5.5.1,4 Each State of a Functional Group
Couples to the States of Another
Functional Group by Contributing to and
Being Affected by the Oil-like Character
of the Polymer
Recall also from section 5.3 that the value of Tj
provides one measure of the oil-Uke character
or hydrophobicity of the model protein. A
lower value of Tt means a more oil-like charac-
ter for the model protein. Accordingly, the cou-
pling of one functional group to another, that is,
the conversion of energy represented by one
functional group to the form represented by
another functional group, occurs because each
energy change has the common action of effect-
ing and being affected by the change in free
energy of hydrophobic association,
AGHA-
5.5.2 Hydrophobicity (Degree of
Oil-like Character) Positions
Transition Zone
5.5.2.1 Changing the Oil-like Character by
Progressive Addition of More Oil-like Phe
Groups Moves the Temperature Interval
(Transition Zone) to Lower Temperatures
When the transition zone is specifically the tem-
perature interval, any change in oil-like charac-
ter of the model protein-based machine moves
the temperature range over which the temper-
ature interval occurs. Specifically, the replace-
ment of a less hydrophobic valine (Val, V)
residue by the more hydrophobic phenylala-
nine (Phe, F) residue lowers and narrows the
temperature interval over which hydrophobic
association occurs. This is schematically repre-
sented in Figure S.IQA,"^ which shows what
happens to a curve like that of Figure 5.5A,
when the polymer involved is subjected to a
systematic change in its oil-like character. As
discussed below, any change in oil-like charac-
ter can move the transition zone of any process
that can itself change the temperature interval
(i.e.,
move the Tj-divide; see sections
5.1.3.4
and
5.3).
5.5.2.2 Changing Oil-like Character by
Progressive Addition of More Oil-like Phe
Groups Moves the Transition Zone,
Increases Affinity of the COOH/COO'
Chemical Couple for Protons, and
Decreases the Change in Chemical Energy
Required to Protonate
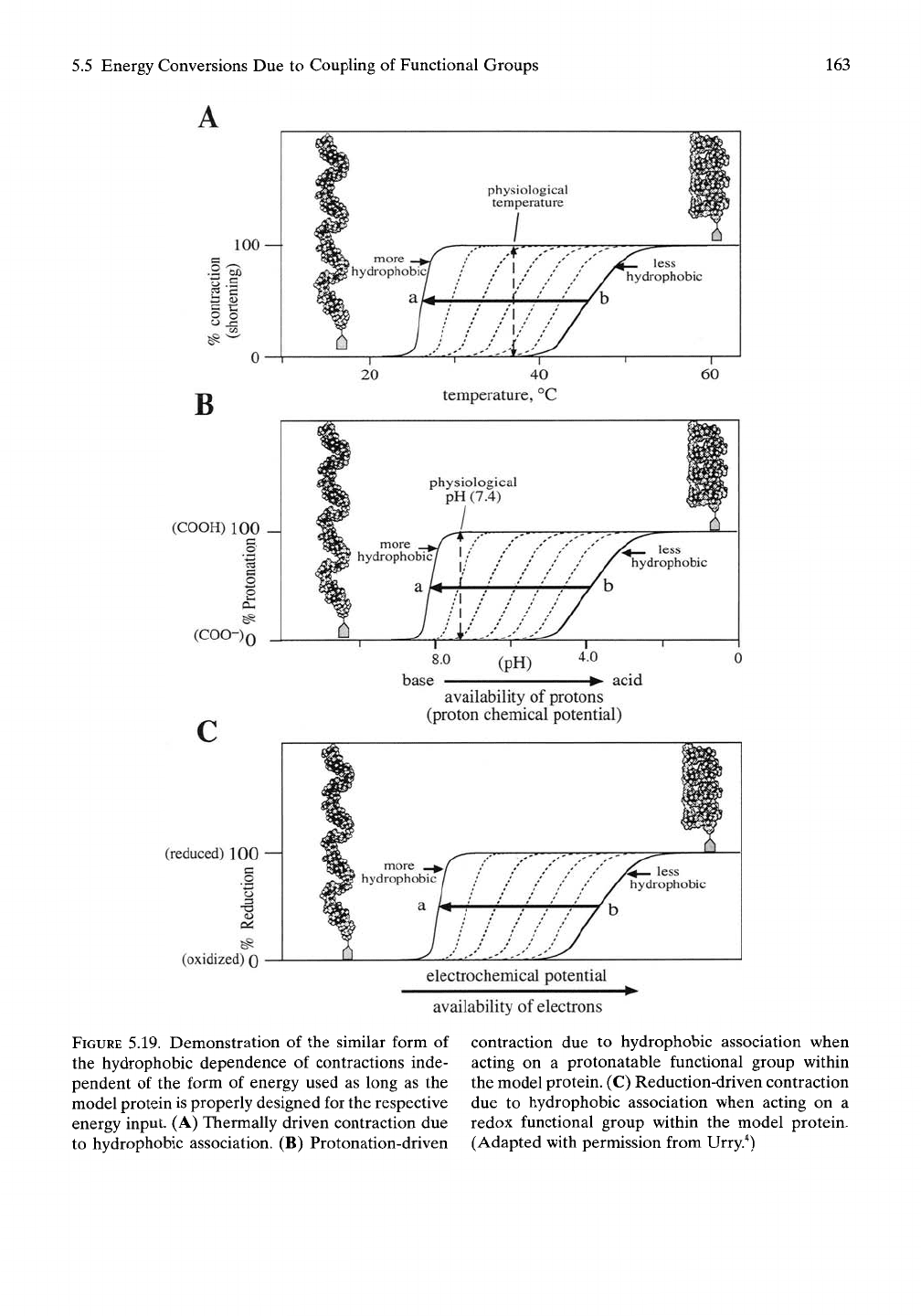
Figure 5.19B represents the series of curves that
result when the polymer series containing the
COOH/COO" chemical couple is designed
with systematic changes in hydrophobicity.'* In
practice the polymer may be from the family
(GVGVP GVGXP GEGXP GVGVP GVGXP
GXGXP)n, where E is the glutamic acid residue
with its carboxyl function and X may be either
the less hydrophobic V or the more hydropho-
bic F residue. The less oil-like polymer would
have V at all positions indicated by X. Then, as
more positions indicated by X have their V
residue replaced by the more oil-like F residue,
the curves shift to indicate that lower concen-
trations of acid are able to protonate the
carboxyl, and the protonation occurs over a
narrower transition zone. The same family of
curves is obtained for (GVGVP GVGXP
GKGXP GVGVP GVGXP GXGXP)^, where
K is the lysine residue with its amine chemical
couple (-NH2/-NH3^) but where the indepen-
dent variable involves the concentration of
base (the hydroxyl ion, OH"). Both families of
curves take on the same form as found when
the energy input was thermal in Figure 5.19A.
5.5 Energy Conversions Due to Coupling of Functional Groups
163
100—^
P. o
B
(COOH) 100 J
§
§
(COO-)o
less
hydrophobic
—T"
60
physiological
pH (7.4)
/_.
more
^ , /* /' ,- ,- ,- yi
less
hydrophobi?/
[/ / / / /
/^droptobic
a
14
/ /''
/' /' /' /b
/ ' -' /
f ••* '' I—^ r
8.0 (pH) 4.0
base w acid
availability of protons
(proton chemical potential)
(reduced) 100 H
a
o
•Z3
(oxidized) 0
more
_|
'
hydrophobic
less
hydrophobic
electrochemical potential
availability of electrons
FIGURE
5.19. Demonstration of the similar form of
the hydrophobic dependence of contractions inde-
pendent of the form of energy used as long as the
model protein is properly designed for the respective
energy input. (A) Thermally driven contraction due
to hydrophobic association. (B) Protonation-driven
contraction due to hydrophobic association when
acting on a protonatable functional group within
the model protein. (C) Reduction-driven contraction
due to hydrophobic association when acting on a
redox functional group within the model protein.
(Adapted with permission from Urry."^)

164
5.
Consilient Mechanisms for Diverse Protein-based Machines
5.5.2.3 Changing the Oil-like Character by
Progressive Addition of More Oil-like Phe
Groups Moves the Transition Zone,
Increases Affinity of the NMeN/NMeN^
Redox Couple for Electrons, and
Decreases the Electrical Energy Required
to Reduce
Figure 5.19C represents the series of curves that
result when the polymer series containing the
NMeN/NMeN^ redox couple is also designed
with systematic changes in hydrophobicity."^ The
same family of polymers can be designed as
used for the lysine residue, but in this case the
lysine residue is utilized for attachment of the
N-methyl nicotinamide (NMeN), that is,
(GVGVP GVGXP GK[NMeN]GXP GVGVP
GVGXP GXGXP)n. Again, as less oil-like Val
(V) residues are systematically replaced by
more oil-like Phe (F) residues, a lower concen-
tration of electrons (a lower electrochemical
potential) reduces the oxidized state of the
redox couple. Again, the family of curves take
on the same form as occurs when chemical
energy was the input energy and when thermal
energy was the input energy, as in Figure
5.19A,B.
5.5.2.4 Changing the Oil-like Character of
a Model Protein by Any Energy Input, %,
That Moves the Transition Zone Can Be
Used to Change the Energy of a
Functional Group That Is Also Capable of
Changing the Oil-like Character of the
Model Protein
Additional input energies can be light energy
interacting with a chromophore to change its
hydrophobicity and pressure-volume energy
acting most effectively on aromatic groups to
change their expression of hydrophobicity.
Many other interaction energies come from
the electromagnetic frequency spectrum. They
can come from outside of the ultraviolet,
visible, and infrared frequency ranges. All that
is required is an element of the model protein
in water that is able to take up the energy. The
dipole moment of the peptide group with its
positive end at the NH and its negative end at
the oxygen of the CO provides one site of inter-
action. The acoustic and radio frequency ranges
of the electromagnetic radiation spectrum rep-
resent two frequency ranges that are funda-
mental to our elastic model proteins capable of
exhibiting inverse temperature transitions.
When subjected to oscillating electric fields
near
3
kHz (acoustic frequency) and
5
MHz
(radio frequency), the peptide group of the
protein backbone reorients its dipole moment
and oscillates with the appUed oscillating elec-
tric fields in these frequency ranges. The uptake
of these energies by the elastic model proteins
change the internal chain dynamics and are
expected to alter the force exerted by a slightly
extended polymer. Of more direct concern to a
change in free energy of hydrophobic associa-
tion dependent on the amount or potential
amount of hydrophobic hydration is the 5 GHz
absorption (microwave frequency) exhibited by
hydrophobic hydration itself (see section 5.7).
Energy absorbed directly by hydrophobic
hydration is expected to affect the TAS
controlling solubility (i.e., hydrophobic
association)
The innumerable ways in which the value of
Tt can be changed constitute the number of
energy inputs, x, that can drive protein-based
machines.
5.5.3 Coupling of Functional Groups
by Means of the Consilient
Mechanism and by Means of Their
Common Dependence on the Gibbs
Free Energy of Hydrophobic
Association,
AGHA
The energy input to a first functional group
causes a change in the free energy of the
hydrophobically associated state,
AG^A-
That
AG/M then causes a change in the free energy of
a second functional group. This is the coupling
of functions by the consilient mechanism. It rep-
resents the most explicit expression of the com-
prehensive hydrophobic effect.
Section 5.4.3 and Tables 5.2 to 5.4 list many
calculated estimates of AGHA arising from many
different energy
inputs.
The estimated values of
AGHA
provide a quantitative measure of how a
given energy input changes the energy of the
hydrophobically associated state. This provides
