Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


5.2 Molecular Structure and Elasticity of Model Protein
125
detailed treatment than the brief comments
given here.
5.2.1 The Sequence of Amino Acid
Residues: The Primary Structure
This journey of personal enchantment leading
to the consilient mechanism of protein-based
energy conversion began with the parent model
protein (Gly-Val-Gly-Val-Pro)n.^^ This five
amino acid residue repeat, referred to as a pen-
tamer, recurs 11 times in the elastic fibers of the
pig and cow, that is, the subscript n is
11.^^'^^
In
our designed model proteins the pentamer gen-
erally repeats some 200 times or more, that is,
the subscript n is often greater than 200. These
protein-based polymers may also be called
polypentapeptides. The general way to indicate
this large repeating pentamer is poly(GVGVP)
or (GVGVP)n, where the value of n is specified
for a given preparation. When made by genetic
engineering, polymers of a precise number of
repeats are obtainable. In Figure 5.1, the data
represent those of a polypentapeptide com-
prised of 251 repeats, that is, (GVGVP)25i.
5.2.2 Secondary, Tertiary, and
Quaternary Structures
5.2.2.1 Hydrogen Bonds Between
Backbone Peptide Groups: The
Secondary Structure
The molecular structure of poly(GVGVP)
is one of a series of P-turns, one in each
pentamer, inserted by the Pro-Gly sequence
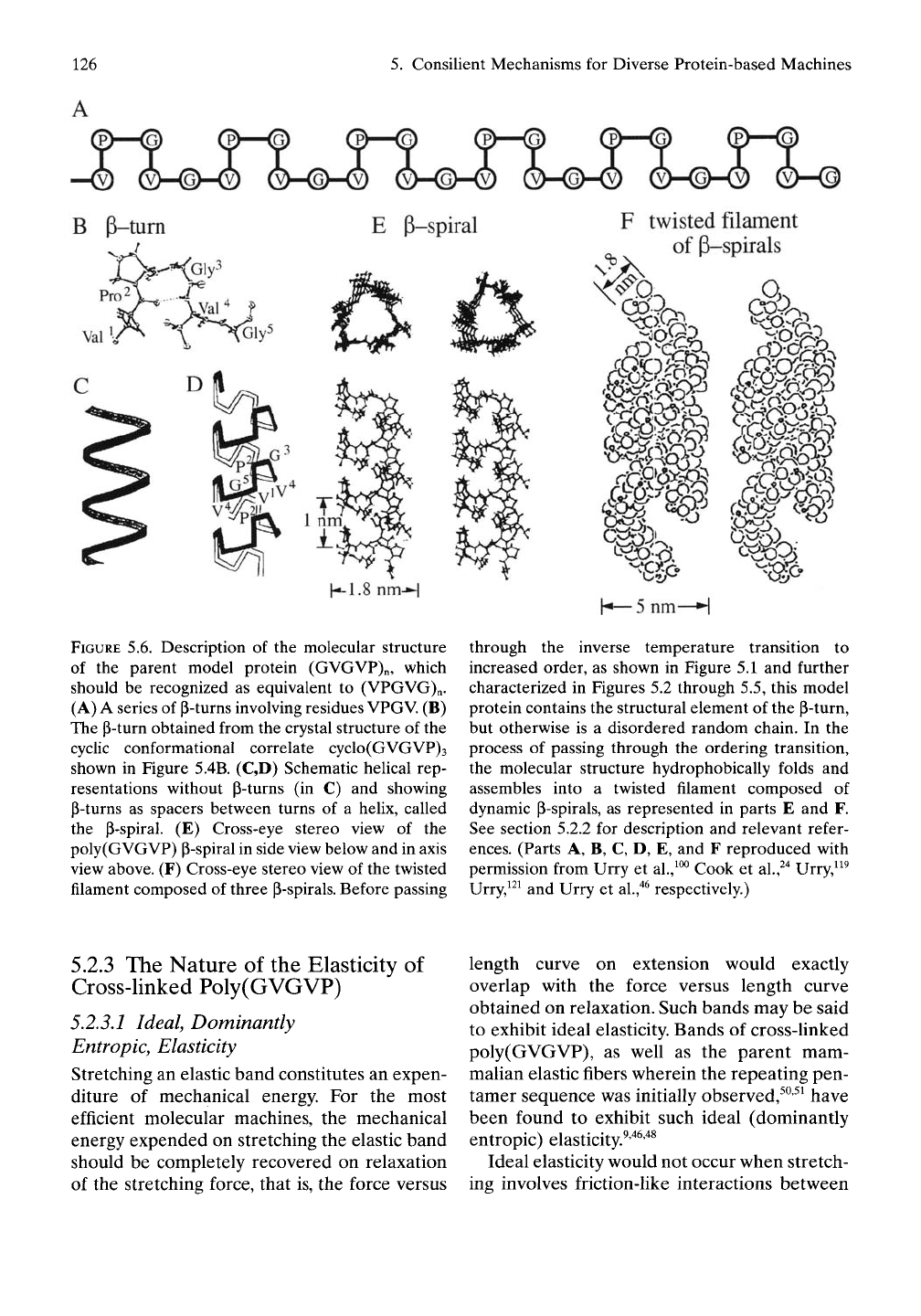
as schematically indicated in Figure 5.6A.
Because of the location of the p-turn, which is
a 10-atom hydrogen bonded ring involving
the Val^C-O-HNVal"^, early representations of
the polypentapeptide were written as
poly(VPGVG) or (Val'-Pro'-Gly^-Val'-Gly^n.^^
The detailed p-turn shown in Figure 5.6B was
obtained from the crystal structure of the cyclic
molecule comprised of three pentamers^"^ and
was developed independently by the physical
methods of nuclear magnetic resonance and
circular dichroism and by the computational
methods of molecular mechanics and dynamics.
The p-turn, the 10-atom hydrogen bonded ring
involving the Val^-CO to the Val'^-NH shown in
Figure 5.6B, is the single secondary structural
feature, the only hydrogen bond, in the repeat-
ing pentamer. Studies using Raman scattering
indicate the presence of the j8-turn below Tt,
before the phase transition, and indicate no
change in secondary structure, that is, no
change in hydrogen bonding during the
hydrophobic folding and assembly process
attending the phase change.^^ This means
during the phase transition that the significant
changes within and between the chain mole-
cules are changes in hydrophobic association.
5.2.2.2 Hydrophobic Association
Represents Tertiary and Quaternary
Structure
On raising the temperature above 25° C, the
series of p-turns optimizes hydrophobic con-
tacts within a single chain by wrapping up into
a helical structure, called a ^-spiral, as shown in
detail by stereo pair in Figure 5.6E. The forma-
tion of intramolecular oil-like contacts, for
example, the interaction between the PropCH2*
of pentamer i and the ValyCHs'^^ of pentamer i
-h
3 in the sequence, may be considered changes
in tertiary structure attending the inverse
temperature transition. Finally, hydrophobic
association between chains results in a twisted
filament structure as indicated in Figure 5.6F.
These are quaternary structural changes as
developed from electron micrographs of
negatively stained incipient aggregates formed
on heating, from optical diffraction of the
micrographs,^"^ and from molecular mechanics
and dynamics calculations.^^'^^ In reahty, the
hydrophobic contacts within and between
chains develop simultaneously each assisting
the other, that is, in a cooperative manner.
For simpHcity, we often display a single chain
in the p-spiral structure; in reality, however, we
do not expect such to form readily in isolation,
but rather as part of a twisted filament as in
Figure 5.6F. Accordingly, the schematic repre-
sentations of the P-spiral structure of Figures
5.6C-E are regularly used to simplify but ade-
quately make the intended point.
126
5.
Consilient Mechanisms for Diverse Protein-based Machines
E P^spiral
F twisted filament
of P-spirals
K1.8nm-H
FIGURE 5.6. Description of the molecular structure
of the parent model protein (GVGVP)n, which
should be recognized as equivalent to (VPGVG)n.
(A) A series of P-turns involving residues
VPGV.
(B)
The p-turn obtained from the crystal structure of the
cyclic conformational correlate cyclo(GVGVP)3
shown in Figure 5.4B. (C,D) Schematic helical rep-
resentations without P-turns (in C) and showing
p-turns as spacers between turns of a helix, called
the p-spiral. (E) Cross-eye stereo view of the
poly(GVGVP) P-spiral in side view below and in axis
view above. (F) Cross-eye stereo view of the twisted
filament composed of three P-spirals. Before passing
through the inverse temperature transition to
increased order, as shown in Figure 5.1 and further
characterized in Figures 5.2 through 5.5, this model
protein contains the structural element of the p-turn,
but otherwise is a disordered random chain. In the
process of passing through the ordering transition,
the molecular structure hydrophobically folds and
assembles into a twisted filament composed of
dynamic p-spirals, as represented in parts E and F.
See section 5.2.2 for description and relevant refer-
ences.
(Parts A, B, C, D, E, and F reproduced with
permission from Urry et al.,^^^ Cook et al.,^"^ Urry,^^^
Urry,^^^ and Urry et
al.,"^^
respectively.)
5.2.3 The Nature of the Elasticity of
Cross-Hnked Poly(GVGVP)
5.2.3.1 Ideal, Dominantly
EntropiCy
Elasticity
Stretching an elastic band constitutes an expen-
diture of mechanical energy. For the most
efficient molecular machines, the mechanical
energy expended on stretching the elastic band
should be completely recovered on relaxation
of the stretching force, that is, the force versus
length curve on extension would exactly
overlap with the force versus length curve
obtained on relaxation. Such bands may be said
to exhibit ideal elasticity. Bands of cross-linked
poly(GVGVP), as well as the parent mam-
malian elastic fibers wherein the repeating pen-
tamer sequence was initially observed,^^'^^ have
been found to exhibit such ideal (dominantly
entropic) elasticity.^'^^'^^
Ideal elasticity would not occur when stretch-
ing involves friction-like interactions between

5.2 Molecular Structure and Elasticity of Model Protein
127
the load-bearing chain and surrounding
nonload-bearing chains. In such cases some
of the mechanical energy expended during
stretching is not recovered on removal of the
stretching force. Chains having complex sec-
ondary (hydrogen bonded), tertiary, and qua-
ternary structures provide mechanisms for the
energy expended on extension to be dissipated
away from the load-bearing chain segments.
5.2.3.2 The Decrease of Internal Chain
Motions on Stretching
Stretching of the molecular structure described
in Figure 5.6 does not involve the disruption of
complex secondary, tertiary, and quaternary
structures nor does significant frictional drag
appear to occur between the chain bearing the
load and adjacent nonload-bearing chains. In
the structure most easily seen in stereo in
Figure 5.6E, suspended segments, involving the
VGV sequence, occur between the p-turns
within a single chain. The two peptide groups,
-CONH-, within VGV sequence are quite free
to rotate or rock about the nearly colinear
C-CONH-C bonds in the structure in Figure
5.6E. This motion of the backbone constitutes
chain entropy. On stretching, the amplitude of
these rocking motions decreases, and this
causes the decrease in entropy that provides
the restoring force after stretching. This mech-
anism of elasticity has been called the damping
of internal chain dynamics on extension.^^y^lh^n
the energy of stretching the elastomer primar-
ily decreases internal chain motions, the elas-
tomer becomes an entropic elastomer. This type
of elasticity allows for more efficient energy
conversions.
This mechanism of elasticity provides a
"common groundwork of explanation" for the
elasticity of all chain molecules regardless of
composition and structure as long as there
exists an internal chain motion that becomes
decreased on deformation. For this reason, it
too is a consilient mechanism. To delineate this
consilient mechanism for elasticity from the
consilient mechanism for hydrophobic associa-
tion, as treated extensively in this volume, it
will be referred to as the elastic consilient
mechanism.
5.2.3.3 Importance of the Elastic
Consilient Mechanism to Efficiency of
Energy Conversion
An important element of an ideal elastomer is
that the energy of deformation of an otherwise
kinetically free chain must remain entirely in
the backbone modes where it can be recovered
on relaxation. Should the energy of deforma-
tion find its way into side chain motional
modes, this can be dissipated into the solvent
and into adjacent nonforce sustaining mole-
cules and, therefore, be lost to the recovery
during relaxation. The experimental result of
this dissipation is hysteresis. Cross-linked
poly(GVGVP) exhibits near ideal elasticity;
the extension and relaxation curves overlap
well. Single-chain force-extension curves of
(GVGVP)502 can exhibit extension and
relaxation curves that overlap perfectly
within instrumental limits."^^ On the other hand,
cross-linked poly(GVGIP) always exhibits
hysteresis; the relaxation curve falls well below
the extension curve. Because the work of exten-
sion is the area under the force-extension curve,
when the force-relaxation curve falls below the
force-extension curve, the energy represented
by the difference between extension and
relaxation is lost. Expectedly, therefore, single-
chain force-extension curves of (GVGIP)nx26o
do exhibit marked hysteresis; however, under
conditions of extreme dilution occasional
curves of (GVGIP)nx26o can be obtained
wherein the extension and relaxation curves
superimpose.^^ (GVGIP) nx26o with the greater
tendency for hydrophobic association between
chains, due to the bulkier more hydrophobic
isoleucyl residue in each pentamer resulting
from an added CH2 moiety, provides an
example of energy loss through side chain
motions and interactions.
Hanging a weight on an elastic band of cross-
linked poly(GVGVP), as described in section
5.1.4, constitutes a extending force; the band
increases in length until the area under the
force-extension curve becomes equivalent to
the mechanical energy of the lifted weight.
For elastic-contractile bands operating under
the consilient mechanism, heating the band
through the temperature interval or adding salt

128
5.
Consilient Mechanisms for Diverse Protein-based Machines
to move through the transition zone of Figure
5.5 Ufts a weight a certain distance and results
in the performance of a given amount of
mechanical w^ork. For bands that exhibited
hysteresis, the distance a weight is lifted, on
raising the temperature from below to above
the phase transition for hydrophobic associa-
tion, would be less than for the bands that
exhibited a near ideal elasticity. The former
bands would not be very good molecular
machines for biological energy conversion. All
of the energy conversions of the consilient
mechanism involve changes in hydrophobic
association, whether or not mechanical energy
is the input or output. Because of this, all of the
energy conversions are more efficient when the
protein-based machine is a dominantly entropic
elastomer.
Significantly, for elastin the majority of the
heat and entropy change during chain exten-
sion results from changes in hydrophobic
hydration.^^ Reversibility of an ideal elastomer,
recovery to the same structure on relaxation,
allows that the same change in hydrophobic
hydration occur. To understand energy conver-
sion adequately by the consilient mechanism,
that is, by means of inverse temperature
transitions, solvent entropy changes attending
changes in hydrophobic association require
delineation from changes in chain entropy for
the development of elastic force.
5.2.4 Relationship Between Nature of
Elasticity
and Contractility
Historically, the question of mechanism of
elasticity has been one of evaluating the rela-
tive contributions of three different proposed
mechanisms: (1) the random chain network
(classic rubber elasticity) theory,^^'^^ (2) the
solvent entropy theory,^^"^^ and (3) the damping
of internal chain dynamics on extension.^"^^'^^'^^
The first is due to the Flory school; the second
was initiated by Weis-Fogh and Andersen, and
the third is due to the present author and
coworkers of the last quarter century.
The atomic force microscopy (AFM) single-
chain force-extension studies on the elastic
model proteins^'"^^ demonstrate entropic elastic-
ity for single chains without the presence of
random chain networks with Gaussian distrib-
utions of end-to-end chain lengths as would
have been required for the random chain
network theory.^^ This leaves the proper delin-
eation of solvent entropy and internal chain
dynamics. The solvent entropy theory of elas-
ticity requires consideration in the case of our
elastic model proteins, because changes in
solvent entropy attend changes in hydrophobic
association during stretching and because most
recent adherents of this view base their per-
spective on calculations of (GVGVP)n.^^'^^ In
addition, a clear understanding of energy con-
version by means of the inverse temperature
transitions exhibited by elastic model proteins
requires accurate delineation of the roles of
chain and solvent entropy. Experimental delin-
eation immediately follows.
5.2.4.1 Experiment to Determine Fraction
of Ideal (Entropic) Elasticity
The classic experiment to estimate the amount
of total elastic force,/, derived from the inter-
nal energy component of force,
/E,
and the
entropic component of force,/s, where/=/£; +
fs,
examines the temperature dependence of
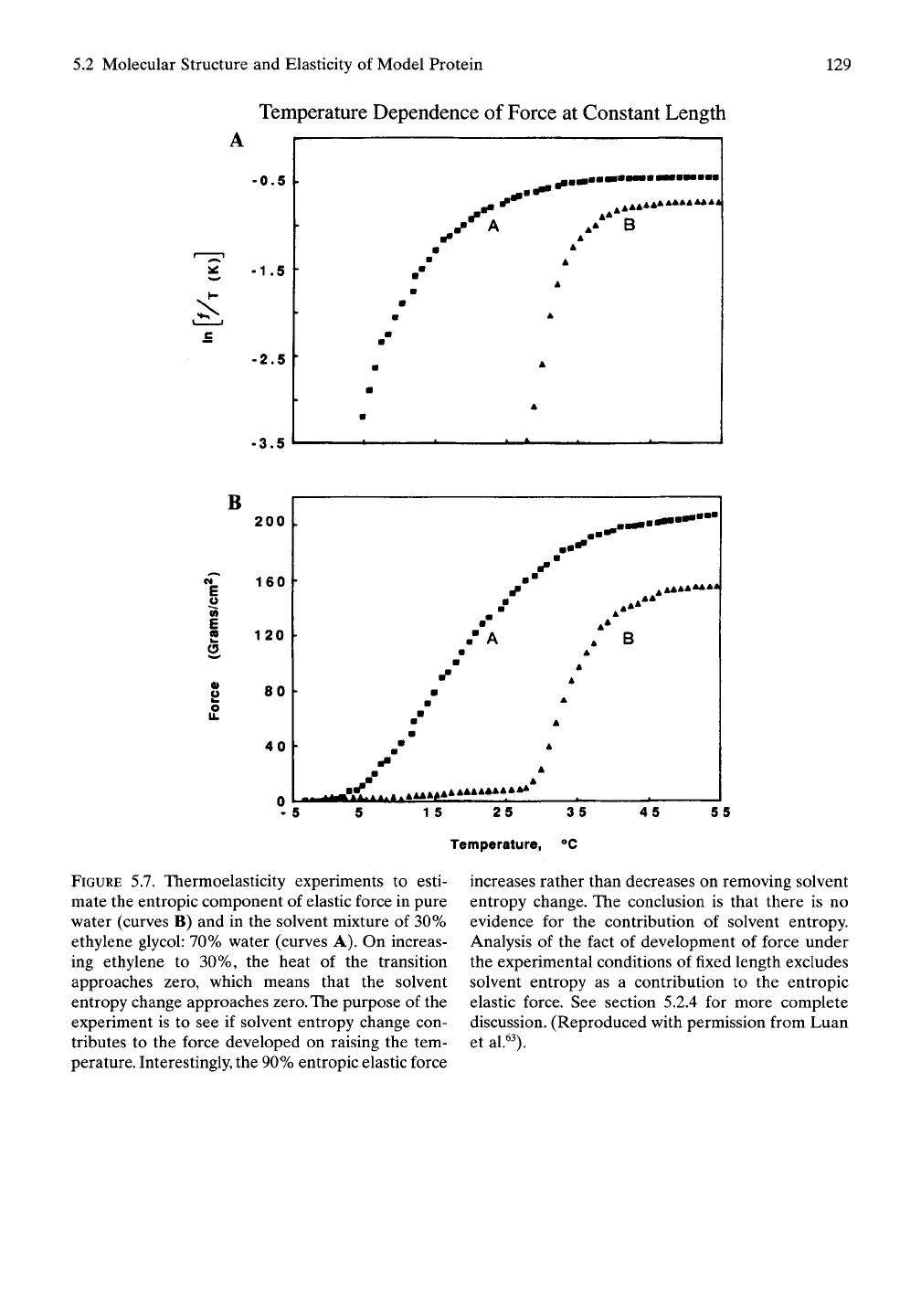
force at constant length. On plotting
ln[//T],
with T in Kelvin, as a function of temperature
while maintaining the elastomer element at
fixed length, the slope of the plot multiplied by
(-T K) provides the f^lf ratio. Such data are
given in Figure 5.7A, where the/E//ratio above
40° C is found to be 0.9.^^ Whether in ethylene
glycoliwater (30:70), curve A, or pure water,
curve B, the elastic band of cross-linked
poly(GVGVP) exhibits an elastic force that
is 90% entropic. When the solvent entropy
change through the temperature interval of the
inverse temperature transition is reduced to
near zero, as occurs in ethylene glycol:water
(30:70),^^
the entropic elastic force actually
increases. This is contrary to the solvent
entropy theory of elasticity. As analyzed below,
however, the increase in entropic elastic force
on going from 25° to 40° C eliminates solvent
entropy change as a direct source of the esti-
mated entropic component elastic force.
5.2 Molecular Structure and Elasticity of Model Protein
Temperature Dependence of Force at Constant Length
129
>
-0.5 I
-1.5
-2.5 r
-3.5
B
E
o
M
E
o
o
u.
200
160
120
80
40
n
m
m
/A
•
•
•
•
•
•
1
iiiaiiiiiiii
.AAAAAAAAAAAAAA4AA
•
•
A
A
A
A
A
A
A
•
.AAAAAAAJ
**
B
1 1
15 25 35
Temperature,
*C
45 55
FIGURE
5.7. Thermoelasticity experiments to esti-
mate the entropic component of elastic force in pure
water (curves B) and in the solvent mixture of 30%
ethylene glycol: 70% water (curves A). On increas-
ing ethylene to 30%, the heat of the transition
approaches zero, which means that the solvent
entropy change approaches zero. The purpose of the
experiment is to see if solvent entropy change con-
tributes to the force developed on raising the tem-
perature. Interestingly, the 90% entropic elastic force
increases rather than decreases on removing solvent
entropy change. The conclusion is that there is no
evidence for the contribution of solvent entropy.
Analysis of the fact of development of force under
the experimental conditions of fixed length excludes
solvent entropy as a contribution to the entropic
elastic force. See section 5.2.4 for more complete
discussion. (Reproduced with permission from Luan
et
al.^^).
130
5.
Consilient Mechanisms for Diverse Protein-based Machines
5.2.4.2 Implications of Force Changes at
Constant Length
For poly(GVGVP) the temperature interval
for hydrophobic association is 25° to 40° C. It
is over this temperature range that hydropho-
bic hydration becomes higher entropy bulk
water as the hydrophobic groups associate.
For this temperature interval the AS for
solvent is clearly positive. As shown in Figure
5.7, an elastic force develops over this temper-
ature interval that is 90% entropic. Now,
because /s = -T(9S/3L)yT,n, entropy change
for entropic elastic force development is nega-
tive.
This means that the solvent entropy
change is not contributing to the observed
increase in the entropic component of elastic
force.
The same argument applies for isometric
contraction (force development at fixed length)
driven by protonation of carboxylates (see
Figure
1.4).^'^"^
In this case, protonation of car-
boxylates in the cross-linked elastic matrix
drives hydrophobic association with an increase
in entropic elastic force due to a negative
change in elastomer entropy. Under these
conditions, however, hydrophobic hydration
converts to bulk water; the solvent entropy
change is positive, exactly the wrong sign to
contribute to the entropic elastic force. How
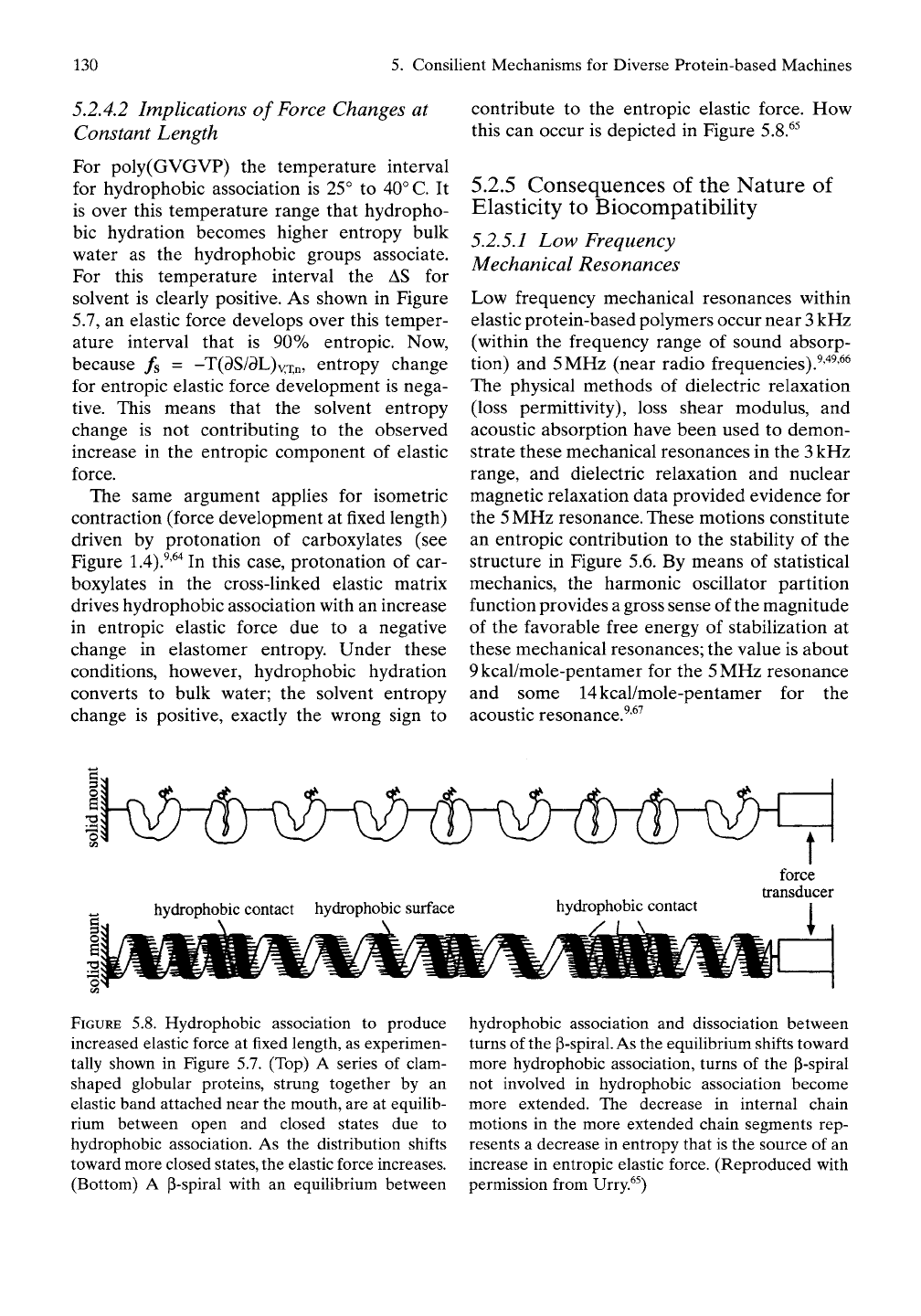
this can occur is depicted in Figure 5.8.^^
5.2.5 Consequences of the Nature of
Elasticity to Biocompatibility
5.2.5.1 Low Frequency
Mechanical Resonances
Low frequency mechanical resonances within
elastic protein-based polymers occur near
3
kHz
(within the frequency range of sound absorp-
tion) and 5MHz (near radio frequencies).^"^^'^^
The physical methods of dielectric relaxation
(loss permittivity), loss shear modulus, and
acoustic absorption have been used to demon-
strate these mechanical resonances in the
3
kHz
range, and dielectric relaxation and nuclear
magnetic relaxation data provided evidence for
the
5
MHz resonance. These motions constitute
an entropic contribution to the stability of the
structure in Figure 5.6. By means of statistical
mechanics, the harmonic oscillator partition
function provides a gross sense of the magnitude
of the favorable free energy of stabilization at
these mechanical resonances; the value is about
9kcal/mole-pentamer for the
5
MHz resonance
and some 14kcal/mole-pentamer for the
acoustic resonance.^'^^
II
hydrophobic contact hydrophobic surface
hydrophobic contact
force
transducer
FIGURE 5.8. Hydrophobic association to produce
increased elastic force at fixed length, as experimen-
tally shown in Figure 5.7. (Top) A series of clam-
shaped globular proteins, strung together by an
elastic band attached near the mouth, are at equilib-
rium between open and closed states due to
hydrophobic association. As the distribution shifts
toward more closed
states,
the elastic force increases.
(Bottom) A p-spiral with an equilibrium between
hydrophobic association and dissociation between
turns of the
p-spiral.
As
the equilibrium shifts toward
more hydrophobic association, turns of the P-spiral
not involved in hydrophobic association become
more extended. The decrease in internal chain
motions in the more extended chain segments rep-
resents a decrease in entropy that is the source of an
increase in entropic elastic force. (Reproduced with
permission from Urry.^^)

5.3 Cataloging the Energy Resources Available to the Consilient Mechanism of Energy Conversion 131
5.2.5.2 Interaction, as in Antigen-
Antibody Complexation, Would Increase
Free Energy
For a molecular species to be antigenic, the
antibody must bind to the antigen in the for-
mation of a complex that would identify the
molecular species as foreign. This necessitates
suppression of the motions of the elastomer, of
the mechanical resonances, at a cost of increas-
ing the free energy of the complex. If the com-
plexation free energy increase were less than
one-half of that calculated by the harmonic
oscillator partition function, the probability of
interaction would be decreased by a factor of
10 million, making its formation insignificant.
Because of this, the elastic protein-based poly-
mers are expected, as they have been found
(see Chapter 9) to be remarkably biocompati-
ble.
This property contributes to the usefulness
of these elastic protein-based polymers over a
large range of medical applications.
5.3 Cataloging the Energy
Resources Available to the
Consilient Mechanism of
Energy Conversion
5.3.1 Each Means of Changing Tt
Represents an Energy Source for
Protein-based Machines
5.3.1.1 Point of
View
of
Greatest
Simplicity
"One of the principal objects of theoretical
research in any department of knowledge is to
find the point of view from which the subject
appears in its greatest simplicity.''^^ This state-
ment was written by J. Willard Gibbs in 1881,
the same Gibbs that gave us the Gibbs free
energy, AG, so central to the above analyses.
The significance of the quotation is com-
pounded here. We propose the Tt-perspective
to be a point of view of greatest simplicity, and
in section
5.1.3.4
we derived approximations to
the change in Gibbs free energy for hydropho-
bic association, AGHA(%)- From this expression,
we recognize the change in Tt from a reference
Tt to a new value of Tt caused by an energy
input represented by x to provide a measure of
the change in Gibbs free energy for hydropho-
bic association of the protein-based polymer.
Therefore, Tt, the onset temperature for the
inverse temperature transition, represents an
intrinsic property of the hydrophobic consilient
mechanism of energy conversion.
5.3.1.2 Simplicity of the Observations
For our model proteins, determination of Tt
is straightforward. The approximate value of
Tt is evident to the unaided eye. A glass tube
containing the parent model protein,
poly(GVGVP), dissolved in water and at a
comfortable room temperature, forms a clear
solution (see the clear tube in Figure 5.1A). On
grasping the test tube in the palm of one's hand
for a few moments, the clear solution becomes
cloudy, as represented in the middle tube in
Figure 5.1A. On standing at palm temperature,
two separate phases appear. Simply warming
the tube from room temperature to body tem-
perature causes the model protein to fold,
assemble, and phase separate due to the asso-
ciation of oil-Uke groups.
Now, cross-linking the elastic model protein
in the phase-separated state results in elastic
bands.
Similarly warming the band, swollen at
room temperature (just below Tt), to body
temperature (some 15 degrees above Tt) causes
the band to contract with the performance of
mechanical work. The band pumps iron on
raising the temperature from below to above
Tt. As scientific accounts go, the Tt perspective
exemplifies simpUcity.
5.3.1.3 Inputs That Move the Trdivide
Represent Energy Resources for the
Consilient Mechanism of Energy
Conversion Without Changing
Temperature
Any change in Tt indicates a change in free
energy for the folded (hydrophobically associ-
ated) state of the polymer. Therefore, in the first
approximation the effectiveness of the energy
input is obvious from the magnitude of the
change in
Tt.
As a particularly relevant example.

132
5.
Consilient Mechanisms for Diverse Protein-based Machines
the most dramatic increase in Tj occurs on phos-
phorylation. Accordingly, phosphate binding
provides the most effective energy input to
drive unfolding (hydrophobic disassociation),
and phosphate removal imparts the greatest
output for contraction by favoring hydrophobic
association. A list of the Trvalues resulting from
the full range of energy inputs to a reference
model protein becomes the catalog of energy
resources available to the consilient mechanism
for protein-based machines. Changes in the
value of Tt, therefore, constitute the nuts and
bolts of energy conversion by the consilient
mechanism. Most significantly^ the following
Tt-based cataloging of energy resources avail-
able to the consilient mechanism coincides with
the catalog of the energy resources that run
biology's machines.
hydration, or by water that has been oriented
for hydration of charged groups, or by no water
as when in an insoluble state, that is, hydropho-
bically associated.
This section develops the foundation with
which to utilize these different states in engi-
neering protein function and in understanding
the function of proteins as molecular machines.
It does so by developing a scale that determines
the relative effectiveness of chemical groups
associated with protein to change the tem-
perature at which the phase transition of
poly(GVGVP) takes place, that is, to move the
Tt-divide in Figure 5.3. That relative effective-
ness in changing the location of the Tt-divide,
as noted above, provides a measure of the
relative effectiveness of the energy input that
caused the ATf
5.3.1.4 Impact of the Relative Oil-like
Character of Functional Groups of
Proteins on the Trdivide
The capacity to exist in two different states
defines a functional group. Here we divide the
two different states into more and less
hydrophobic or, from the opposite perspective,
more and less polar. The clearest examples of
functional groups are those groups that can be
either uncharged or charged, that is, either
neutral or ionized. The most obvious examples
of ionizable functional groups of protein are
the carboxyl/carboxylate, -COOH/-COO", and
the amino/ammonium, -NH2/-NH3"^, chemical
couples. A change in the amount of acid, H^,
present in the solution can change the state of
the chemical couple from one into the other.
Importantly, the interaction between oil-like
side chains and these functional groups changes
the amount of acid required to convert from
one state to the other (see Figures 1.2 and 5.20B
and section 5.7.7). In other words, the extent of
the oil-like character of the domain in which
the functional group occurs can change the
state of a functional group. From this perspec-
tive,
the oil-like residues, normally considered
nonfunctional, exhibit functionality by their
capacity to change the state of a functional
group. Oil-like groups can also be in different
states;
they can be surrounded by hydrophobic
5.3.2 The Tt-based Hydrophobicity
Scale for Amino Acid Residues
5.3.2.1 The Preferred Site of Substitution
For our model protein, poly(GVGVP),^^
replacement of either of the Gly residues or
the Pro residue markedly alters the structure
and properties of the resulting protein-based
polymer. For example, substitution of the Gly
and Pro residues can destroy the favorable elas-
ticity of the polymers. These positions will not
be used for the comparisons of interest here.
Substitution of the Val preceding the Pro is pos-
sible by most amino acid residues, but not all.
Fortunately, any one of the 20 naturally occur-
ring amino acid residues can replace the V of
GVG without significant change to the basic
structure and function.
5.3.2.2 An Expression for Substitution in
Chemically Synthesized Model Proteins
The approach, therefore, is to introduce a guest
residue in place of the V residue in the GVG
sequence by preparing the pentamer GXGVP
where X is any of the naturally occurring amino
acid residues or a chemical modification of bio-
logical interest. (See Table 5.1 for identification
of each of the R-groups of the 20 natural amino
acid residues.) The pentamer with the guest
5.3 Cataloging the Energy Resources Available to the Consilient Mechanism of Energy Conversion 133
residue, X, is mixed in the reaction solution with
the parent pentamer, GVGVP, at different
ratios in different reaction vessels, and each
mixture is polymerized. The result is a series of
model proteins with different amounts of the
guest residue.
The general expression for the composition
of this series of model proteins becomes
poly[f,(GXGVP),fv(GVGVP)], where f, and fv
are called mole fractions wherein fx + fv = 1. By
way of example, if fx were 0.2 and, therefore, fv
were 0.8 (i.e., 1 - 0.2), there would be four
GVGVP pentamers for each GXGVP pen-
tamer, which means that on the average there
would be 1 substitution, that is, one X residue,
for every 25 residues or 4 substituted residues
in every 100 residues.
5.3.2.3 The Experimental Observation
As long as the model proteins are not too oil-
like,
they are soluble at low temperature in a
biological salt solution (physiological saline,
0.15NNaCl), that is, the experiment begins at
low temperature with a clear solution. The solu-
tion is heated; the temperature increases, and,
at a critical temperature unique to the compo-
sition of the model protein, the sample becomes
cloudy. This is shown in Figure 5.IB, where
aggregation of the model protein causes cloudi-
ness.
A graph representing clear solutions of a
number of different model protein composi-
tions that turn cloudy at different temperatures
on increasing the temperature is shown in
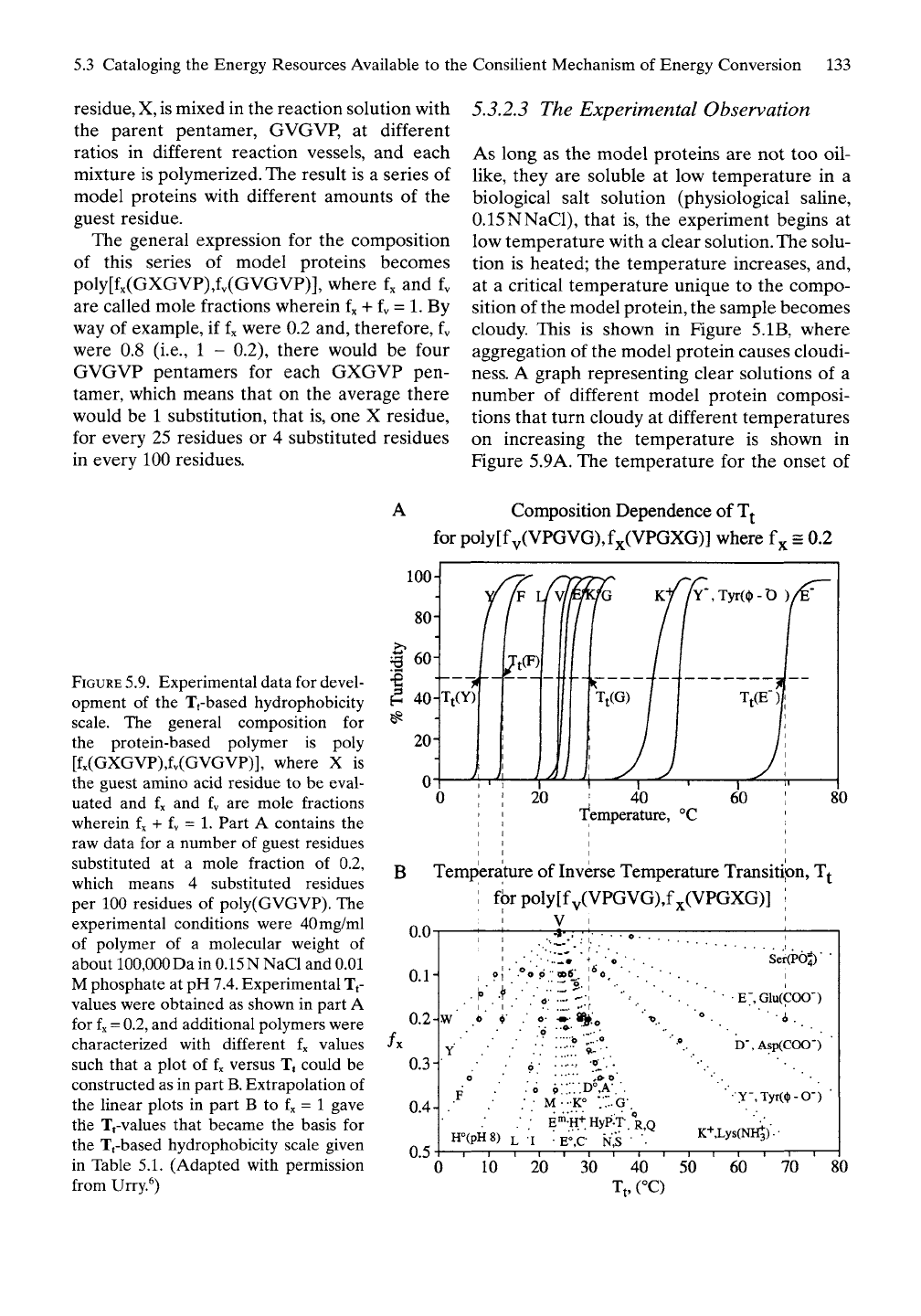
Figure 5.9A. The temperature for the onset of
FIGURE
5.9.
Experimental data for devel-
opment of the Tt-based hydrophobicity
scale. The general composition for
the protein-based polymer is poly
[fx(GXGVP),fv(GVGVP)], where X is
the guest amino acid residue to be eval-
uated and fx and fy are mole fractions
wherein fx + fv = 1. Part A contains the
raw data for a number of guest residues
substituted at a mole fraction of 0.2,
which means 4 substituted residues
per 100 residues of poly(GVGVP). The
experimental conditions were 40mg/ml
of polymer of a molecular weight of
about 100,000 Da in 0.15N NaCl and 0.01
M phosphate at pH
7.4.
Experimental Tt-
values were obtained as shown in part A
for
fx =
0.2,
and additional polymers were
characterized with different fx values
such that a plot of fx versus Tj could be
constructed as in part
B.
Extrapolation of
the linear plots in part B to fx = 1 gave
the Tt-values that became the basis for
the Tt-based hydrophobicity scale given
in Table 5.1. (Adapted with permission
from Urry.^)
Composition Dependence of T|.
for poly[f ^(VPGVG),fx(VPGXG)] where f^ = 0.2
, 40 60
Temperature, °C
80
B Temperature of Inverse Temperature Transition, T|.
: forpoly[f^(VPGVG),fx(VPGXG)] ;
(\ H-,
Kj.y)
0.1"
0.2-
0.3-
0.4-
0.5-
, i V 1
1
yr":/-,^^ '•:
W .6 * ." o- •^- 85^.0 ^.-
Y •••;•.•. »-••
•6-
• -.v-
0 • • ,©• 0
.• • 0 P::;;;D°,A;.
; • M •••K° V"G-.
• E™H+HyP-r R,Q
H°(pH8) LI • E°,C N,S "
1 1 1 1 1 1 i 1 1
Ser(i>6t) •
E7,Glu(COO')
« . • i .
*. D',Asp(COO')
•••Y-,Tyr(<|)-0-) '
K+,Lys(NH5)'-
•
—1 1 1 1 1 1
0 10 20 30 40 50 60 70 80
Tf (°C)

134
5.
Consilient Mechanisms
for
Diverse Protein-based Machines
aggregation
(of
cloudiness) changes remark-
ably with substitution
of
only
4
guest residues
in
100
residues.
As
established above
in
section
5.1.3.4,
an
increased oil-like character lowers
the temperature
for the
onset
of
oil-like aggre-
gation,
and an
increased vinegar-like character
raises
the
temperature
for
onset
of
oil-like
aggregation.
5.3.2.4
A
Scale
for
Measuring Relative
Oil-like Character
Establishing
a
scale requires
the
choice
of ref-
erence conditions. We choose
one
guest residue
per pentamer
as the
reference.
Now, for the
most oil-like residues
the
temperature
for
onset
of aggregation
for one
guest residue
per pen-
tamer
is
below
the
freezing point
of
water,
and
for the
very vinegar-like residues
the
tem-
perature
for
aggregation
for one
vinegar-like
residue
per
pentamer would
be
above
the
boiling point
of
water.
In
such cases, fewer guest
residues
are
used,
for
example,
one in
five
pen-
tamers
and one in ten
pentamers,
and the
onset
temperatures
for
aggregation
are
plotted.
The
plots give straight lines
as
shown
in
Figure
5.9B,
as
the
fraction
of
guest residue increases.
The
lines
can be
extended
to the
value expected
for
one guest residue
per
pentamer, that is,
for f^ =
1,
and
this value
of Tt
becomes
the
measure
of
relative oil-Hke character.
The relative oil-like character
of
each amino
acid residue
is
given
in
terms
of the
Tt-based
hydrophobicity scale
in
Table
5.1. Tj is
mea-
sured
by
plotting
as
indicated
in
Figure 5.9;
and
the technical term
for
oil-like, hydrophobic
(meaning water fearing),
is
used.
The
more
commonly used technical term, hydrophobicity,
replaces
the
equivalent statement
of
oil-like
character.
5.3.2.5 Effect
of
Making R-groups More
Oil-like Axiom
I
Most simply stated, oil
is a
string
of
CH2 groups.
Adding
a
CH2 group,
or
more generally only
hydrocarbons (carbons,
C, and
hydrogens,
H),
to
the
R-group
of a
protein clearly represents
an increase
in
oil-like character. Changing
the
R-group from
the -CH3 of
alanine (Ala,
A) to
the -CH(CH3)2
of
vaUne
(Val, V) and to the
-CH2CH(CH3)2
of
leucine
(Leu, L)
progres-
sively creates more oil-like model proteins.
Replacing
the X in
poly(GXGVP)
by A,
V,
or
L progressively lowers
the
temperature
for
onset
of
aggregation
of
the model proteins from
45°
C to
24°
C and to
5° C, respectively,
as
shown
by
the
data
in
Figure
5.9 and
listed
in
Table 5.1.
Accordingly,
a
stepwise increase
in
oil-like
character
of the
R-group stepwise lowers
the
temperature
for
aggregation!
Henceforth,
any
R-group substitution cap-
able
of
lowering
the
temperature
for
aggrega-
tion
is
recognized
as
more oil-like than
the
R-group
it
replaced.
A
perspective
so self-
evident warrants statement
as an
axiom.
In the
following axiom,
the
transition
is
defined
by the
temperature interval, where
the
onset tempera-
tures,
noted above, identify
the low
tempera-
ture sides
of the
respective temperature inter-
vals,
as
shown
in
Figures
5.1C and 5.5.
Axiom 1:
The
change
in
temperature interval,
over which occurs
the
oil-like folding
and
assembly transition
of a
host model protein
on introduction
of
different guest substituents,
becomes
a
functional measure of relative oil-like
character
of
the substituents, that
is,
of
their rel-
ative hydrophobicity,
and it
provides
a
measure
of the change
in
free energy
of
the hydrophobi-
cally associated state.
5.3.2.6 Effect
of
Changing
the
State
of
Vinegar-like Side Chains
Above,
the
increase
in
oil-like character
lowered
the
value
of Tt and, by
Equation
(5.9)
for
AGHA(%), lowered
the
free energy
of
the hydrophobically associated state.
The
reverse occurs
for a
series
of
side chains that
increase
the
vinegar-like character. Addition
of
R-groups containing charged amino groups,
-NHB^, like that
of
lysine (Lys,
K) or
containing
charged carboxylates, -COO", like that
of
glutamic acid
(Glu, E)
into poly(GXGVP)
increases
the
temperature
for
aggregation
to
extrapolated values
of
120°
C for X = K and
250°
C for X = E
under
the
conditions described
in more detail below. Accordingly,
a
stepwise
increase
in the
vinegar-like character
of the R-
group stepwise raises
the
temperature
for
