Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


5.1 Introduction 115
Generally applicable statements result on
simply subtracting Equation (5.2) from Equa-
tion (5.1) to give^
AGHA
ix) = [Tt (ref) ASt (ref) - T, (;f) AS^ (x)]
(5.10a)
and as more recently derived^
AGHA
(%) - [AH, (ref) - AH, (%)]
(5.10b)
One should realize that Equation (5.10b)
includes the exothermic heat of van der Waals
contacts due to the folding and assembly of the
model protein during the transition. The asso-
ciation of model protein during the phase sep-
aration would be exothermic. The endothermic
heats obtained from the inverse temperature
(phase) transition arise from the conversion of
hydration of oil-like groups to bulk water.
Accordingly, the AGHA(Z) values calculated
directly from the heat of the inverse tempera-
ture transition, as in Equations (5.10a) and
(5.10b),
would be minimal values when consid-
ered as the change in Gibbs free energy for con-
version of hydrophobic hydration to bulk water
(See Figure 8.1 and associated text).
5.1.3.4.4 Moving the Trdivide by Replacing V
by Other Naturally Occurring Amino Acids
Whatever amino acid replaces the valine (Val,
V) residue, there results a change in the value
of
Tj.
This dependency of T, becomes a measure
of the relative hydrophobicity of the different
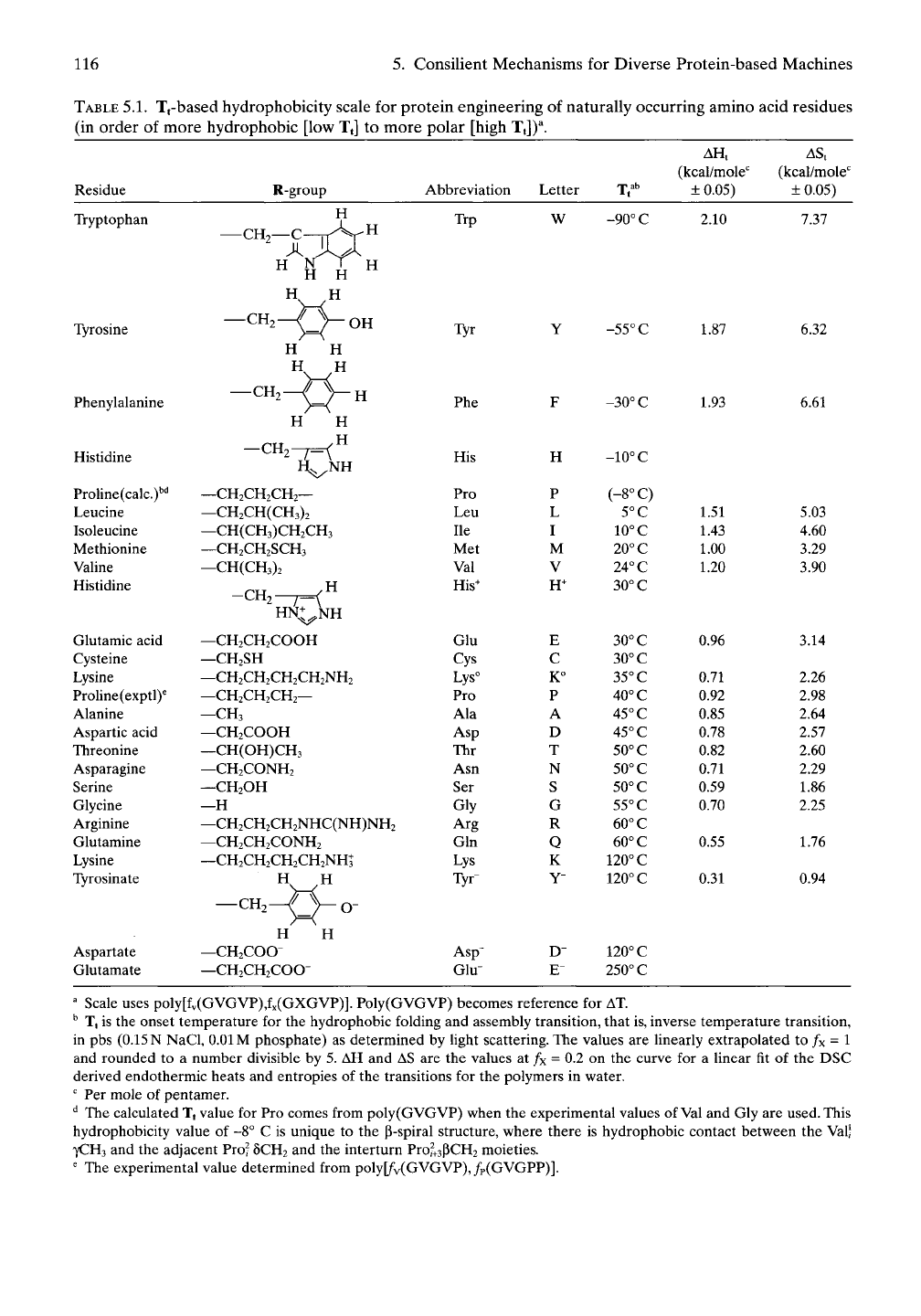
naturally occurring amino acid residues (see
Table 5.1), called the Tj-based hydrophobicity
scale."^ Design of protein function by use of the
Tt-based hydrophobicity scale requires knowl-
edge of Tt only. Changing Tt provides a simple
on-off switch for achieving function. Lowering
Tt from above to below the operating temper-
ature drives hydrophobic association, and
raising Tt from below to above the operating
temperature results in hydrophobic
dissociation.
5.1.3.4.5 Moving the Tt-divide Without
Changing the Amino Acid Composition
There are many ways, in addition to changing
the amino acid composition of the model
protein, to change the location of the Tt-divide.
They fall into two main classes. One class is to
have functional groups attached to the protein
that can exist in two different states, for
example, charged or uncharged, oxidized or
reduced, phosphorylated or dephosphorylated,
and so forth. The second class is to change the
composition of the solvent in ways that alter the
solvation of the oil-like groups without acting
by changing the state of a functional side chain.
Each of these means for moving the Tt-divide
constitute energy inputs, and AGHA(X), ^S iden-
tified above, becomes the energy output. Each
energy input resulting in a
AGHA(3C),
depending
on the sign of AGHA(X), drives hydrophobic
association (when negative) or dissociation
(when positive). For cross-linked matrices, this
is equivalent to contraction or relaxation,
respectively, to produce motion. A negative
AGHA(Z)
produces contraction, and a positive
AGHA(Z)
results in relaxation. Examples of
many energy inputs, as evidenced by changing
Tt, are very briefly noted immediately below.
5.1.3.4.6 Moving the Tt-divide by Adding
Protons to Carboxylates
Protonation, adding a proton, to the carboxy-
late group of the glutamic acid residue, or an
aspartic acid residue, lowers the value of Tt and
represents a chemical energy input that is one
of the most effective ways to drive association
of oil-hke groups, that is, to result in the
mechanical energy output resulting from con-
traction. Protonation of a carboxylate, -COO"
-h H^ ^ -COOH, represents an energy input
similar to reduction of a nicotinamide, and
these are exceeded only by removal of a phos-
phate group in their effectiveness in lowering
the value of Tt and consequently in producing
a negative change in AGHA(Z), with which to
drive hydrophobic association and its equiva-
lent of contraction (see sections 5.14 and 5.15).
5.1.3.4.7 Moving the Tt-divide by Adding
Electrons to Bound Oxidized Group
Reduction of an oxidized nicotinamide drives
association of oil-like groups and is similar
in effectiveness to protonation of a carboxy-
late in lowering the value of Tt. In oxidative
116
5.
Consilient Mechanisms for Diverse Protein-based Machines
TABLE
5.1. Tj-based hydrophobicity scale for protein engineering of naturally occurring amino acid residues
(in order of more hydrophobic [low TJ to more polar [high TJ)^.
Residue R-group Abbreviation Letter
AHt
(kcal/mole''
Tt^' ± 0.05)
ASt
(kcal/mole''
± 0.05)
Tryptophan
H
-c„,-c
H
Trp
Tyrosine
Phenylalanine
Histidine
Proline(calc.)^'^
Leucine
Isoleucine
Methionine
Valine
Histidine
Glutamic acid
Cysteine
Lysine
Proline(exptl)^
Alanine
Aspartic acid
Threonine
Asparagine
Serine
Glycine
Arginine
Glutamine
Lysine
Tyrosinate
Aspartate
Glutamate
H :^ J_ H
H H
H H
—™2^p^OH
H H
H H
H H
H^NH
—CH2Cri2Crl2—
-CH2CH(CH3)2
—CH(CH3)CH2CH3
—CH2CH2SCH3
-CH(CH3)2
-CH,^=("
HN;^NH
—CH2CH2C00H
—CH2SH
—CH2CH2CH2CH2NH2
—Cri2CH2Cri2—
—CH3
—CH2COOH
—CH(OH)CH3
—CH2CONH2
—CH2OH
—H
—CH2CH2CH2NHC(NH)NH2
—CH2CH2CONH2
—CH2CH2CH2CH2NH^
H H
H H
—CH2COO-
—CH2CH2COO-
Tyr
Phe
His
Pro
Leu
He
Met
Val
His^
Glu
Cys
Lys°
Pro
Ala
Asp
Thr
Asn
Ser
Gly
Arg
Gin
Lys
Tyr-
Asp'
Glu-
w
H
D-
E"
-90° C
-55° C
-30° C
-10°
C
120° C
250° C
2.10
1.87
1.93
p
L
I
M
V
H^
E
C
K°
P
A
D
T
N
S
G
R
Q
K
Y-
(-8°C)
5°C
10° C
20° C
24°
C
30°
C
30°
C
30°
C
35° C
40° C
45° C
45°
C
50°
C
50° C
50° C
55°
C
60°
C
60°
C
120°
C
120°
C
1.51
1.43
1.00
1.20
0.96
0.71
0.92
0.85
0.78
0.82
0.71
0.59
0.70
0.55
0.31
7.37
6.32
6.61
5.03
4.60
3.29
3.90
3.14
2.26
2.98
2.64
2.57
2.60
2.29
1.86
2.25
1.76
0.94
^ Scale uses poly[fv(GVGVP),fx(GXGVP)]. Poly(GVGVP) becomes reference for AT
^ T| is the onset temperature for the hydrophobic folding and assembly transition, that is, inverse temperature transition,
in pbs
(0.15
N NaCl,
0.01
M phosphate) as determined by light scattering. The values are linearly extrapolated to /x = 1
and rounded to a number divisible by 5. AH and AS are the values at fx = 0.2 on the curve for a linear fit of the DSC
derived endothermic heats and entropies of the transitions for the polymers in water.
"^ Per mole of pentamer.
'^ The calculated Tt value for Pro comes from poly(GVGVP) when the experimental values of Val and Gly are used. This
hydrophobicity value of -8° C is unique to the p-spiral structure, where there is hydrophobic contact between the Val)
7CH3 and the adjacent Pro? 5CH2 and the interturn Pro^+3pCH2 moieties.
' The experimental value determined from poly[/v(GVGVP),/p(GVGPP)].

5.1 Introduction 117
phosphorylation of the inner mitochondrial
membrane, the relationship between the
change of state of a redox group and proton
transport is central to the process of develop-
ing the proton gradient used to drive phospho-
rylation. As discussed in Chapter 8, section
8.3.4, during analysis of the structure and func-
tion of Complex III: ubiquinone: cytochrome c
oxidoreductase (the cytochrome bci complex)
of the electron transport chain of the inner
mitochondrial membrane, the oxidation of
ubiquinol to produce a positively charged
species and the reduction of ubiquinone to
produce a negatively charged species both
disrupt hydrophobic association to effect the
release of two protons from the former and the
uptake of two protons to the latter in effecting
proton transport. In this manner and in the
context of Tt, both the formation of positive
charge and the formation of negative charge
raise the value of Tt to disrupt hydrophobic
association and thereby to allow, respectively,
proton egress into the cytosol and ingress from
the matrix for net transport across the mem-
brane. In a different context, irreversible oxi-
dation of protein components represents a
means of inactivating protein function by irre-
versibly driving hydrophobic dissociation.
5.1.3.4.8 Moving the Trdivide by
Phosphorylation/Dephosphorylation
Phosphorylation, the covalent attachment of a
phosphate to an OH group, has been to date the
most effective way to raise the temperature of
the Tt-divide.Thus, dephosphorylation, removal
of phosphate, has been the most effective way
to lower the temperature of the Tt-divide and
thereby the most effective way to drive
hydrophobic association and its equivalent of
contraction. This is similar to a primary event in
muscle contraction (see Chapters 7 and
8).
The
shift in the Tj-divide on binding of ATP can be
as great as or greater than simple phosphoryla-
tion, depending on the interactions of ATP at
the binding site. As discussed in Chapter 8,
section 8.5, ATP binding drives hydrophobic
dissociation, whereas loss of phosphate drives
hydrophobic association both for the attach-
ment to actin and for the power stroke to
produce motion by the myosin II motor of
muscle contraction.
5.1.3.4.9 Moving the Tt-divide by Ion
Pairing with Oppositely Charged Vinegar-like
R-groups
The ion pairing of sodium ion, Na^, with nega-
tive carboxylate, COO", that is, COO"* • •Na^,
and the ion pairing of the ammonium ion func-
tional group of lysine (Lys, K) with the chloride
ion, that is, -NHa^ • • •CI", markedly lower
the Tt-divide. The effectiveness of ion pairing
in lowering the Tt-divide increases as the
hydrophobicity (oil-like character) of the
domain increases with which the charged
group is associated. Interestingly, chloride ion
(CI") bridges between the Val^(a-NH3^) and the
Arg^^^(guanidinium^) in the ion-pairing
network between subunits of deoxyhemoglo-
bin, and the hydrophobic association of the
deoxy state is further stabilized by the replace-
ment of the a-NHs^* • •CI" with the carbamate,
R-NH-COO", resulting from CO2 pick up in the
tissues (see Chapter 7). The most effective ion
pairing involves association, within a hydropho-
bic domain, of calcium ion with a pair of car-
boxylates, that is, -COO"* • •Ca^^* • •OOC-.
Again, this has a familiar ring as the triggering
event for muscle contraction (see Chapters 7
and 8).
5.1.3.4.10 Moving the Tfdivide by Changing
the Solvent, That Is, Adding Salt to
the Solution
Even when there are no vinegar-like (func-
tional) groups in the model protein, as is the
case for poly(GVGVP), adding salt, NaCl,
lowers the Tt-divide. This effect is very anemic,
however, requiring an order of magnitude
(some 10 times) more salt than when there is a
functional group with which to ion pair in order
to drive hydrophobic association.
5.1.3.4.11 Moving the Tt-divide by Changing
the Solvent, That is, Adding Organic Solutes
to the Solution
The most effective organic solute in raising the
Tfdivide is sodium dodecyl sulfate (SDS).

118
5.
Consilient Mechanisms for Diverse Protein-based Machines
Much less effective is guanidinium hydrochlo-
ride,
followed by urea. Glycerol and ethylene
glycol have only a small effect in lowering the
Tfdivide, and trifluoroethanol is somewhat
more effective in lowering the Tfdivide. On this
basis,
the effectiveness of SDS polyacrylamide
(SDS-PAGE) gels in achieving protein separa-
tions based on size becomes apparent.
5.1.3.4.12 Moving the Tfdivide by Increasing
the Pressure
Especially when there are amino acid residues
with aromatic side chains, such as tryptophan
(Trp,
W), phenylalanine (Phe, F), and tyrosine
(Tyr, Y), increasing the pressure raises the tem-
perature of the Tj-divide and favors protein
unfolding (see the structures of the R-groups in
Table 5.1).
5.1.3.4.13 Moving the Tfdivide by a Bound
Chromophore That Changes Its Oil-like
Character on Absorbing Light
Any chromophore attached to the protein
that changes its hydrophobicity on absorbing a
photon of light will change the temperature of
the Tt-divide. The absorption of light is unique
among the energy conversions of the consilient
mechanism in that it is irreversible, for example,
stretching does not generally cause the emis-
sion of
light.
To
the best of our knowledge, there
is no simple reversal of a consilient process
driven by the absorption of light that can result
in the emission of the same frequency of elec-
tromagnetic energy. The energy of a relevant
photon is some 70kcal/mole, whereas the
energy output, AGHA(X)' will be an order of
magnitude less, that is, the energy changes of
biology are generally within ±8kcal/mole.^^
5.1.3.5 What Causes Loss of Solubility on
Raising the Temperature from Below to
Above the Trdivide?
By definition of the change in Gibbs free
energy, AG, solubiUty occurs when AG(solubil-
ity) is negative, and solubiUty is lost as AG(sol-
ubility) becomes positive. Here we restate that
shown very early by Butler.^^ AH is negative
and -TAS is positive when oil-like groups dis-
solve in water, that
is,
when hydrophobic hydra-
tion forms. Specifically, the addition of the four
CH2 moieties in the soluble alcohol series from
methanol to n-pentanol results in an average
AH/CH2 of -1.4kcal/mole-CH2, that is, forma-
tion of hydrophobic hydration results in a
favorable release of heat. But (-TAS/CH2) is
-hl.7kcal/mole-CH2. This is why solubility in
water is ultimately lost when enough CH2 moi-
eties have been added such that AG has become
positive.
Accordingly, for a fixed amount of hydropho-
bic hydration, simply raising the temperature
linearly increases the magnitude of the positive
(-TAS) term until it becomes greater than the
negative AH term, that is, until AG becomes
positive and solubility is lost. The result is the
association of hydrophobic groups as an inte-
gral part of the inverse temperature transition of
the hydrophobic folding and assembly transi-
tion. Solubility is lost, which is readily seen in
our model proteins as
a
phase separation, simply
because increasing the temperature increases
the magnitude of the positive
(-TAS)
term until
it becomes greater than the negative AH term.
Contrary to common description, solubility is
not lost because of thermal destructuring,
melting, or disordering of the hydrophobic
hydration.^^ As shown below in section 5.7.6
and Figure 5.27, the Tj-divide occurs at a higher
temperature when the amount of hydrophobic
hydration for a model protein composition
becomes less.
5.1.3.6 An Experimental Method
for Following Changes in
Hydrophobic Hydration
Improved insight into the role of hydrophobic-
ity in protein folding, assembly, and function
requires an experimental means with which to
estimate and follow changes in hydrophobic
hydration as a function of different variables.
Since the work of Butler^^ and of Frank and
Evans,^^ the search has been for an experimen-
tal method with which to characterize changes
in hydrophobic hydration and particularly to
do so during changes in functional state of
the protein. This goal was reached with the

5.1 Introduction
119
model proteins of focus here in part because
conditions are possible where most of the
water in the system can be hydrophobic
hydration.^^
The experimental method is microwave
dielectric relaxation, which uses the same
energy as that of a kitchen microwave oven.
Hydrophobic hydration absorbs energy in the
microwave frequency range at a slightly lower
frequency than liquid water absorbs energy in
microwave ovens to heat foods. It is now possi-
ble to follow the loss of hydrophobic hydration
as insolubility takes place, that is, as hydropho-
bic association occurs, which includes intra-
molecular hydrophobic folding and/or inter-
molecular assembly. Most significantly, in
section 5.7.3 and Figures 5.24 and 5.25, we can
see the loss of hydrophobic hydration as
hydrophobic association occurs and as vinegar-
hke groups ionize and take limited hydration
away from hydrophobic groups in the process
of obtaining their own hydration.
5.1.3.7 In Summary, Hydrophobic
Hydration Disappears for Two
Different Reasons with Opposite
Consequences of Insolubility
and Solubility
5.1.3.7.1 Hydrophobic Hydration Increases
with Increased Number of Oil-Uke Groups
Until Solubility Is Lost and Then
Hydrophobic Hydration Disappears
Hydrophobic hydration increases as the
number of oil-like groups increases, and then
it abruptly disappears when insolubility is
reached. As shown above with the expression
AG(solubility) = AH - TAS, insolubility occurs
as the positive (-TAS) term becomes greater
than the negative AH term. This is one reason
for the disappearance of hydrophobic hydra-
tion. Knowledge of the source of insolubility
due to hydrophobic association provides one of
the two primary keys to understanding changes
in protein folding during function. De novo
design of elastic-contractile model proteins for
diverse energy conversions using the Tt-based
hydrophobicity scale demonstrates the useful-
ness of this view.
5.1.3.7.2 Hydrophobic Hydration is
Destroyed as Proximal Vinegar-like Species
Achieve Hydration on Becoming Charged
Formation of charged species, by the very act
of requiring more hydration when ionized,
destroys proximal hydrophobic hydration in
the process of becoming charged. Consider a
spatially localized association of oil-like
domains in water. As the clusters of oil-like
groups representing each domain begin to dis-
sociate, hydrophobic hydration forms. As too
much hydrophobic hydration forms, the posi-
tive (-TAS) term becomes larger than the neg-
ative AH term, resulting in a positive AG, and
the association reforms. If, however, a proximal
charged species should form concurrently, the
nascent hydrophobic hydration is destroyed as
the ionizing vinegar-like species gains its hydra-
tion. The result is a negative AG and the disso-
ciation stands, that is, solubility occurs. To be
sure,
water molecules can continue to be adja-
cent to the exposed hydrophobic groups, but in
this case the water molecules may no longer
have the structure represented in Figure 2.8. In
our view, this is the second of the two primary
keys to understanding changes in protein
folding in the process of function. Experimen-
tal studies on model proteins, designed to
perform diverse energy conversions, as reviewed
in this chapter, exemplify these explanations.
5.1.4 Heating/Cooling Pumps Iron!
5.1.4.1 Raising the Temperature from
Below to Above the Temperature Interval
of the Phase Transition Produces Folding
and Association with the Result of Motion
The phase separated state of the parent elastic
protein-based polymer, (GVGVP)n/^ at 37° C is
about 50% polymer and 50% water by weight.
This was the composition for the transitions
represented in Figure 5.2. On exposure of this
state to 20 Mrad^^ of y-irradiation, a nearly ideal
elastic band forms^^ with properties similar to
those of the major elastic arteries. On lowering
the temperature to 25° C or below, the elastic
band swells and increases its volume 10-fold.
A return to body temperature, 37° C (98.6° F),

120
5.
Consilient Mechanisms for Diverse Protein-based Machines
causes the band to contract by hydrophobic
association, which is to deswell. The y-
irradiation cross-Unked (GVGVP)n forms an
elastic-contractile band.
We then hang a weight on the elastic band at
25° C
and it stretches until the elastic force due
to deformation matches the attached weight.
We then raise the temperature to
37°
C;
the
band contacts and lifts the weight. Insignificant
contraction occurs below
25°
C,
and insignifi-
cant contraction occurs above
37°
C.
In the tem-
perature interval from 25° to
37°
C,
thermal
energy converts to the mechanical work of
lifting the weight (see Figures 2.4, 2.5, and
2.6A). This is pumping iron, which we all rec-
ognize as work. When thermal energy pumps
iron,
the technical term becomes thermo-
mechanical transduction. Thermal energy, by
way of this model protein-based machine, con-
verts into mechanical work.
5.1.4.2 Graphic Representation of
a
Transition from One State to Another as
Temperature Is Raised from Below to
Above a Temperature Interval
Figure 5.5A plots the independent variable,
temperature, and the resultant change in the
dependent variable, contraction. As noted
above, Httle change occurs below and little
change occurs above the temperature interval.
Also,
as noted below, adding chemical energy in
the form of increased concentration of salt
lowers the temperature range of the tempera-
ture interval.This
represents in graphic form the
depiction of thermally driven contraction in
Figure 2.4.
5.1.5 Pumping Iron without
Changing Temperature
5.1.5.1 Using Salt with an Uncharged
Polymer to Move the Temperature Interval
Over Which Thermally Driven
Contraction Occurs
The addition of
58
grams of NaCl, table salt, to
1 liter of water containing the elastic band of
cross-linked (GVGVP)n lowers the
temperature
interval for the phase separation by
15°
C.
(This is approximately equal to dissolving the
entire contents of the common grocery store
container of table salt in 2 gallons of water.)
Now, raising the temperature from 10° to
25°
C
in this 1N salt solution causes the elastic strip
to contract and lift a weight. Under these
circumstances no significant contraction
occurred below
10°
C,
and no significant con-
traction occurs above
25°
C.
In the temperature
interval
from 10° to
25°
C,
thermal energy again
performs mechanical work. Now the work of
lifting the weight is complete at
25°
C in
IN NaCl rather than at 37°C in salt-free
water.^^
When we again hang the weight on the
swollen elastic band at
25° C
in pure water and
then add the above noted quantity of salt, the
elastic band contracts and lifts the weight
without heating and without a change in tem-
perature. Further addition of salt at
25°
C has
only a limited effect. Addition of salt consti-
tutes an input of chemical energy, and, under
these circumstances, the addition of chemical
energy results in the performance of mechani-
cal work. The technical term is chemo-
mechanical transduction. Chemical energy, by
means of our model protein-based machine,
produces mechanical work without a change in
temperature. In the absence of a significant
vinegar-Uke R-group, an energy other than heat
can be used to produce mechanical work. In
this case, however, performance of a modest
amount of mechanical work required much
chemical energy. With the correct vinegar-like
R-group and chemical energy input, more effi-
cient chemo-mechanical transduction becomes
possible.
As shown in Figure 5.5B, the indepen-
dent variable can be chemical potential, the
change in Gibbs free energy per mole of chem-
ical added.
5.1.5.2 Using a Chemical Couple to
Lower the Temperature Interval from
Above to Below a Given Temperature
Rather Than Raising the Temperature
Living organisms function without the changes
in temperature required for thermomechanical
transduction by the consilient mechanism.
What makes the mechanism consilient or per-
vasive, however, is that many different energy

5.1 Introduction 121
inputs acting on many different vinegar-like R-
groups change the temperature interval over
which contraction occurs, and the resulting
energy conversions can be very efficient. Now,
as noted with the salt-driven contraction above,
it is emphasized that energy conversions occur
without a change in temperature.
In general, a change in polymer composition
provides a particular vinegar-like group with
which to access other energies. In this general
case,
the other energy input changes the
vinegar-like group from a less to a more oil-like
state.
The functional group of the major com-
ponent of vinegar provides a very effective
chemical couple, namely, -COOH/-COO".
The addition of acid,
H^,
to -COO" converts the
very vinegar-like carboxylate, -COO", to the
more oil-like -COOH. This protonation of a
single carboxylate group in the 100 residues of
poly(GVGVP) can lower the temperature
interval by tens of degrees centigrade and can
require far less chemical energy to pump iron
than is required in the NaCl example above for
the uncharged polymer. Protonation/deproto-
nation very efficiently pumps iron.
Alternatively, the amino/ammonium chemi-
cal couple, -NH2/-NH3^, functions similarly,
although the change from -NH2 to -NHs^ is
only about half as effective in changing the
value of Tt as is the -COOH/-COO" chemical
couple (see Table 5.1).
5.1.5.3 Electrons, Added to a Bound
Redox Couple, Lower the Temperature
Interval from Above to Below a Given
Temperature
Certain vitamins like B2 (riboflavin) and B3
(niacin) become chemically dressed up for
attachment to proteins. As such these attached
vitamins become redox couples that can accept
electrons (become reduced) and give up elec-
trons (become
oxidized).
A change in the redox
state changes the temperature interval for the
phase transition, that is, moves the Tt-divide.
Just as protonation of a carboxylate lowers the
temperature interval, so too does adding elec-
trons to the oxidized state of a redox couple.
Lowering the temperature interval from above
to below the operating temperature by reduc-
tion drives contraction and performs mechani-
cal work. The technical term is electro-
mechanical transduction. In short, reduction/
oxidation pumps iron.
5.1.5.4 Graphic Representation of the
Generalized Transition Zone for
Contraction Due to a Generalized
Independent (Intensive) Variable
As mentioned above in reference to Figure
5.5A, as the temperature is raised, contraction
of a band composed of elastic-contractile
model protein occurs. Contraction occurs as the
temperature is raised through a temperature
interval. Crossing over the Tfdivide, defined in
Figure 5.3, is to pass through the temperature
interval over which contraction occurs; it is the
result of the phase separation, specifically of
the inverse temperature transition. Further-
more, the temperature interval for contraction
occurs at a lower temperature when the model
protein is more hydrophobic and at a higher
temperature when the model protein is less
hydrophobic.
As noted above, the temperature interval
shifts on changing the concentration of a chem-
ical, that is, on changing the chemical potential
of a chemical for which the model protein is
responsive, as in protonation of a carboxylate.
Similarly, the temperature interval shifts on
changing the availability of electrons, that
is,
on
changing the electrochemical potential, such
that an oxidized component of a redox couple
becomes reduced.
Now, instead of plotting temperature, the
chemical potential or electrochemical potential
can be plotted, and contraction occurs as the
concentration of a chemical passes through the
critical range for reaction or as the availability
of electrons passes through a critical range to
achieve reduction. The range over which the
contraction occurs is the transition zone, and
the plot looks the same as in Figure 5.5A for
temperature. Thus, the graphic representation
in Figure 5.5B is common for all of the inde-
pendent variables, such as temperature, pres-
sure,
and chemical potential, that can drive
contraction, in which case contraction becomes
the dependent variable.

122
5.
Consilient Mechanisms for Diverse Protein-based Machines
In this perspective, the independent variables
are the intensive variables of the free energy.
The input energy to drive contraction, for
example, is the product of two quantities, the
intensive variable times the extensive variable.
In the case of protonation of a carboxylate, the
intensive variable of chemical potential is pro-
portional to the change in concentration of the
protons over which the contraction occurs, and
the extensive variable is the number of protons
utilized in driving the contraction. In the case
of the intensive variable of temperature, the
extensive variable is the entropy of the transi-
tion (ASt), that is, the amount of heat (calories)
divided by the temperature, and the input
energy, of course, is simply the heat of the tran-
sition, AHt (= TtASt) at the Tj-divide.
5.1.6 Energy Conversions in Addition
to Pumping Iron
5.1.6.1 Energy Conversions Not Involving
Mechanical Work
There are at least six kinds of free energy that
interconvert by the consilient mechanism. They
are mechanical, thermal, pressure-volume,
chemical, electrical, and electromagnetic
energy, for example, light, and the correspond-
ing intensive variables are mechanical force,
temperature, pressure, chemical potential, elec-
trochemical potential, and electromagnetic
radiation frequency. Above we noted only three
energies (thermal, chemical, and electrical) of
the five that can provide input energy for the
performance of mechanical work. In fact any
pair of the five, leaving mechanical energy
aside, interconvert one into the other by the
consilient mechanism, as treated more fully
below in section 5.5. Immediately below we
note interconversion of the pair of energies,
chemical and electrical. In fact, some 18 classes
of pairwise energy conversions occur by the
consilient mechanism (see section 5.6).
5.1.6.2 Conversion of Electrical Energy
into Chemical Work and Vice Versa
When a properly designed model protein con-
tains both protonation/deprotonation chemical
couples and reduction/oxidation redox couples,
reduction drives contraction and protonation
drives contraction. Most importantly, how-
ever, reduction (electrons) drives proton (acid)
uptake, and protonation drives electron uptake.
The former is electro-chemical transduction,
and the latter is chemo-electrical transduction.
This is the nature of the consilient mechanism
as further described below. Two distinct energy
sources, each of which performs mechanical
work, can interconvert by effecting the changes
in hydrophobic folding and assembly of the
consilient mechanism.
5.1.7 The Consilient Mechanism in
a Nutshell: The Comprehensive
Hydrophobic Effect
5.1.7.1 Hydrophobic Replaces Oil-like
Hydrophobic, meaning water fearing, is the
more technical term for oil-like. Instead of
speaking of water surrounding oil-like group-
ings of atoms, or oil-like hydration, the term
hydrophobic hydration is generally used. Even
in the scientific community, however, those
not directly active in the field find the term
hydrophobic hydration contradictory and
troublesome. Nonetheless, hydration of hydro-
phobic groups does occur, as shown in the
pentagonal arrangements of water molecules in
Figure 2.8 surrounding an oil-like group, and
understanding the energetics of hydrophobic
hydration is central to the consilient mecha-
nism for function of protein-based machines.
Several key aspects of hydrophobic hydration
that constitute the comprehensive hydrophobic
effect are briefly noted in this section and then
expanded upon in section 5.7.
5.1.7.2 The Comprehensive Hydrophobic
Effect Results from the Actual or Potential
Hydrophobic Hydration That Can Occur
for a Pair of Associable Hydrophobic
Surfaces or Domains
If a pair of hydrophobic domains capable of
association and dissociation would have too
much hydrophobic hydration for a given tem-

5.1 Introduction 123
perature when fully exposed to water, the result
is insolubility of the domains, that is, hydropho-
bic association of the domains with loss of the
hydrophobic hydration. In our analysis we use
the change in Gibbs free energy, AG, which
governs solubility under common experimental
conditions of constant temperature and pres-
sure.
To reiterate, AG involves two terms. The
first term is the heat change (AH) that occurs,
for example, as the molecule dissolves in water.
When heat is released on dissolution, AH is neg-
ative. Release of heat on addition to water indi-
cates solubility. The second term is -TAS, where
AS is the entropy change on dissolution. In par-
ticular, AG(solubility) = AH - TAS. For the
result of insolubility of hydrophobic domains,
the (-TAS) term is positive and of greater mag-
nitude than the negative AH term.
5.1.7.3 Competition for Hydration
Between Apolar (Hydrophobic) and Polar
(e.g., Charged) Groups Controls Amount
of Hydrophobic Hydration
If a charged species appears proximal to
the associated pair of hydrophobic domains
(within a few nanometers) as the pair of
domains exhibit an opening fluctuation, the
ionizing species destructures the nascent
hydrophobic hydration in achieving its own
hydration."^'^^ The decrease in hydrophobic
hydration (in the number of pentagonally
arranged water molecules) results in a smaller
positive (-TAS) term, the negative AH term
dominates, and the hydrophobic domains dis-
sociate. Also, of course, association occurs at a
higher temperature when the positive (-TAS)
term becomes larger than the negative AH
term, that is, Tt is at a higher temperature.
5.1.7.4 The Competition for Hydration
Also Is Responsible for Large
Hydrophobic-induced pKa Shifts with
Positive Cooperativity That Results in
Efficient Protein-based Machines
Competition for hydration between polar (e.g.,
charged) and hydrophobic (apolar) groups,^^
called the apolar-polar repulsive free energy of
hydration, AGap, controls the amount
of hydrophobic hydration."^'^^ Competition for
limited hydration can result in large pKa
shifts,
because the vinegar-like group must do
the work of destroying hydrophobic hydration
in the process of obtaining its own adequate
hydration."^^'"^^ In particular, carboxyls occur as
part of hydrophobically associated domains
that undergo opening and closing fluctuations.
During an opening fluctuation, a carboxyl has
the opportunity to ionize, but if the hydropho-
bic hydration is too great it recovers its proton.
This continues until the pH is so high that the
chance of proton recovery is too small, and a
carboxylate forms, destructuring hydrophobic
hydration to do
so.
The first carboxylate to form
reached out a significant distance in gathering
its hydration shell. The next carboxyl that
has the opportunity to form a carboxylate
during an opening fluctuation finds it has less
hydrophobic hydration to destructure, and
as a consequence its probability of remaining
ionized increases. Thus the probability for the
third carboxyl to remain ionized is even
greater, and so on. This is positive cooperativity
described at the molecular level. The positive
cooperativity arises out of competition for
hydration between hydrophobic and charged
groups, and the mechanism requires that the
energetics of hydrophobic-induced pKa shifts
parallel the energetics reflected in the Hill
coefficient that describes cooperativity. As
shown in Figures 1.2 and 1.3 for Model proteins
i, ii, iii, iv, and v and as will be further con-
sidered in section 5.8, nonlinear increases in
pKa resulting from linear increases in hydro-
phobicity parallel nonlinear increases in Hill
coefficients.
5.1.7.5 Competition by Positively and
Negatively Charged Species for Hydration
that Is Too Limited by Strongly Held
Hydrophobic Hydration is Responsible for
Ion Pair Formation in Protein Folding and
Assembly
Consider an ion pair-containing hydrophobic
association induced to open, due to the emer-
gence of a new proximal polar (e.g., charged)
species. These newly emergent charged species

124
5.
Consilient Mechanisms for Diverse Protein-based Machines
(formerly ion paired in the associated hydro-
phobic domains) propagate destruction of
hydrophobic hydration, causing further re-
moved hydrophobic domains to dissociate. In
this manner, hydrophobic disassociation with
emergence of separated positive and negative
groups propagates hydrophobic dissociation
outv^ard many nanometers in a domino-like
effect. As a consequence, hydrophobic dissoci-
ations occur in a positively cooperative manner
with the result of efficient free energy
transduction."^^
Thus,
common in the consilient mechanism is
formation of ion pairs, the association of nega-
tively charged with positively charged vinegar-
like groups, each of which are part of an oil-like
domain. The association of oppositely charged
groups from two separate oil-like domains
occurs as the oil-like property of the domains
gains dominance, but then dissociation occurs
as the pair of oil-like domains lose dominance
on introduction of a new more polar group, for
example, as a charged vinegar-like component
is introduced into the vicinity of ion-pair-
containing hydrophobic domains.
5.1.7.6 Multidimensional Representations
of Protein-based Polymer Function as
Molecular Machines
5.1.7.6.1 Seven-dimensional Phase
Transitional Space for Protein-based Polymer
Function as Molecular Machines
The position of the Tt-divide that separates
soluble from insoluble (hydrophobically associ-
ated) states in the phase diagram depends on
seven variables: on the six intensive variables"^^
of temperature, chemical potential, electro-
chemical potential, mechanical force, pressure,
and electromagnetic radiation, and on polymer
volume fraction or concentration. Therefore,
diverse protein-catalyzed energy conversions
by the consilient mechanism result from
designs that control the location of the Tfdivide
in this seven-dimensional phase transitional
space. Complete mathematical description has
yet to be written for representation of the Tt-
divide in seven-dimensional phase transitional
space, but it may prove to be more relevant to
molecular machines to develop representation
of energy conversion in multidimensional free
energy space.
5.1.7.6.2 Multidimensional Free Energy
Space for Protein-based Polymer Function as
Molecular Machines
On inclusion of the six energies interconverted
by the consilient mechanism (see Figure 5.2,
below) with the concentration dependence
results in a seven-dimensional free energy
space. In this connection a relationship between
the magnitude of the shift of the Tj-divide
and the change in Gibbs free energy of the
protein-based polymer is given in Equation
(5.9).
Knowledge of the relevant ASt(reference)
in Equation (5.9) for a given variable, %,
would allow calculation of the complete %-
dimensional, energy conversion landscape of
relevance to a specific protein-based machine.
More comprehensive yet would be to break
down each of the six energies into their
intensive and extensive variables. In this case,
the general description for energy conver-
sion becomes a 13-dimensional, free energy
landscape for energy conversion by diverse
protein-based machines. In practice, graphic
representations usually plot two dimensions
or at most three dimensions. Nonetheless,
complete description of this multidimensional
dependency is required for the desired level of
understanding of protein function and in order
most effectively to engineer diverse protein-
based machines.
5.2 Molecular Structure and
Elasticity of Model Protein
Early reviews are available on the development
of the molecular conformation of the tetrapep-
tide,
pentapeptide, and hexapeptide repeating
sequences of elastin'^^'*^' and on the mechanism
of elasticity of the basic elastic-contractile
model protein."^^'"^^ These may be sought for
historical background. Recent reviews of
the mechanism of elasticity"^^"^^ and its relation
to contractility^ may be examined for a more
