Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.

5.1 Introduction
105
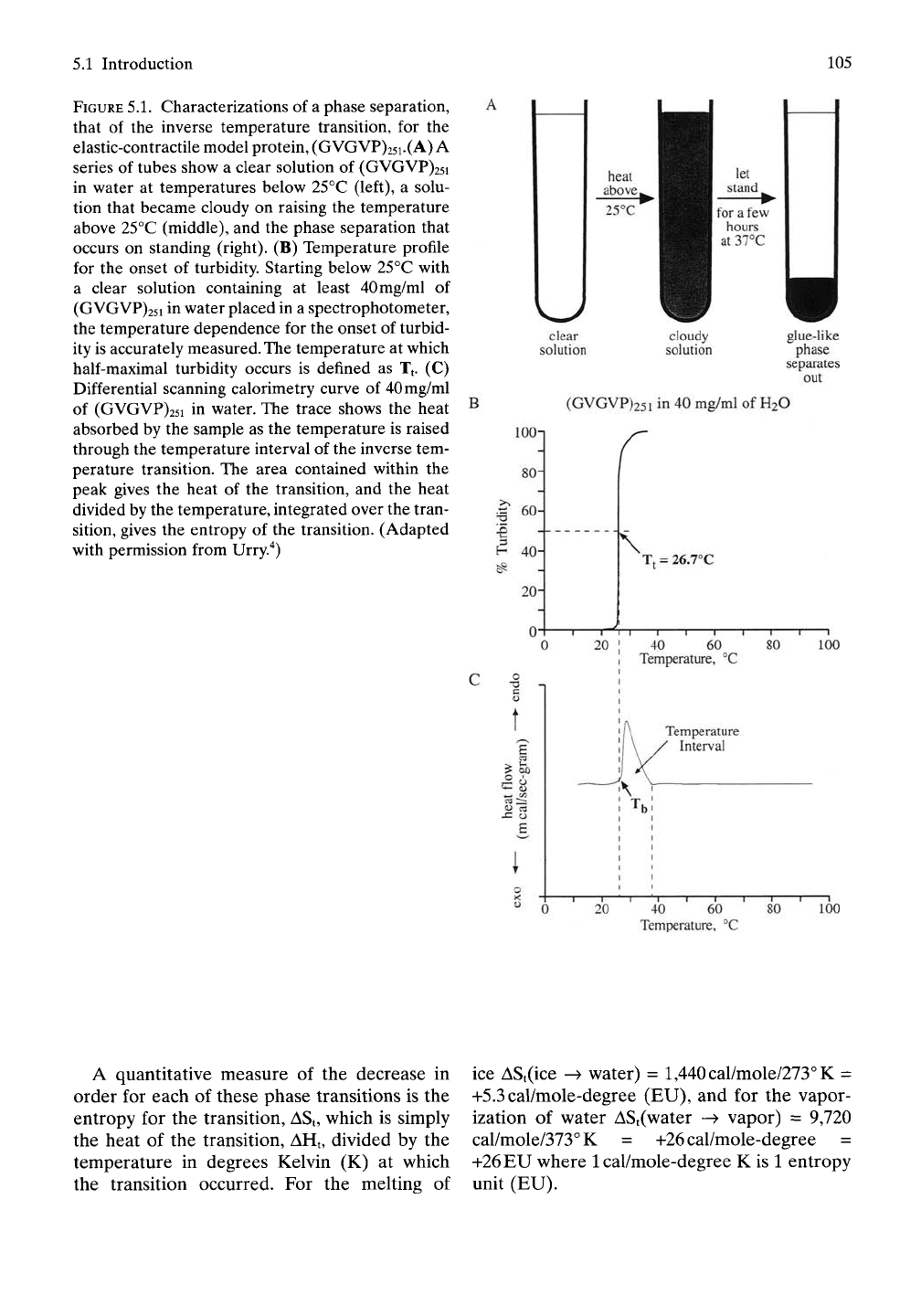
FIGURE
5.1.
Characterizations of
a
phase separation,
that of the inverse temperature transition, for the
elastic-contractile model protein, (GVGVP)25i.(A) A
series of tubes show a clear solution of (GVGVP)25i
in water at temperatures below 25°C (left), a solu-
tion that became cloudy on raising the temperature
above 25°C (middle), and the phase separation that
occurs on standing (right). (B) Temperature profile
for the onset of turbidity. Starting below 25°C with
a clear solution containing at least 40mg/ml of
(GVGVP)25i in water placed in a spectrophotometer,
the temperature dependence for the onset of turbid-
ity is accurately
measured.
The
temperature at which
half-maximal turbidity occurs is defined as Tj. (C)
Differential scanning calorimetry curve of 40mg/ml
of (GVGVP)25i in water. The trace shows the heat
absorbed by the sample as the temperature is raised
through the temperature interval of the inverse tem-
perature transition. The area contained within the
peak gives the heat of the transition, and the heat
divided by the temperature, integrated over the tran-
sition, gives the entropy of the transition. (Adapted
with permission from Urry."^)
clear
solution
cloudy
solution
glue-like
phase
separates
out
(GVGVP)25i in 40 mg/ml of H2O
20 40 60 80 100
Temperature, °C
A quantitative measure of the decrease in
order for each of these phase transitions is the
entropy for the transition, ASt, which is simply
the heat of the transition, AHt, divided by the
temperature in degrees Kelvin (K) at which
ice ASt(ice -^ water) = 1,440 cal/mole/273°K =
-1-5.3
cal/mole-degree (EU), and for the vapor-
ization of water ASt(water -> vapor) =
9,720
cal/mole/373° K =
-i-26
cal/mole-degree =
-1-26
EU where
1
cal/mole-degree K is 1 entropy
the transition occurred. For the melting of unit (EU).
106
5.
Consilient Mechanisms for Diverse Protein-based Machines
Schematic Representation of Transitions of the
poly(GVGVP)-water system
(0
Q.
(0
O
c5
o
X
/u-^
60-
50-
40-
30-
20-
10-
0-
-2
3 ASt(soln-^hase separated) = AH/Tt
E +1.2/303%+4.0 EU; Factor 409/18 = 23
(DO 1 ,_
-1 .i
O
A « 1
°-- IS 2
Bo 2 <D
2 -a 3 ^1
1
1 >3 -c
/ c ^
1 -
>
A
1 k_
CO
•D
3
.5"
Jl :: ^Jq
0
T 1 1 1 1 r
0 20 40 60 80 IOC
)
1^
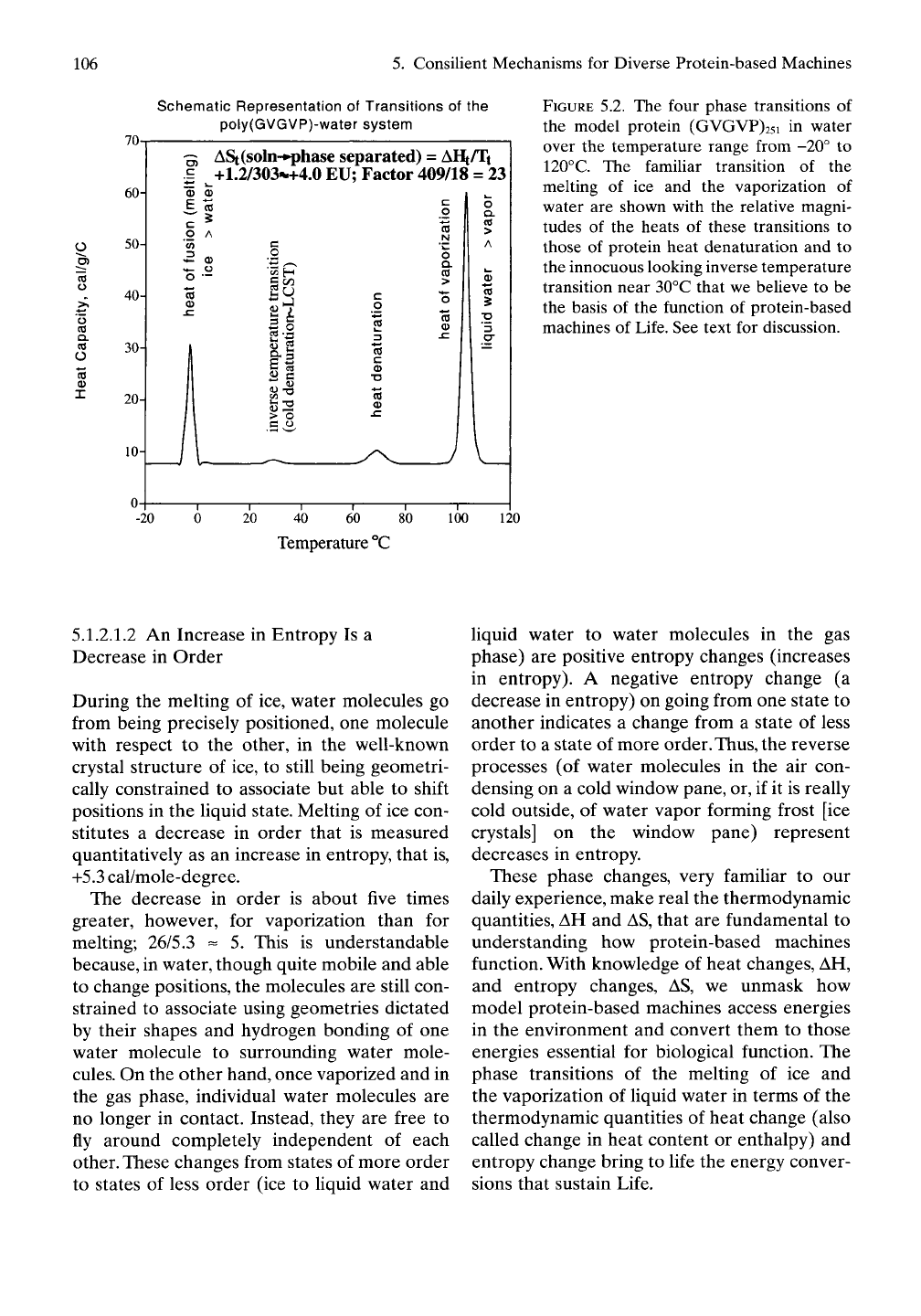
FIGURE 5.2. The four phase transitions of
the model protein (GVGVP)25i in water
over the temperature range from -20° to
120°C. The familiar transition of the
melting of ice and the vaporization of
water are shown with the relative magni-
tudes of the heats of these transitions to
those of protein heat denaturation and to
the innocuous looking inverse temperature
transition near 30°C that we believe to be
the basis of the function of protein-based
machines of Life. See text for discussion.
Temperature °C
5.1.2.1.2 An Increase in Entropy Is a
Decrease in Order
During the melting of ice, water molecules go
from being precisely positioned, one molecule
with respect to the other, in the well-known
crystal structure of ice, to still being geometri-
cally constrained to associate but able to shift
positions in the liquid state. Melting of ice con-
stitutes a decrease in order that is measured
quantitatively as an increase in entropy, that is,
+5.3
cal/mole-degree.
The decrease in order is about five times
greater, however, for vaporization than for
melting; 26/5.3 ~ 5. This is understandable
because, in water, though quite mobile and able
to change positions, the molecules are still con-
strained to associate using geometries dictated
by their shapes and hydrogen bonding of one
water molecule to surrounding water mole-
cules.
On the other hand, once vaporized and in
the gas phase, individual water molecules are
no longer in contact. Instead, they are free to
fly around completely independent of each
other. These changes from states of more order
to states of less order (ice to liquid water and
liquid water to water molecules in the gas
phase) are positive entropy changes (increases
in entropy). A negative entropy change (a
decrease in entropy) on going from one state to
another indicates a change from a state of less
order to a state of more order.
Thus,
the reverse
processes (of water molecules in the air con-
densing on a cold window pane, or, if it is really
cold outside, of water vapor forming frost [ice
crystals] on the window pane) represent
decreases in entropy.
These phase changes, very familiar to our
daily experience, make real the thermodynamic
quantities, AH and AS, that are fundamental to
understanding how protein-based machines
function. With knowledge of heat changes, AH,
and entropy changes, AS, we unmask how
model protein-based machines access energies
in the environment and convert them to those
energies essential for biological function. The
phase transitions of the melting of ice and
the vaporization of liquid water in terms of the
thermodynamic quantities of heat change (also
called change in heat content or enthalpy) and
entropy change bring to life the energy conver-
sions that sustain Life.

5.1 Introduction 107
5.1.2.2 Phase Transitions of Proteins
5.1.2.2.1 Heat Denaturation of Protein
Along the temperature scale shown in Figure
5.2, sandwiched between commonplace phase
changes of the melting of ice at low tempera-
ture and the vaporization of liquid water at
high temperature, are transitions exhibited by
proteins in water. The higher temperature
transition, shown near 70° C, represents the
denaturation of proteins. Below the denatura-
tion transition temperature, the protein has a
regular structure, where each amino acid
residue of the protein chain occurs in a defined
space. Above the denaturation transition tem-
perature, the protein can be described as a dis-
ordered, irregular, and randomized tangle of
chains. The location in space of a given amino
acid residue in one chain with respect to other
amino acids in the chain is different for each
protein chain. During cooking (heating) of an
egg, the proteins of the clear gel, surrounding
the yellow yolk, become disordered, more
sohdified, and white on heat denaturation.
5.1.2.2.2 Low Heat of the Intermediate
Transition at Lower Temperature
The lower temperature transition, occurring
near 30° C for our basic elastic-contractile
model protein, is difficult to detect, as plotted,
because of the relatively small amount of heat
involved in this transition. The representations
in Figure 5.2 provide a visual bridge from the
more familiar transitions of water, considered
above, to the less familiar transitions central
to the function of our model protein-based
machines. Figure 5.2 is a composite of the tran-
sitions for pure or nearly pure water with the
curve for poly(GVGVP) with the relative areas
of the curves representing the circumstance for
1 gram of protein dissolved in 1 gram of water.
The relative areas are plotted for equal weights
of water and model protein, yet the differences
in the areas of the peaks, which measure the
relative heats of the transitions, are dramatic.
This seemingly innocuous transition, shown
here peaking near
30"^
C for poly(GVGVP), is
the source of the consilient mechanism for
energy conversion. We believe this inconspicu-
ous transition represents the primary physical
process enabling the energy conversions that
sustain Life.
5.1.2.2.3 Heat Maturation of Protein
For our model contractile protein,
poly(GVGVP), AHt = -hl.2kcal/mole-pentamer
and ASt(solution -^ phase separated) = +1.2/303
kcal/mole-degree K ~ +4.0cal/mole-pentamer-
degree (EU).'''' Because 1 mole of (GVGVP)
is 409 grams and 1 mole of water is 18 grams,
on a per gram basis (4.0/409 = 0.0098 and
5.3/18
= 0.29) the entropy change for the melting of
ice is 30 times greater (0.29/0.0098) than that of
the transition for poly (GVGVP).
Despite the absorption of heat for the tran-
sition and the overall increase in entropy of
+4.0
EU for the water plus protein, the protein
component actually increases in order on
raising the temperature. As unambiguously
demonstrated by crystallization of a cyclic
analog (see Figure 2.7), in this case the protein
component of the water plus protein system
becomes more ordered as the temperature is
raised. For this and additional reasons,
noted below in section 5.1.3, we call this tran-
sition exhibited by our model protein, poly
(GVGVP), an inverse temperature transition.
5.1.2.2.4 The Inconspicuous Inverse
Temperature Transition Is Equivalent to Cold
Denaturation of Protein
In the early 1900s, it was recognized that low-
ering the temperature could cause enzymes to
lose their catalytic activity due to an unfold-
ing and/or disassembly of proteins and their
subunits.^^ This is called cold denaturation.^^
Similarly with our model protein, lowering the
temperature from above to below the tempera-
ture interval of the inverse temperature transi-
tion causes the model protein to unfold and
disassemble, that is, to dissolve. In the case
of our model protein, the reverse process of
raising the temperature from below to above
the temperature of the inverse temperature
transition causes the massive, visible changes
in solubility represented in Figure 5.1A. The
phase diagram, discussed immediately below,
provides systematic characterization of this

108
5.
Consilient Mechanisms for Diverse Protein-based Machines
phase separation. The phase separation analy-
sis,
however, accommodates many other
energy inputs in addition to thermal energy,
because many other energy inputs drive phase
separation.
5.1.3 Phase Diagram for Inverse
Temperature Transitions and
Related Analyses
5.13.1 The Inverse Temperature
Transition of Model Proteins
The model proteins of interest here are poly-
mers of a distinctive family of repeating peptide
sequences where the repeating unit may be
as few as two residues or as many as several
hundred residues. These elastic-contractile
model proteins exhibit remarkably functional
and distinctive reversible phase transitional
behavior to increased polypeptide order on
raising the temperature and on this basis was
called an inverse temperature transition.^^ This
inverse order-disorder transition demonstrable
by elastic-contractile model proteins is the basis
for many different energy conversions, includ-
ing, we believe, the energy conversions existing
in biology."^'^
5.1.3.2 Inverted Phase Transitional
Behavior of Inverse Temperature
Transitions
In general, solubility in a solvent, of whatever
molecular species, increases with heating. The
solubility of petroleum-based polymers in
organic solvents and most petroleum-based
polymers in water represent such cases. In
general, solubility of the polymers increases on
raising the temperature. For the case of the
amount of solute within a solvent, phase dia-
grams graphically represent the change in sol-
ubility as a function of temperature. In the case
of the phase diagram, the boundary between
insolubiUty and solubiUty is called the binodal
or coexistence
line.^^
At the temperature of the
coexistence line soluble and insoluble states
coexist. In general, insolubility occurs at lower
temperatures, and solubility takes place on in-
creasing the temperature.
5.1.3.2.1 Our Model Proteins Become
Insoluble on Increasing the Temperature
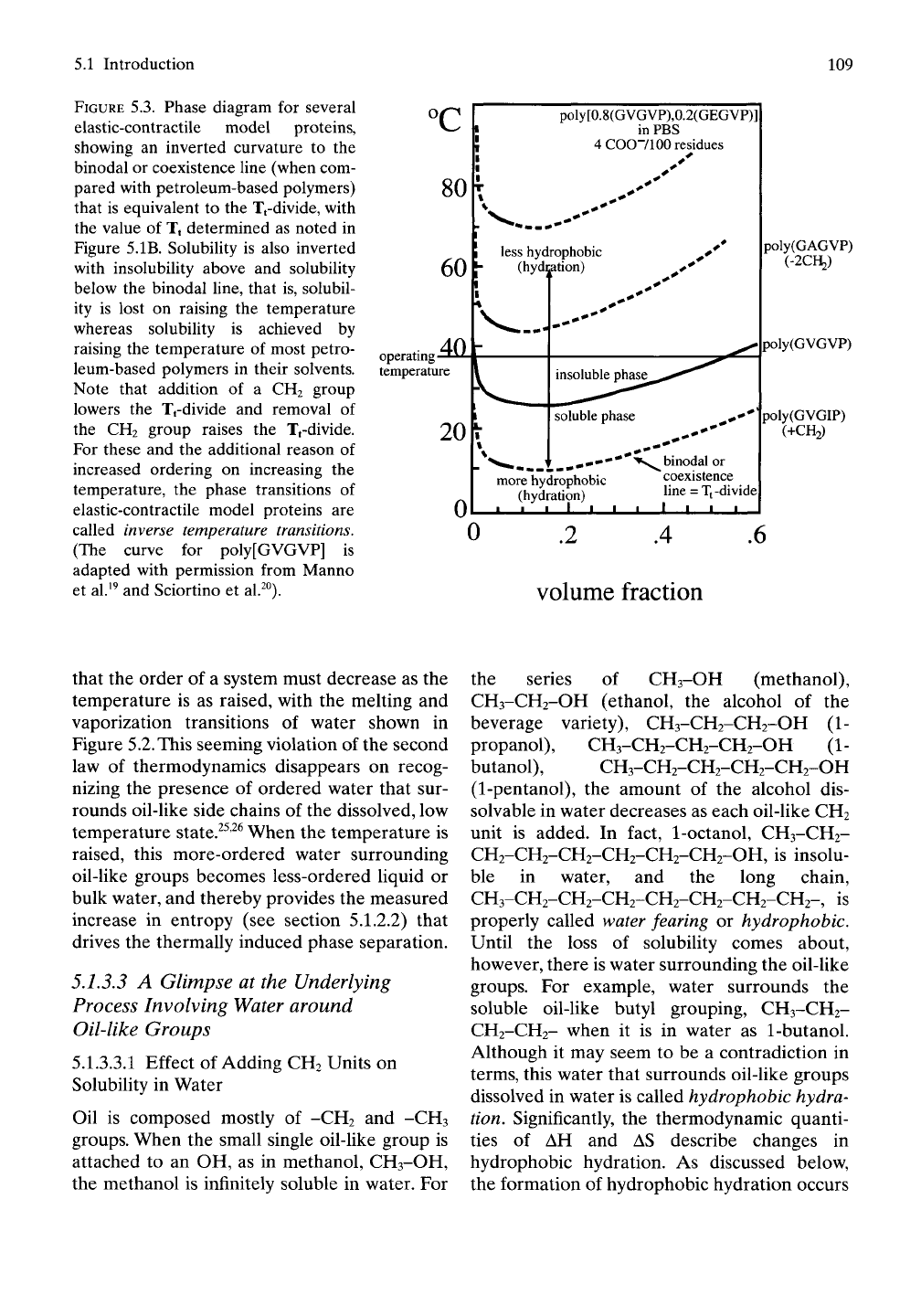
As demonstrated in Figure 5.3 for several
model proteins, essentially unUmited solubiUty
occurs at low temperature, and phase separa-
tion (insolubility) occurs as the temperature is
raised. Also, for our model protein composi-
tions,^^'^^
the curvature of the coexistence line is
inverted, having the shape of a valley instead of
a smooth mountain peak. Because of this we
call the phase transition, exhibited by elastic-
contractile model proteins, an inverse tempera-
ture transition. Even more compelling reasons
exist for the inverse temperature transition label.
5.1.3.2.2 Our Model Proteins Increase Order
on Raising the Temperature
A vital property of these model proteins is that
they are more ordered above the transition
temperature defined by the binodal or coexis-
tence line in Figure 5.3. The polymer compo-
nent of this water-polypeptide system becomes
more ordered or structured on increased tem-
perature from below to above the transition.
This behavior is the inverse of that observed for
most systems, as discussed above. In particular,
we developed the term inverse temperature
transition when the precursor protein and
chemical fragmentation products of the mam-
malian elastic fiber changed from a dissolved
state,
and therefore when molecules were ran-
domly dispersed in solution, to a state of paral-
lel-aligned twisted filaments as the temperature
was raised from below to above the phase
transition.^^'^^'^^
Chemically synthesized high molecular
weight polymers of repeating sequences found
within the elastic fiber similarly form parallel-
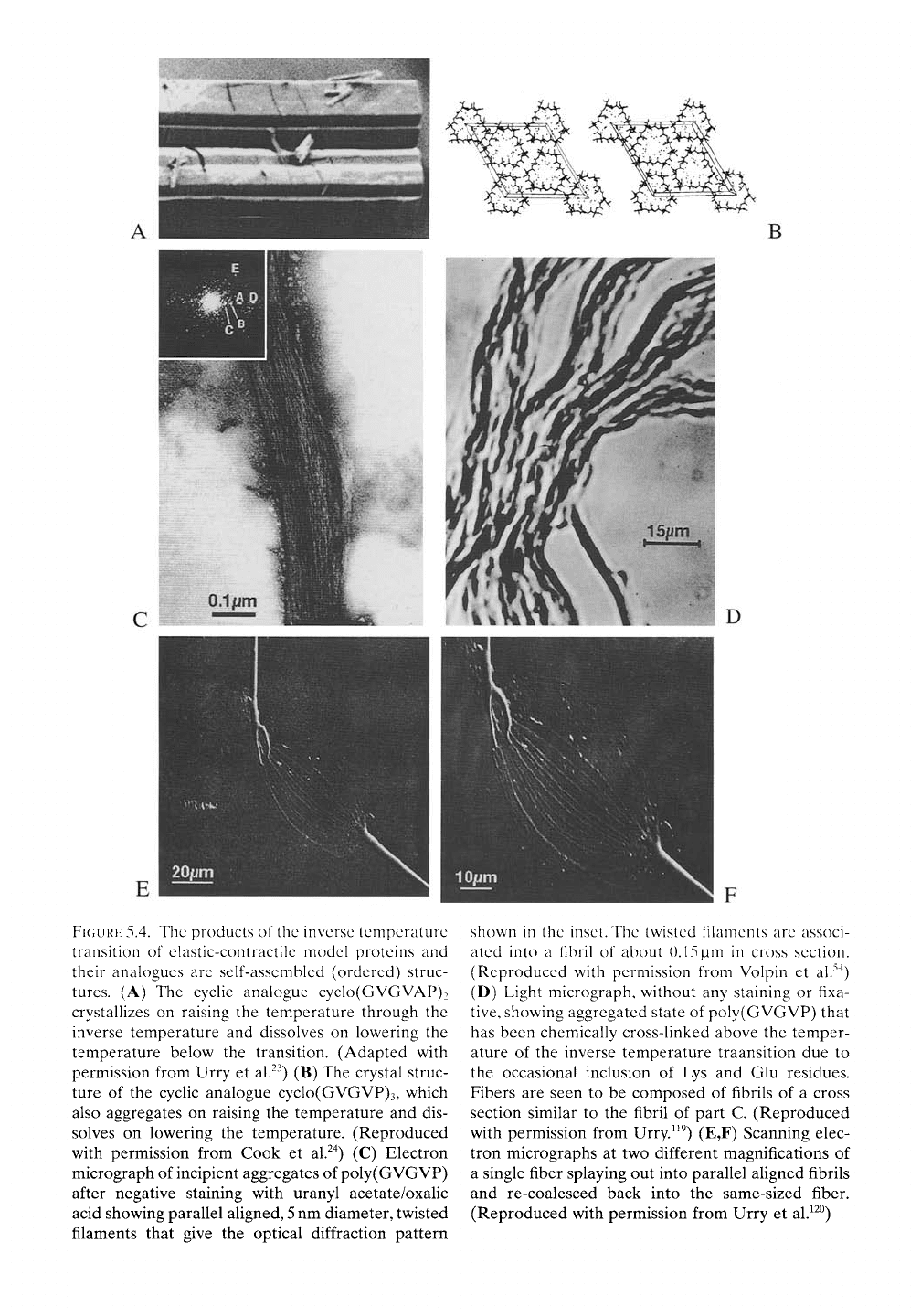
ahgned twisted filaments (see Figure 5.4C)
when the temperature is raised from below to
above the coexistence line. Most definitively,
cyclic analogues of the repeating peptide
sequences crystallize when the temperature is
raised above the transition temperature (see,
e.g.. Figure 2.5 and Figure 5.4A,B) and dissolve
when the temperature is lowered below the
transition.^^'^^
At first thought, this appears to contradict
the second law of thermodynamics, which states
5.1 Introduction 109
FIGURE 5.3. Phase diagram for several
elastic-contractile model proteins,
showing an inverted curvature to the
binodal or coexistence line (when com-
pared with petroleum-based polymers)
that is equivalent to the Tfdivide, with
the value of Tt determined as noted in
Figure 5.IB. Solubility is also inverted
with insolubility above and solubility
below the binodal line, that is, solubil-
ity is lost on raising the temperature
whereas solubility is achieved by
raising the temperature of most petro-
leum-based polymers in their solvents.
Note that addition of a CH2 group
lowers the Tfdivide and removal of
the CH2 group raises the Tfdivide.
For these and the additional reason of
increased ordering on increasing the
temperature, the phase transitions of
elastic-contractile model proteins are
called inverse temperature transitions.
(The curve for poly[GVGVP] is
adapted with permission from Manno
et al.^^ and Sciortino et al.^°).
°C
80
60
Operating-
temperature
0
poly[0.8(GVGVP),0.2(GEGVP)]
in PBS
4 COO"/100 residues
less hydrophobic
(hydration)
more hydrophobic
(hydration)
-I
I I I I I
binodal or
coexistence
line = Tt-divide
1 I I I
poly(GAGVP)
(-2C1^)
poly(GVGVP)
poly(GVGIP)
(+CH2)
0
.2 .4
volume fraction
that the order of a system must decrease as the
temperature is as raised, with the melting and
vaporization transitions of water shown in
Figure
5.2.
This seeming violation of the second
law of thermodynamics disappears on recog-
nizing the presence of ordered water that sur-
rounds oil-like side chains of the dissolved, low
temperature state.^^'^^ When the temperature is
raised, this more-ordered water surrounding
oil-like groups becomes less-ordered liquid or
bulk water, and thereby provides the measured
increase in entropy (see section 5.1.2.2) that
drives the thermally induced phase separation.
5.1.3.3 A Glimpse at the Underlying
Process Involving Water around
Oil-like Groups
5.1.3.3.1 Effect of Adding CH2 Units on
Solubility in Water
Oil is composed mostly of -CH2 and -CH3
groups. When the small single oil-like group is
attached to an OH, as in methanol, CH3-OH,
the methanol is infinitely soluble in water. For
the series of CH3-OH (methanol),
CH3-CH2-OH (ethanol, the alcohol of the
beverage variety), CH3-CH2-CH2-OH (1-
propanol), CH3-CH2-CH2-CH2-OH (1-
butanol), CH3-CH2-CH2-CH2-CH2-OH
(1-pentanol), the amount of the alcohol dis-
solvable in water decreases as each oil-like CH2
unit is added. In fact,
1-octanol,
CH3-CH2-
CH2-CH2-CH2-CH2-CH2-CH2-OH, is insolu-
ble in water, and the long chain,
CH3-CH2-CH2-CH2-CH2-CH2-CH2-CH2-, is
properly called water fearing or hydrophobic.
Until the loss of solubility comes about,
however, there is water surrounding the oil-like
groups. For example, water surrounds the
soluble oil-like butyl grouping, CH3-CH2-
CH2-CH2- when it is in water as
1-butanol.
Although it may seem to be a contradiction in
terms,
this water that surrounds oil-like groups
dissolved in water is called hydrophobic hydra-
tion.
Significantly, the thermodynamic quanti-
ties of AH and AS describe changes in
hydrophobic hydration. As discussed below,
the formation of hydrophobic hydration occurs
B
FIGURE
5.4. The products of the inverse temperature
transition of ehistic-contractile model proteins and
their analogues are self-assembled (ordered) struc-
tures.
(A) The cyclic analogue cyclo(GVGVAP)2
crystallizes on raising the temperature through the
inverse temperature and dissolves on lowering the
temperature below the transition. (Adapted with
permission from Urry et al.^^) (B) The crystal struc-
ture of the cyclic analogue cyclo(GVGVP)3, which
also aggregates on raising the temperature and dis-
solves on lowering the temperature. (Reproduced
with permission from Cook et al.^"^) (C) Electron
micrograph of incipient aggregates of poly(GVGVP)
after negative staining with uranyl acetate/oxalic
acid showing parallel aligned,
5
nm diameter, twisted
filaments that give the optical diffraction pattern
shown in the inset. The twisted filaments are associ-
ated into a fibril of about ().15)im in cross section.
(Reproduced with permission from Volpin et al.""^)
(D) Light micrograph, without any staining or fixa-
tive,
showing aggregated state of poly(GVGVP) that
has been chemically cross-linked above the temper-
ature of the inverse temperature traansition due to
the occasional inclusion of Lys and Glu residues.
Fibers are seen to be composed of fibrils of a cross
section similar to the fibril of part C. (Reproduced
with permission from Urry.^^^) (E,F) Scanning elec-
tron micrographs at two different magnifications of
a single fiber splaying out into parallel aligned fibrils
and re-coalesced back into the same-sized fiber.
(Reproduced with permission from Urry et aV^^)
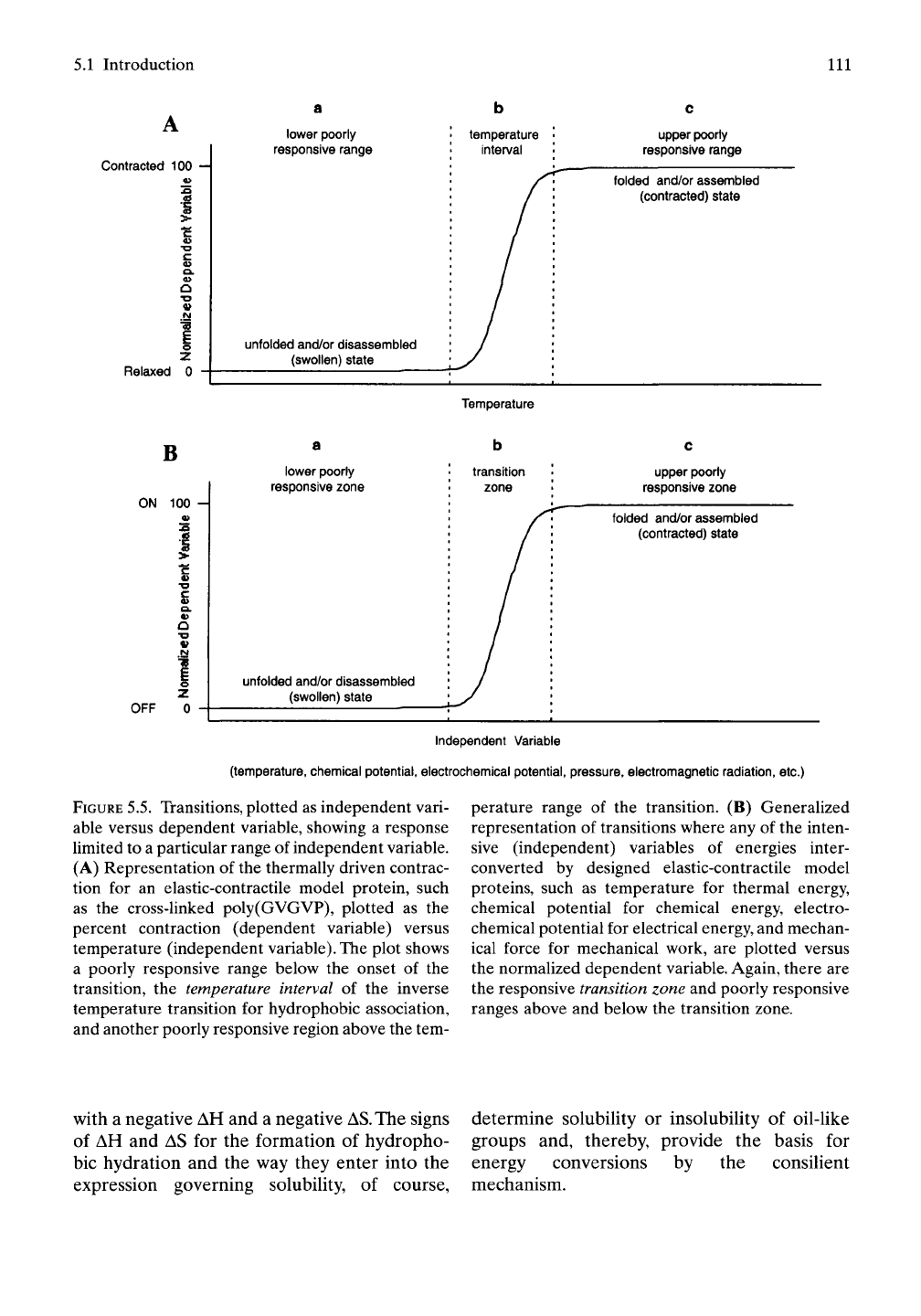
5.1 Introduction
111
Contracted 100 —
a>
.a
1
>•
"c
c
<u
O
"O
V
o
2
Relaxed 0 —
lower poorly
responsive range
unfolded and/or disassembled
(swollen) state
1
temperature
Interval
i
1
upper poorly
responsive range
folded and/or assembled
(contracted) state
Temperature
B
ON 100 H
>-
c
OFF
lower poorly
responsive zone
unfolded and/or disassembled
(swollen) state
transition
zone
upper poorly
responsive zone
folded and/or assembled
(contracted) state
Independent Variable
(temperature, chemical potential, electrochemical potential, pressure, electromagnetic radiation, etc.)
FIGURE
5.5.
Transitions,
plotted as independent vari-
able versus dependent variable, showing a response
limited to a particular range of independent variable.
(A) Representation of the thermally driven contrac-
tion for an elastic-contractile model protein, such
as the cross-linked poly(GVGVP), plotted as the
percent contraction (dependent variable) versus
temperature (independent
variable).
The plot shows
a poorly responsive range below the onset of the
transition, the temperature interval of the inverse
temperature transition for hydrophobic association,
and another poorly responsive region above the tem-
perature range of the transition. (B) Generalized
representation of transitions where any of the inten-
sive (independent) variables of energies inter-
converted by designed elastic-contractile model
proteins, such as temperature for thermal energy,
chemical potential for chemical energy, electro-
chemical potential for electrical energy, and mechan-
ical force for mechanical work, are plotted versus
the normalized dependent variable. Again, there are
the responsive
transition
zone and poorly responsive
ranges above and below the transition zone.
with a negative AH and a negative
AS.
The signs
of AH and AS for the formation of hydropho-
bic hydration and the way they enter into the
expression governing solubility, of course,
determine solubility or insolubility of oil-like
groups and, thereby, provide the basis for
energy conversions by the consilient
mechanism.

112
5.
Consilient Mechanisms for Diverse Protein-based Machines
5.1.3.3.2 Thermodynamic Expression for
Solubility: The Gibbs Free Energy
(AG = AH - TAS)
The thermodynamic quantity that determines
solubiHty is the Gibbs free energy, AG It is
made up of two terms, AH, the change in heat
on dissolution, and -TAS where T is the tem-
perature in degrees Kelvin (K) and AS quanti-
fies the change in order attending dissolution.
(These are the same thermodynamic quantities
discussed above in relation to the phase
changes exhibited by water and by proteins in
water.) Accordingly, AG(solubility) = AH -TAS.
When AG(solubility) for dissolution is negative,
solubility occurs. As AG(solubility) becomes
positive, solubility decreases.
In general, substances that form ions on dis-
solving in water evolve much heat; AH is nega-
tive.
An example is caustic soda, NaOH. When
water is carefully added to a test tube contain-
ing pellets of NaOH, they dissolve as Na^ and
OH" ions with the production of much heat.
The test tube becomes hot to the touch. In
general, hydration of ions is an exothermic
reaction. The same happens for glacial acetic
acid (the major component of vinegar,
CH3-COOH, at a very high concentration).
Glacial acetic acid must be added slowly and
very carefully to water, because it dissolves as
H^ and CH3-COO" with the release of so much
heat. AH for dissolution is large and negative.
The full hydration of ions in water is a very
exothermic, that is, very favorable, process.
5.1.3.3.3 The Thermodynamic Properties of
Hydrophobic Hydration
Remarkably, in the above series of alcohols,
addition of an oil-Uke CH2 unit increases the
release of heat when dissolved in water. Dis-
solving CH3-CH2-CH2-CH2-OH in water
releases more heat per mole added than does
dissolving CH3-CH2-CH2-OH in water. The
dissolution of these oil-like CH2 units in water is
exothermic. Therefore, formation of hydropho-
bic hydration is exothermic. Nonetheless, solu-
bility decreases as the number of oil-like CH2
units increases, despite a continuing favorable
release of heat on dissolution. Now we must
remember that AG(solubility) = AH - TAS is
the expression that governs solubility; and
solubility occurs when AG is negative and
decreases as AG becomes positive. Therefore,
the loss of solubility, as the number of oil-like
CH2 units increases, is due to the sign and mag-
nitude of the (-TAS) term. Based on the data
of Butler,^^ the average change in the AH and
(-TAS) terms for each of the four CH2 units
added on going from CH3-OH to
CH3-CH2-CH2-CH2-CH2-OH is a favorable
-1.4kcal/mole-CH2 for AH and an unfavorable
-hl.7kcal/mole-CH2 for (-TAS).Thus, insolubil-
ity ultimately happens when enough CH2 units
are added due to the larger and positive (-TAS)
term. It measures the contribution to the Gibbs
free energy due to an increase in order of water
as it goes from being bulk water to being
hydrophobic hydration. This ordering effect is
so pronounced that Frank and Evans^^ referred
to hydrophobic hydration as "icebergs" sur-
rounding oil-like groups.
5.1.3.3.4 The Inverse Temperature
Transition: A Hydrophobic Folding and
Assembly Transition
The protein-based polymer is soluble in water
at temperatures below its coexistence line
where the hydrophobic residues are sur-
rounded by hydrophobic hydration. As the
positive (-TAS) term due to hydrophobic
hydration becomes larger than the negative AH
term, simply due to increasing the value of T,
solubility of a protein-based polymer is lost,
and it hydrophobically folds and assembles. The
inverse temperature transition is a hydrophobic
association transition.
5.1.3.4 Capacity to Move the T^divide
as a Measure of Energy Available to the
Consilient Mechanism (That Is, Moving
the Temperature Interval)
5.1.3.4.1 Defining the Tt-divide
At the coexistence line of a phase transition,
such as that plotted in Figure 5.3, soluble and

5.1 Introduction 113
insoluble states coexist. Heat added at this tem-
perature, for an ideal case like ice dissolving in
water, converts one state to the other rather
than increases the temperature. At the temper-
ature of the phase transition when there are
two phases, the Gibbs free energy per mole of
the molecules in the two phases are the same,
that is, the chemical potential,
|Li
= AG/mole, is
the same for the molecules in solution and for
the molecules in the phase-separated state. For
the inverse temperature transition, the chemi-
cal potential for molecules in solution, that is,
for molecules in the hydrophobically dissoci-
ated state,
\iHD,
is the same as the chemical
potential of the molecules in the separated,
hydrophobically associated phase, IIHA, that is,
\iHD
=
\IHA'
This means for the transition that
AGt = 0 = AHt - TtASt, where the subscript t
stands for the phase transition. Therefore, AHt
= TtASt, and Tt = AHt/ASt, where Tt is the tem-
perature for the transition. Accordingly, the
coexistence line may also be called the Tr
divide between soluble and insoluble states (see
Figure 5.3).^^ Importantly for the transition,
AGt = 0 = AHt - TfASf at the Tf-divide and
AHt = TASt.
5.1.3.4.2 Dependence of Tt on Chain Length
and on Concentration
Meaningful comparison of the different means
whereby the value of Tt may be moved requires
definition of a common polymer chain length
and concentration to function as a reference
state.^^'^^'^^'^^
Definition of the reference condi-
tions occurred nearly two decades ago with
chemically synthesized polymers,^^ before the
following systematic comparisons began. In
particular, chemically synthesized polymers
dialyzed against 50,000 Da cut-off dialysis
membranes^^ at 4°C (i.e., a temperature sub-
stantially below Tt) exhibit a lowering of Tt on
increasing the concentration until a concentra-
tion of 40mg/ml was reached. Increasing the
concentration further had little effect on the
value of Tt such that this became the concen-
tration for comparison. This was extensively
substantiated in the process of developing the
phase diagrams for poly(VPGVG),^^'^^'^^ as the
phase diagram is a plot of temperature versus
concentration.
The chain length dependence was also deter-
mined on chemically synthesized polymers
using dialysis membranes. ^^ Subsequently,
protein-based polymers using recombinant
DNA technology were prepared with 41, 141,
and
251
repeats of the pentamer.^^ Again, above
100,000 Da or over 200 repeats of the pentamer,
the chain length dependence of Tt becomes
limited. Accordingly using polymers of approx-
imately 100,000 Da at concentrations of
40mg/ml have become the reference conditions
for the comparisons noted below.
5.1.3.4.3 Moving the Tt-divide by Adding a
CH2 Unit, That Is, Replacing Val by He (an
Approximation to the Change in Gibbs Free
Energy for Hydrophobic Association,
AGHA)
From the data of Butler,^^ we can predict how
the Tt-divide will change as CH2 units are added
or removed from the polymers plotted in
Figure 5.3. If we add a CH2 group to the R-
group of the second Val residue of (GVGVP)n,
it becomes (GVGIP)n, that is, the R-group for
the V residue, -CH(CH3)2, becomes
-CH(CH3)-CH2-CH3 for an I residue. Because
AHt = TtASt for both compositions at their
respective Tt-divides, the relevant equations
become
AHt(GVGVP) = Tt(GVGVP)ASt(GVGVP)
(5.1)
and
AH,(GVGIP) = T,(GVGIP)AS,(GVGIP).
(5.2)
Due to the experimental work of Butler and for
reasons of the additive nature of the compo-
nents within AH and of the components that
make up
AS,
we separate the effect of the addi-
tion of a single CH2 group and write that
AHt(GVGIP) = AHt(GVGVP) +AHt(CH2)
(5.3)
and

114
5.
Consilient Mechanisms
for
Diverse Protein-based Machines
T,
(GVGIP)
AS,
(GVGIP)
=
T,
(GVGIP)[
AS,
(GVGVP) +
AS,(CH2)]
(5.4)
In general, therefore,
AGHA
(X)
= [T. (ref)
-
T.
(z)]AS,
(ref)
(5.8)
Substitution
of
Equations (5.3)
and
(5.4) into
Equation
(5.2) and
subtracting Equation
(5.1)
gives
AH,(CH2)-T,(GVGIP)AS,(CH2)
=
[T,
(GVGIP)
-
T,
(GVGVP)]
AS,
(GVGVP)
(5.5)
Again,
if the
data
of
Butler^^
for the
effect
of
addition
of a CH2
group
on
solubiUty
are
apphcable,
the
hydrophobic association
of the
phase separation would
be
such that
the
mag-
nitude
of the
Tt(GVGIP)ASt(CH2) term would
be larger than that
of the
AHt(CH2) term,
and
for hydrophobic association, that
is, for
insolu-
bilization, ASt(GVGVP)
is
positive
as
more
ordered hydrophobic hydration becomes less
ordered bulk water
on
hydrophobic associa-
tion. This means that Tt(GVGIP)ASt(CH2)
>
AHt(CH2), such that
Tt
(GVGVP) >
Tt
(GVGIP) (5.6)
This
is
found experimentally
for the
phase sep-
aration
of
these model proteins. Addition
of a
CH2 group lowers
the
value
of
Tt, that
is,
lowers
the Tfdivide
for
(GVGIP)n
as
shown
in
Figure
5.3.
The
experimental estimates
of Tt are
nominally 25°
C for
(GVGVP)^
and
10°
C for
(GVGIP)n
in
keeping with Equation
(5.6).
Also,
removal
of
two CH2 units,
by
replacing
the
first
V residue
of
(GVGVP) with
an
alanine residue,
A, having
an
R-group
of
-CH3, results
in a Tt
value
of
45°
C.
As expected from Equation (5.6)
and from
the
data
of
Butler, Tt(GVGVP)
<
Tt(GAGVP). This simple relationship between
hydrophobicity
and Tt
provides insight into
the
Tfbased hydrophobicity scale
for
protein func-
tion
and
engineering, developed
in
section
5.3
below.
Returning
to
Equation (5.5),
we
define^^
-AGHA(CH2)
=
AHt(CH2)
-
Tt(GVGIP)ASt(CH2)
and rewrite Equation
(5.5) to
give
AGHA
(CH2)
-
[Tt
(GVGVP)
-
Tt
(GVGIP)]
ASt
(GVGVP)
(5.7)
or
AGHA
(X)
- - ATt
(x)ASt
(ref) (5.9)
where
%
is any variable that moves
the
Tt-divide
from that
of the
reference
(ref)
model protein,
and AGHA(Z) is
the
change
in
Gibbs free energy
for forming
the
hydrophobically associated
state
of the
model protein resulting from that
variable. (Note that
the
change
in
Gibbs free
energy
is
written here
for
hydrophobic associa-
tion, that
is, for
insolubilization, which
has the
opposite sign
of
AG(solubility),
as the
latter
considers
the
reverse process
of
solubilization
or solubility discussed
in
section 5.1.3.3.)
The
approximation
of
Equation
(5.9) is
demon-
strated below
in
Figure
5.10 in
terms
of the
diagonal straight fine through
the
sigmoid
of
data points. Within
the
approximation
of
Equation
(5.9)
ASt(ref) would
be
30cal/
mole-(GXGVP)-deg. That
is the
approxima-
tion
of the
practical Tj-based hydrophobicity
scale.
Equation (5.9) provides
an
initial insight into
the relationship between moving
the
Tt-divide
and changes
in
free energy
of
hydrophobic
association;
it is of
use when
the
value
of
ASt(x),
or AHt(x) from which
it is
derived,
is not
avail-
able.
Nonetheless,
the
limitations
of
Equation
(5.9) become apparent when both ASt(z)
^^^
ASt(ref)
are
known, because reversal
of
roles
of
the reference
and
altered state should simply
reverse
the
sign
of
AGHA(X),
^^^
this
is not the
case
due to the
approximation
of
Equations
(5.3)
and
(5.4).
As will
be
shown below
on
analysis
of the
data
in
Figure 5.10,
a
plot
of
Tt versus AGHA
has
a sigmoid appearance. This means that
the
derivative dTt/dGnA gives
a
bell-shaped curve
equal
to
(dSt)"^
and not a
straight line
as
imphed
by
Equation
(5.9) and
assumed
by the
Tfbased hydrophobicity scale. Equation (5.9),
however, does appear
to be
meaningful when
staying within
the
proper family
of
molecular
systems,
for
example,
as
defined
in
Figure
5.10
by lines
b (for the
aliphatic side chains),
c (for
the aromatic side chains),
and d (for the
effect
of charged species).
