Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.

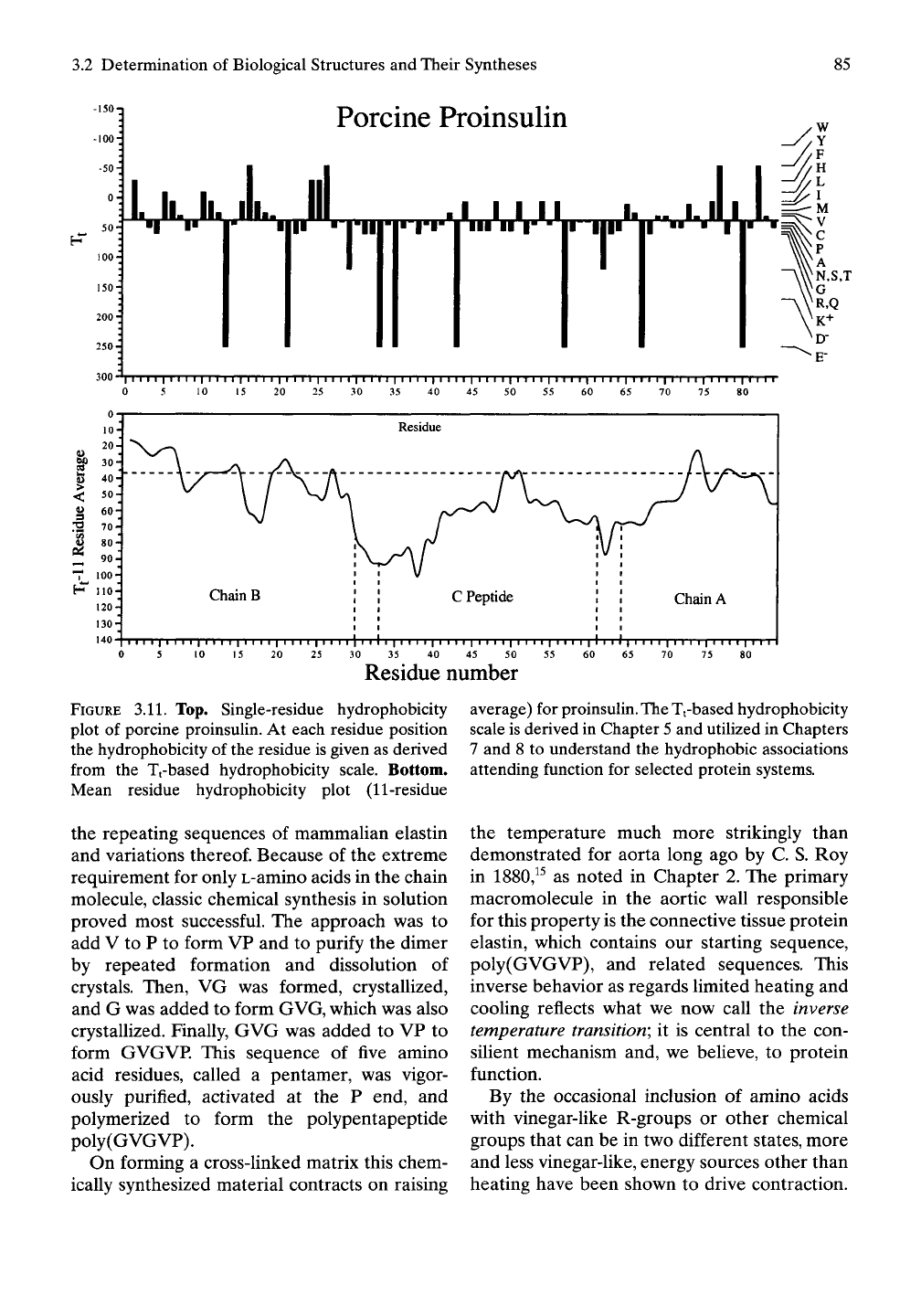
3.2 Determination of Biological Structures and Their Syntheses
85
-150-
-100-
-50-
0-
50-
100-
150-
200-3
250
300-
I. Il-_ll- ill
^TT
Porcine Proinsulin
L..I..I..I.I
|1 TTl
T-l-
Tinrf
l-TIIl
Ult
•••"I
I i I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I
0 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80
Residue
Residue number
FIGURE 3.11. Top. Single-residue hydrophobicity average) for proinsulin.
The
Tfbased hydrophobicity
plot of porcine proinsuUn. At each residue position scale is derived in Chapter
5
and utilized in Chapters
the hydrophobicity of the residue is given as derived 7 and 8 to understand the hydrophobic associations
from the Tt-based hydrophobicity scale. Bottom, attending function for selected protein systems.
Mean residue hydrophobicity plot (11-residue
the repeating sequences of mammalian elastin
and variations
thereof.
Because of the extreme
requirement for only L-amino acids in the chain
molecule, classic chemical synthesis in solution
proved most successful. The approach was to
add V to P to form VP and to purify the dimer
by repeated formation and dissolution of
crystals. Then, VG was formed, crystallized,
and G was added to form GVG, which was also
crystallized. Finally, GVG was added to VP to
form GVGVP. This sequence of five amino
acid residues, called a pentamer, was vigor-
ously purified, activated at the P end, and
polymerized to form the polypentapeptide
poly(GVGVP).
On forming a cross-linked matrix this chem-
ically synthesized material contracts on raising
the temperature much more strikingly than
demonstrated for aorta long ago by C. S. Roy
in 1880,^^ as noted in Chapter 2. The primary
macromolecule in the aortic wall responsible
for this property is the connective tissue protein
elastin, which contains our starting sequence,
poly (GVGVP), and related sequences. This
inverse behavior as regards limited heating and
cooling reflects what we now call the inverse
temperature transition', it is central to the con-
silient mechanism and, we believe, to protein
function.
By the occasional inclusion of amino acids
with vinegar-like R-groups or other chemical
groups that can be in two different states, more
and less vinegar-like, energy sources other than
heating have been shown to drive contraction.
86
3.
The Pilgrimage
These are detailed in Chapter 5. When these
model proteins were subsequently made by
living organisms, using recombinant DNA
technology as described in the Chapter 2
overview, the biosynthesized model proteins
converted energies just as had the chemically
synthesized model proteins. Again, here dis-
appears the delineation of things biological in
origin from those of nonbiological origin so
prevalent when this pilgrimage began with
the accidental synthesis of urea in 1828 by
Wohler.
3.2.9.2 Structure of Elastic-contractile
Model Proteins
The family of dynamic elastic-contractile model
proteins that form the basis for the assertions
of the central message of this volume do not
lend themselves to the precise spatial descrip-
tions of proteins that form crystals. Nonethe-
less,
important structural description is possible
for the poly(GVGVP) family. Indeed, the
experimental and computational elucidation of
structure will be noted in Chapter 5. Here we
briefly provide the information in the context
of the pilgrimage.
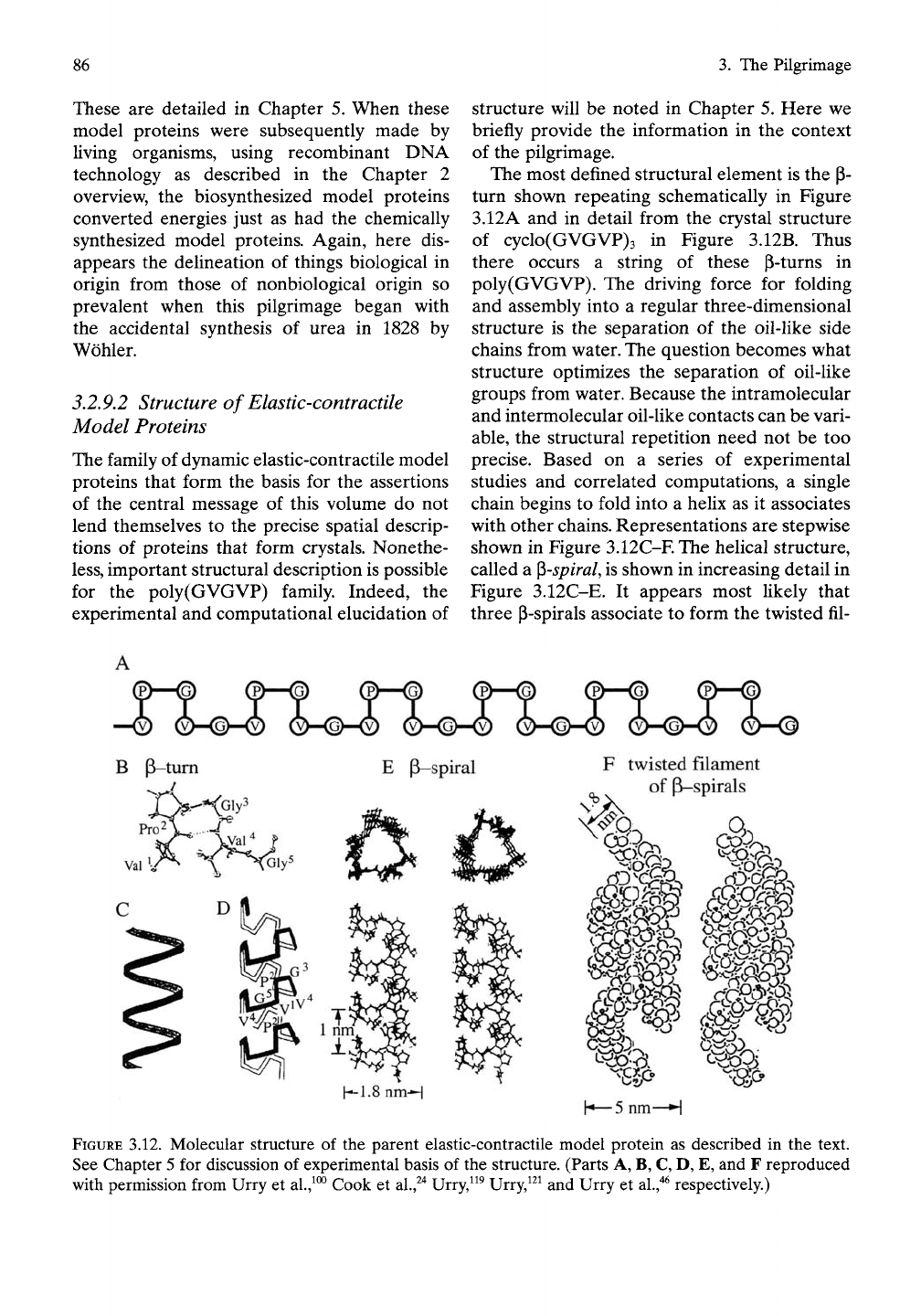
The most defined structural element is the p-
turn shown repeating schematically in Figure
3.12A and in detail from the crystal structure
of cyclo(GVGVP)3 in Figure 3.12B. Thus
there occurs a string of these P-turns in
poly(GVGVP). The driving force for folding
and assembly into a regular three-dimensional
structure is the separation of the oil-like side
chains from water. The question becomes what
structure optimizes the separation of oil-like
groups from water. Because the intramolecular
and intermolecular oil-like contacts can be vari-
able,
the structural repetition need not be too
precise. Based on a series of experimental
studies and correlated computations, a single
chain begins to fold into a helix as it associates
with other chains. Representations are stepwise
shown in Figure 3.12C-F. The helical structure,
called a ^spiral, is shown in increasing detail in
Figure 3.12C-E. It appears most likely that
three P-spirals associate to form the twisted fil-
(p)—<p)
®—^ (p—^ (S—(^ (p—-© ^>—©
—(& ©-^M^ ©-©-® ^-©-(^ ©-©-© ^-©-(^ ®—©
E P-spiral
F twisted filament
of P-spirals
FIGURE 3.12. Molecular structure of the parent elastic-contractile model protein as described in the text.
See Chapter 5 for discussion of experimental basis of the structure. (Parts A, B, C, D, E, and F reproduced
with permission from Urry et
al.,^°^
Cook et
al.,^"^
Urry,^^^ Urry,^^^ and Urry et
al.,"*^
respectively.)
3.2 Determination of Biological Structures and Their Syntheses
87
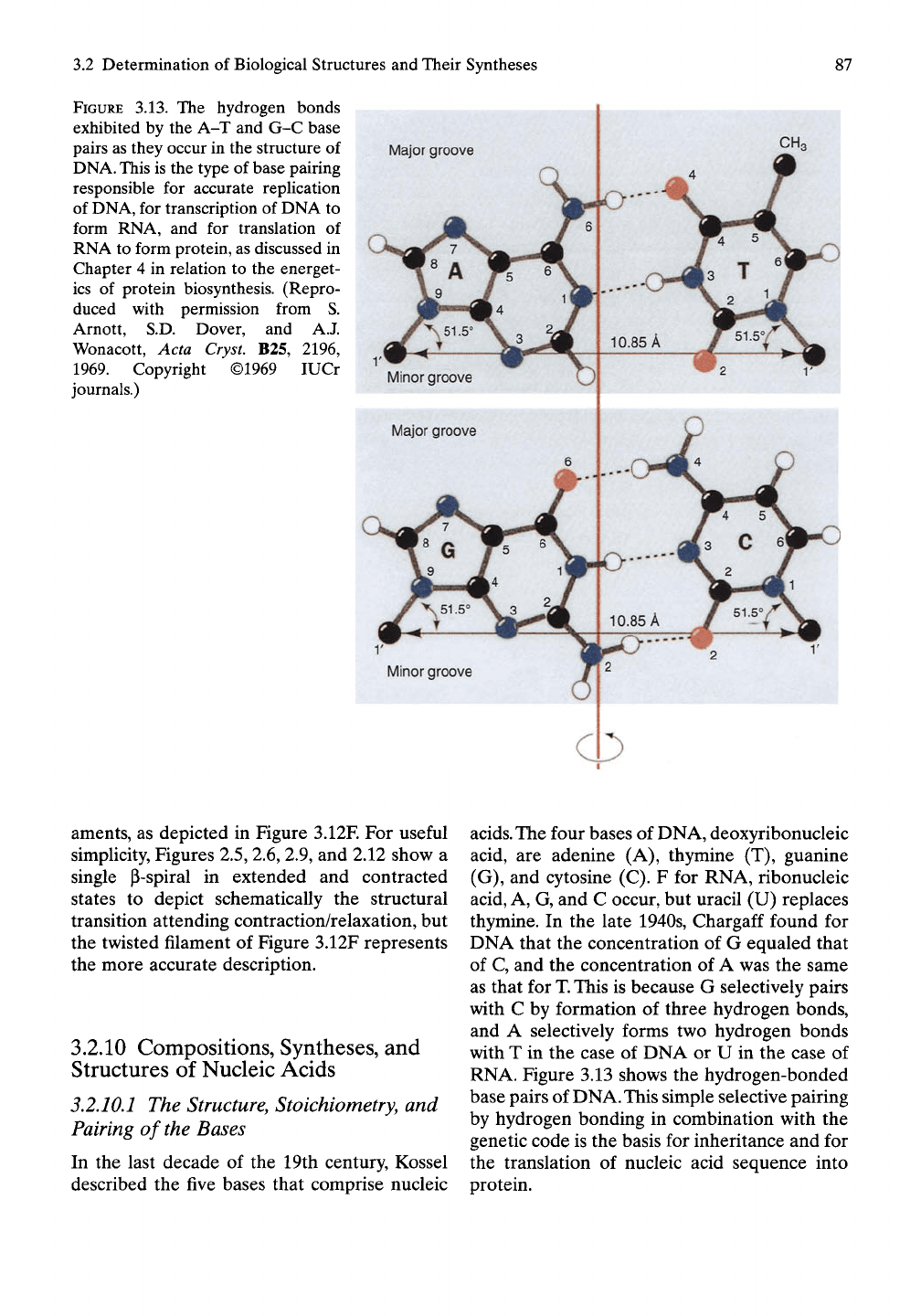
FIGURE 3.13. The hydrogen bonds
exhibited by the A-T and G-C base
pairs as they occur in the structure of
DNA.
This is the type of base pairing
responsible for accurate replication
of DNA, for transcription of DNA to
form RNA, and for translation of
RNA to form protein, as discussed in
Chapter 4 in relation to the energet-
ics of protein biosynthesis. (Repro-
duced with permission from S.
Arnott, S.D. Dover, and A.J.
Wonacott, Acta Cryst. B25, 2196,
1969.
Copyright ©1969 lUCr
journals.)
Major groove
aments, as depicted in Figure 3.12F. For useful
simplicity, Figures 2.5,2.6,2.9, and 2.12 show a
single p-spiral in extended and contracted
states to depict schematically the structural
transition attending contraction/relaxation, but
the twisted filament of Figure 3.12F represents
the more accurate description.
3.2.10 Compositions, Syntheses, and
Structures of Nucleic Acids
3.2.10.1 The Structure, Stoichiometry, and
Pairing of the Bases
In the last decade of the 19th century, Kossel
described the five bases that comprise nucleic
acids.
The four bases of DNA, deoxyribonucleic
acid, are adenine (A), thymine (T), guanine
(G),
and cytosine (C). F for RNA, ribonucleic
acid. A, G, and C occur, but uracil (U) replaces
thymine. In the late 1940s, Chargaff found for
DNA that the concentration of G equaled that
of C, and the concentration of A was the same
as that for T. This is because G selectively pairs
with C by formation of three hydrogen bonds,
and A selectively forms two hydrogen bonds
with T in the case of DNA or U in the case of
RNA. Figure 3.13 shows the hydrogen-bonded
base pairs of DNA. This simple selective pairing
by hydrogen bonding in combination with the
genetic code is the basis for inheritance and for
the translation of nucleic acid sequence into
protein.
3.
The Pilgrimage
32.10,2 Sugar-Phosphate Backbone of
the Nucleic Acids
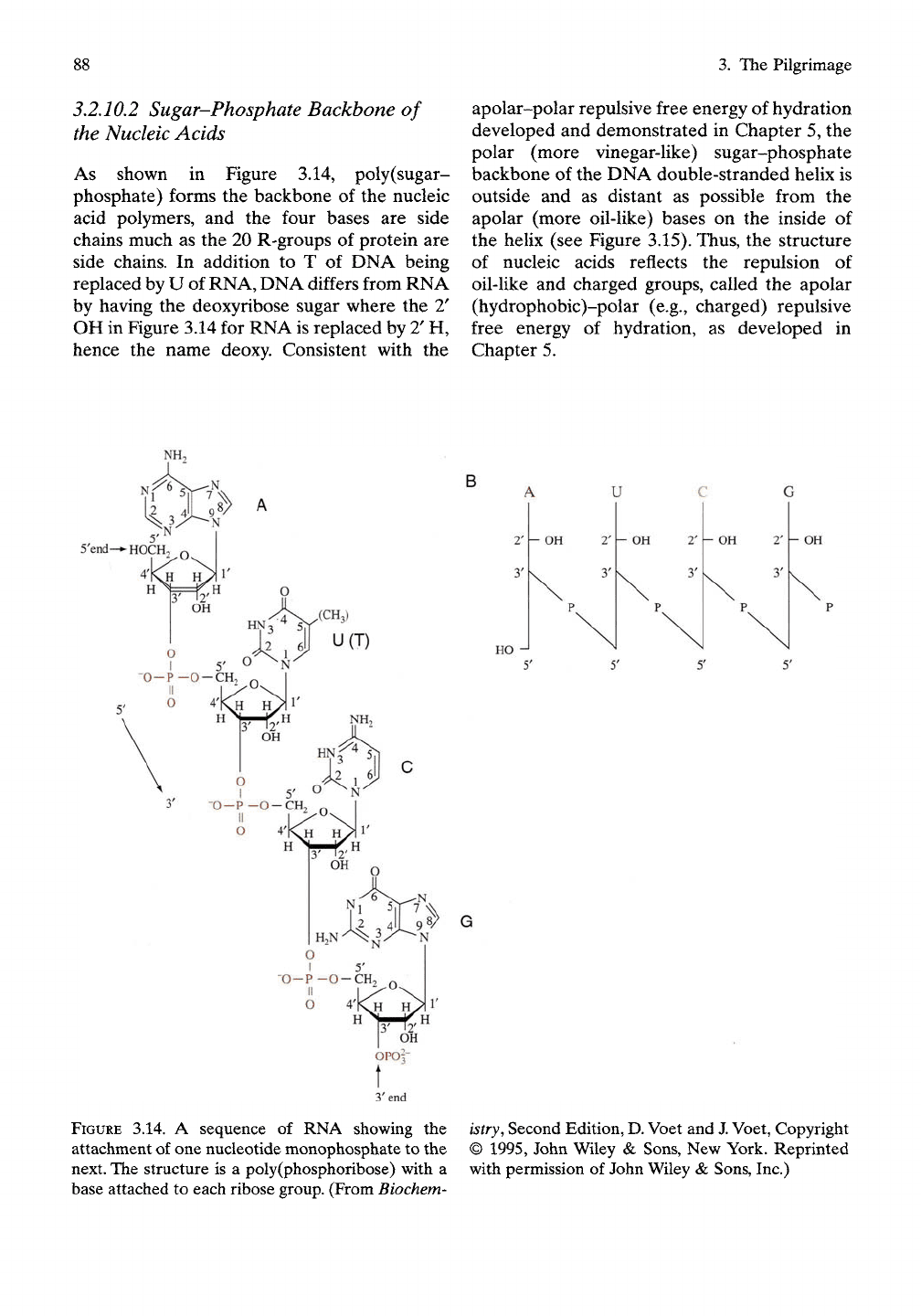
As shown in Figure 3.14, poly(sugar-
phosphate) forms the backbone of the nucleic
acid polymers, and the four bases are side
chains much as the 20 R-groups of protein are
side chains. In addition to T of DNA being
replaced by U of RNA, DNA differs from RNA
by having the deoxyribose sugar where the 2'
OH in Figure 3.14 for RNA is replaced by T H,
hence the name deoxy. Consistent with the
apolar-polar repulsive free energy of hydration
developed and demonstrated in Chapter 5, the
polar (more vinegar-like) sugar-phosphate
backbone of the DNA double-stranded helix is
outside and as distant as possible from the
apolar (more oil-like) bases on the inside of
the helix (see Figure 3.15). Thus, the structure
of nucleic acids reflects the repulsion of
oil-like and charged groups, called the apolar
(hydrophobic)-polar (e.g., charged) repulsive
free energy of hydration, as developed in
Chapter 5.
5'end-
,(CH3)
U(T)
3'end
FIGURE
3.14. A sequence of RNA showing the
attachment of one nucleotide monophosphate to the
next. The structure is a poly(phosphoribose) with a
base attached to each ribose group. (From Biochem-
U
C
2'
y
HO -
OH OH hOH OH
G
istry.
Second Edition,
D.
Voet and
J.
Voet, Copyright
© 1995, John Wiley & Sons, New York. Reprinted
with permission of John Wiley & Sons, Inc.)

3.2 Determination of Biological Structures and Their Syntheses
A
89
FIGURE
3.15. Two representations of the famous
DNA double helix due to Watson and Crick (and
Franklin). (A Reproduced with permission from The
Irving Geis Archives, Rights owned by Howard
Hughes Medical Institute, and A and B from Bio-
chemistry,
Second Edition,
D.
Voet and
J.
Voet,
Copy-
right © 1995, John Wiley & Sons, New York. Re-
printed with permission of John Wiley &
Sons,
Inc.)
3.2.10J The Watson-Crick DNA
Double-stranded Helix
Perhaps standing as the most commanding
symbol of the developments in biology and of
the biotechnological revolution is the double-
stranded helix of DNA shown in Figure 3.15,
the original structure for which was due to
Watson and Crick (Franklin).^^ This structure
stands as the basis of heredity by its own
replication where G pairs with C and A pairs
with T. When being transcribed into RNA,
A pairs with U, and the RNA sequence of bases
is otherwise a copy of the DNA sequence of
bases.
Finally, by the genetic code explicitly
considered in Chapter 6, a given RNA sequence
of three bases encodes for a particular amino
acid in the translation process to produce a spe-
cific protein. The probability and energy costs
for protein biosynthesis are considered in
Chapter 4.
3.2.10.4 Chemical and Enzymatic
Synthesis of DNA
Continuing our pilgrimage to bring the prod-
ucts and components of Life within man's
comprehension, in the 1960s, Gobind Khorana
chemically synthesized nucleic acid, which was
competent to specify synthesis of the appropri-
ate protein, and in 1970 he enzymatically syn-
thesized the first natural gene, a gene of yeast.
Thus it would appear that all of the products
and components of living organisms are within

90
3.
The Pilgrimage
man's capacity to comprehend and to a more
Umited extent within man's capacity to
produce. Now the most ambitious project yet is
unfolding, the determination of the complete
sequence of the human genome with what are
expected to be truly extraordinary implications
to health and to understanding man's relation-
ship to other life forms.
3.3 Determination of the
Human Genome
3.3.1 The Human Genome:
"The Periodic Table of Life"
As noted above, the question posed by Thales
of Miletus 26 centuries ago, "What is the world
made of?'' was answered at a fundamental level
by completion of the Periodic Table of the Ele-
ments. The exact way in which the atomic ele-
ments combined to form the minerals of the
earth's crust, the water of the oceans, the gases
of the atmosphere, and the living organisms of
biology, is not immediately obvious from the
Periodic Table of the Elements, yet the very
groupings within the table dictate the way in
which the atomic elements combine to form
complex combinations of atoms found on earth.
In a somewhat related way, the question of
"What is Life?" would be answered at a certain
level by the elucidation of the genomes of all
living organisms. As the human genome con-
tains many genes that are clearly related to
lower forms of Life and as we pride ourselves
on being the most advanced of creatures, it
would not be too great an exaggeration to refer,
for the sake of brevity, to the human genome as
"The Periodic Table of Life."^^ An important
distinction exists, however. Perhaps a more
accurate description of the genome is The Blue-
print of Life, for it is the proteins for which the
genes encode that perform the essential func-
tions in answering the question of "What
Sustains Life?"
3.3.2 The Difference Between Lower
Animals and Vertebrates
At this stage of analysis of the human genome
data, the evidence indicates that evolution of
vertebrates from lower animals occurred not so
much by the addition of new genes encoding for
new protein domains but rather from the novel
ways that existing protein domains have
evolved into combinations of domains to intro-
duce a new element to function. In particular
Initial examination of the predicted protein comple-
ment of the human genome indicates that verte-
brates have not evolved primarily by addition of new
protein domains but through novel ways of putting
these modules together to make protein. It is mostly
the architecture rather than the building blocks that
distinguishes us from other organisms. ^^
Perhaps the myoglobin protein domain pro-
vides an example. While retaining the same
basic structure of myoglobin, homologous
mutations led to the a-chain and (3-chain vari-
ants that combine to give hemoglobin with new
functional capacity. Our analysis of the amino
acid mutations of myoglobin that gave rise to
the a-chains and P-chains of hemoglobin pro-
vides insight. As is discussed in Chapter 7, it is
hydrophobic association between domains to
form the a2P2-tetramer of hemoglobin that
gives rise to new functions of survival value to
vertebrates. It is the formation of less polar,
more hydrophobic structures that results in the
assembly of these domains to make a protein
with a functional capacity required for a large
animal that must have oxygen transported from
lungs to the
tissues.
When we refer to an oil-like
or hydrophobic domain, this should be recog-
nized as a limited region of a protein domain
that is involved in hydrophobic association
between larger protein domains.
3.3.3 Genomics and Motility
The number of genes involved in the cytoskele-
tal systems (actin filaments, microtubules, and
intermediate filaments) required for motility,
as identified in the pre-human genome, is of
the order of 100.^^ Some additional 14 genes
have been observed from the human genome
sequence, but two actin genes appear to have
been missed. An equivalently large number of
myosin, kinesin, and dynein motor domains
have also been defined by traditional biochem-
ical and genetic methods. Because of the size of

3.4 Design of Living Organisms as Chemical Factories
91
the two former
genes,
the very large introns and
the fragmentary nature of the identifications,
some identified gene segments may result in the
same gene being identified more than once.
Nonetheless, it will continue to be vitalizing as
the gaps are filled and the complete and accu-
rate results emerge. In particular, there emerges
the opportunity to identify and relate the
consilient mechanism among the related motor
domains and their interactions with cytoskele-
tal components.
3.3.4 Evolutionary Genomics
Evolutionary genomics^^ involves the relation-
ship between protein domains (structural or
functional units in a protein) and analyzes the
relationships between lower organisms and
man. Some 50% of the human genome is
thought to result from the sharing or shuffling
of protein domains of lower organisms. For
example, Li et al.^^ count "1,865,
1,218,
1,183
and 973 domain types in human, fruitfly, nema-
tode and yeast, respectively."
Based on the myoglobin/hemoglobin exam-
ple to be discussed in Chapter 7, it is our per-
spective that changes in the oil-like surface of
homologous domains gives rise to new aggre-
gations of the protein domain resulting in new
properties, and a common feature of the new
property would be an increased positive co-
operativity, that is, increased efficiency of
function.
3.3.5 Genomics and Human Disease
The theories that all diseases have a genetic
component and that every individual has
genetic anomalies provide a basis for discussing
the expected impact of the human genome in
the practice of medicine.^^'^^ Based on the
former, targeting of newly designed drugs to
active sites of a diseased protein, preparation
by recombinant DNA technology of peptide
and proteins to target appropriate sites, and
gene delivery become obvious therapeutic
approaches.
Based on the latter, individuals can exhibit
different reactions to medical treatments, pro-
cedures, or
prostheses.
There are now known to
be more than 1,100 genes with disease-related
mutations (to follow this developing informa-
tion, see www.ncbi.nlm.nih.gov/omim).
It has been estimated that 100,000 people die
each year in reaction to medical treatments.^^ In
the past, when a small number of individuals
experience a severe reaction, the medical treat-
ment has been discontinued even though the
vast majority of the population may benefit. In
the future, the people likely to have adverse
reactions will be identified beforehand by their
genetic makeup and be advised against the
treatment, and those who can benefit from the
procedure should be allowed to do so.
As the blueprint of human biology emerges,
our focus turns to the molecular biophysics of
the protein-based machines of biology to
emphasize, as Bacon and Descartes would have
us do, the development of insight into protein
function by means of axioms and graphical rep-
resentations (Chapter
5).
Furthermore, medical
applications of protein-based materials can
provide means for delivering on some of the
promise of medical implications that emerging
knowledge of the human genome forecasts.
Also as a point of clarification in Chapters 4
and 6 from a physical biochemical perspective,
we attempt to alleviate some of the mystery
that has shrouded the production and evolution
of protein-based machines.
3.4 Design of Living Organisms
as Chemical Factories
So commanding has become our understanding
of the products, components, and functions of
living organisms that we can now employ living
organisms as factories to produce complex
molecules of our own design.
Man has demonstrated the capacity to syn-
thesize products and components of living
organisms and, in key ways, to assemble those
components and reconstitute natural function.
He can chemically synthesize sequences of
nucleic acid encoding for a specific protein
sequence of interest.
Holding great promise for the future, man
has learned (1) to produce new genes by chem-

92
3.
The Pilgrimage
ical synthesis of nucleic acids with base pairing
overlaps, (2) to use the renowned enzymatic
polymerase chain reaction (PCR) to extend
and form double-stranded DNA, and (3) to use
the ligase enzyme to splice together large
synthetic gene segments. The synthetic genes
can be introduced into organisms in such a way
as to induce the organism to produce the spec-
ified protein. Man can genetically engineer
living organisms to produce not only proteins
natural to other organisms but also new pro-
teins of man's own design for functions that
evolution never called upon living organisms to
perform.
A few examples are discussed in Chapter 9
wherein the future will see the biosynthesis of
biodegradable protein-based thermoplastics,
materials to prevent postsurgical adhesions,
temporary functional scaffoldings to direct
tissue reconstruction, and drug delivery devices
for new drug release regimens.
Indeed, the period from Wohler's accidental
synthesis of urea in 1828 to the Biotechnologi-
cal Revolution of today presents a remarkable
pilgrimage with the potential for dramatic and
constructive consequences for individual health
and societal development.
References
1.
Our pilgrimage revisits highlights in the devel-
oping understanding of products and compo-
nents of living organisms and thereby provides
historical backdrop for the development of bio-
molecular machines and materials.
2.
D.J. Boorstin, The Seekers: The Story of Man's
Continuing Quest to Understand His
World.
Random House, New York,
1998,
p.
22.
3.
T.R. Malthus, An Essay on the
Principle
of
Pop-
ulation, London, 1798; rev. ed., 1803. Quotation
excerpted from "Darwin'' A Norton Critical
Edition, Third Edition, Selected and Edited by
Philip Appleman,
W.W.
Norton, New
York,
2001,
p.
39.
4.
J. Bronowski,
The
Ascent of
Man.
Little, Brown
and Company, Boston/Toronto, 1973.
5.
So attacked was Vesalius for departing from the
Galenist doctrine that he gave up his research on
anatomy for the practice of medicine only to
attempt
a
return in the year of
his
death at age 50.
6. Harvey counted Kings (James I and Charles I)
and distinguished contemporaries such as Sir
Francis Bacon among his patients. As a result of
publishing his unorthodox views of the circula-
tion of the blood, his prestigious practice is
reported to have suffered.
7.
Ionic ammonium cyanate, NH/»«»OCN~, con-
tains the four most common elements of living
matter, carbon (C), hydrogen (H), oxygen (O),
and nitrogen (N). On heating, there is simply a
rearrangement of the atoms to result in urea,
H2NCONH2. This finding played a significant
role in the debate between mechanists and vital-
ist, a philosophical issue that little interested
Wohler.
8. The term
organic
as used here indicates "of bio-
logical origin." More currently the term organic
chemistry
has come to mean the chemistry of the
carbon atom.
9. The chemical structure of tartaric acid is
HOOC-HCOH-HOCH-COOH, which has two
carbon atoms with four different substituents,
that is, two centers of asymmetry. The natural
tartaric acid is dextrorotatory.
10.
A familiar use of the capacity to distinguish
plane-polarized light is found in the effective-
ness of Polaroid sunglasses. Sunlight scattered
from a road's surface, for example, is polarized
horizontally, and Polaroid sunglasses are
designed such that only vertically polarized light
passes through them and therefore the bright
light scattered from the road's surface does not
enter the eyes. If a dextro-rotatory solution of
tartaric acid or of sugar is placed in a tube and
the tube is placed in front of the polaroid glasses,
the scattered light from the surface of the road
that passes through the tube will also pass
through the sun glasses unless the sun glasses are
rotated clockwise. At the correct amount of
clockwise rotation, the tube becomes dark, and
more of the scattered light from the surface of
the road passes through the portion of the
sunglasses that surround the tube.
11.
L. Pasteur, "On the Molecular Asymmetry of
Natural Organic Products." In Lecons de Chimie
professees en 1860. Chemical Society of Paris,
Paris,
1861. George Stewart and Co., printers,
Edinburgh and London.
12.
Because of his extraordinary knowledge of
chemistry, Fischer was placed in charge of orga-
nizing chemical production in Germany during
the First World War. Among the many mis-
fortunes of that war were the combat deaths of
his two sons. So distraught was Fischer by his
circumstances that he ended his own life shortly
thereafter—in 1919.

References 93
13.
D. Voet and J. Voet, "Electron Transport and
Oxidative Phosphorylation." In Biochemistry,
Second Edition. John Wiley & Sons, New York,
1995.
14.
In the usual manner the Katsoyannis publication
of their work (/. Am. Chem. Soc,
85,
2863,1963)
acknowledged at the end of the publication the
support of the U. S. Public Health Service,
National Institute of Arthritis and MetaboHc
Dis-
eases.
Interestingly,
Niu
and coworkers gave their
acknowledgments in the Introductory Remarks
with the statements "by holding aloft the great
red banner of Mao Tse-tungs's thinking" and
The total chemical synthesis of insulin is a piece
of work which stems directly from the big leap
forward movement. In 1958, when the move-
ment had attained the stage of unbounded
enthusiasm for achievements,
we
took upon our-
selves the task of making synthetic insulin as the
first protein ever to be synthesized, an achieve-
ment which we hope to accomplish in a short
time to serve as a contribution to the scientific
progress of our mother country and also as an
expression of revolutionary initiative. (Kexue
Tongbao,
17,
241,1966)
15.
C.S. Roy, "The Elastic Properties of the Arterial
Wall."
/. Physiol., 3,125-159,1880.
16.
ID. Watson and EH.C. Crick, "Molecular
Structure of Nucleic Acids." Nature, 111, 131-
738,1953.
17.
S. Paabo, "The Human Genome and Our View
of Ourselves."
Science,
291 (February 16), 1219-
1220,2001.
18.
Editorial Comments, Science, 291, 1218,
2001.
19.
T.D. Pollard, "Genomics, the Cytoskeleton and
MotiUty." Nature, 409, 843-845, 2001.
20.
W-H. Li, Z. Gu, H. Wang, and A. Nekutenko,
"Evolutionary Analysis of the Human Genome."
Nature,
409, 847-849,
2001.
21.
L. Peltonen and V.A. McKusick, "Dissecting
Human Disease in the Postgenomic Era."
Science,
291,1224-1229,2001.
22.
G. Jimenez-Sanchez, B. Childs, and D. Valle,
"Human Disease Genes." Nature, 409, 853-855,
2001.
23.
D.
Drell and
A.
Adamson, "Fast Forward to 2020:
What to Expect in Molecular Medicine." In The
Human Genome Project Information: Medicine.
June,
13, 2002 (from online magazine TNTY
Futures).
Likelihood of Life's Protein Machines:
Extravagant in Construction Yet
Efficient in Function
4.1 Introduction
A protein with its specified sequence, where
each position is filled by but 1 of 20 different
amino acid residues, represents an extremely
improbable construct. Perhaps in part because
of this improbability, searches for under-
standing have reasonably considered far-
from-equilibrium conditions and addressed cir-
cumstances whereby order may appear out
of chaos. On the other hand, biochemists with
painstaking commitment have worked their
way down to the component processes of DNA
replication, of transcription of DNA into RNA,
and of translation of RNA into protein
sequence. They dissected each constituent
process into its scores of energy-requiring reac-
tions,
assembled these reactions to provide a
compelling description of each component, and
emerged with a stunning tale of heredity and
protein biosynthesis. Biology's story of protein
biosynthesis is one of such elegant detail that,
no matter how brilliant the mind, it could not
have been derived otherwise. Accordingly, there
is now little mystery in how a protein of speci-
fied sequence can come into being; it simply
requires adequate energy. In fact, the details of
protein biosynthesis reveal an extravagant
process requiring extraordinary amounts of
energy.
We must yet, of course, admit mystery in
how all of those integrated and interdependent
molecular machines came into being in forma-
tion of the first cell with the capacity to synthe-
size protein of specified sequence.
In this chapter we briefly note the improba-
bility of a protein comprised of 20 different
amino acids in specified sequence. Then we step
through the details whereby a protein of spec-
ified sequence comes into being, and, like the
accountant, sum up the cost in terms of the
biological energy currency, adenosine triphos-
phate (ATP), that is, in terms of the number of
ATP molecules consumed in the production of
a protein of unique sequence. Such an essen-
tially ordinary exercise requires inclusion in
this book in order to remove unnecessary
mystery of protein production, to provide an
example whereby molecular machines utilize
energy to produce biological structure, and to
place in perspective the ease with which new
protein-based machines evolve when function-
ing by the consilient mechanism.
4.2 Improbability of a Unique
Protein Sequence
4.2.1 Consideration of a Small 100
Residue Protein
Twenty different amino acid residues become
incorporated into protein. The question may be
stated as, what is the probability of occurrence
of even a small protein of 100 amino acid
residues of specified sequence? The calculation
can be quite simple. For equal availability of all
20 amino acids, the probability of any 1 of the
20 amino acid residues occurring in a single
position is (1/20). The probability for a com-
94
