Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.

2.8 "What Sustains Life?" Becomes "What Sustains Society?"
65
bioelastic matrix stimulates the appropriate
attached cells to produce their own extracellu-
lar matrix, that
is,
to begin the reconstruction of
a natural, functional tissue as shown in a simu-
lated urinary bladder system."^"^
Thus,
the potential exists for preparing bioe-
lastic matrices that match the deformable prop-
erties of the tissue to be reconstructed, and the
potential exists for the matrix to be a tempo-
rary functional scaffolding into which the
natural cells migrate and attach. There the cells
sense the forces that the tissue must sustain and
begin reconstructing the appropriate natural
tissue.
2.8.3 Drug Delivery
In one design for drug delivery, the drug itself
provides the chemical energy to drive folding
and contraction, that is, the drug provides the
energy for its own packaging into the drug
delivery vehicle. When the model protein con-
tains negatively charged carboxylates under the
influence of oil-like R-groups, the affinity for a
positively charged drug increases just like the
affinity increases for
protons.
Therefore, instead
of adding proton, the positively charged drug is
added to drive folding and contraction. The
model protein separates out of solution holding
the drug firmly to form the drug delivery
vehicle. As the drug leaves the vehicle so too
does the vehicle disperse. In effect, the drug
provided the glue that held the delivery device
together.
Many of the drugs that control pain, for
example, the narcotics, are positively charged.
The body has its own painkillers, referred to as
endorphins', these are naturally released by the
body after about 20 to 30 minutes of running
and are considered responsible for the runner's
high. An active part of the endorphins are
the enkephalins. One, called Leu-enkephalin
amide, is positively charged and has been
loaded into a designed model protein with a
strong oil-like sequence and with a carboxylate
occurring every 15 amino acid residues, as
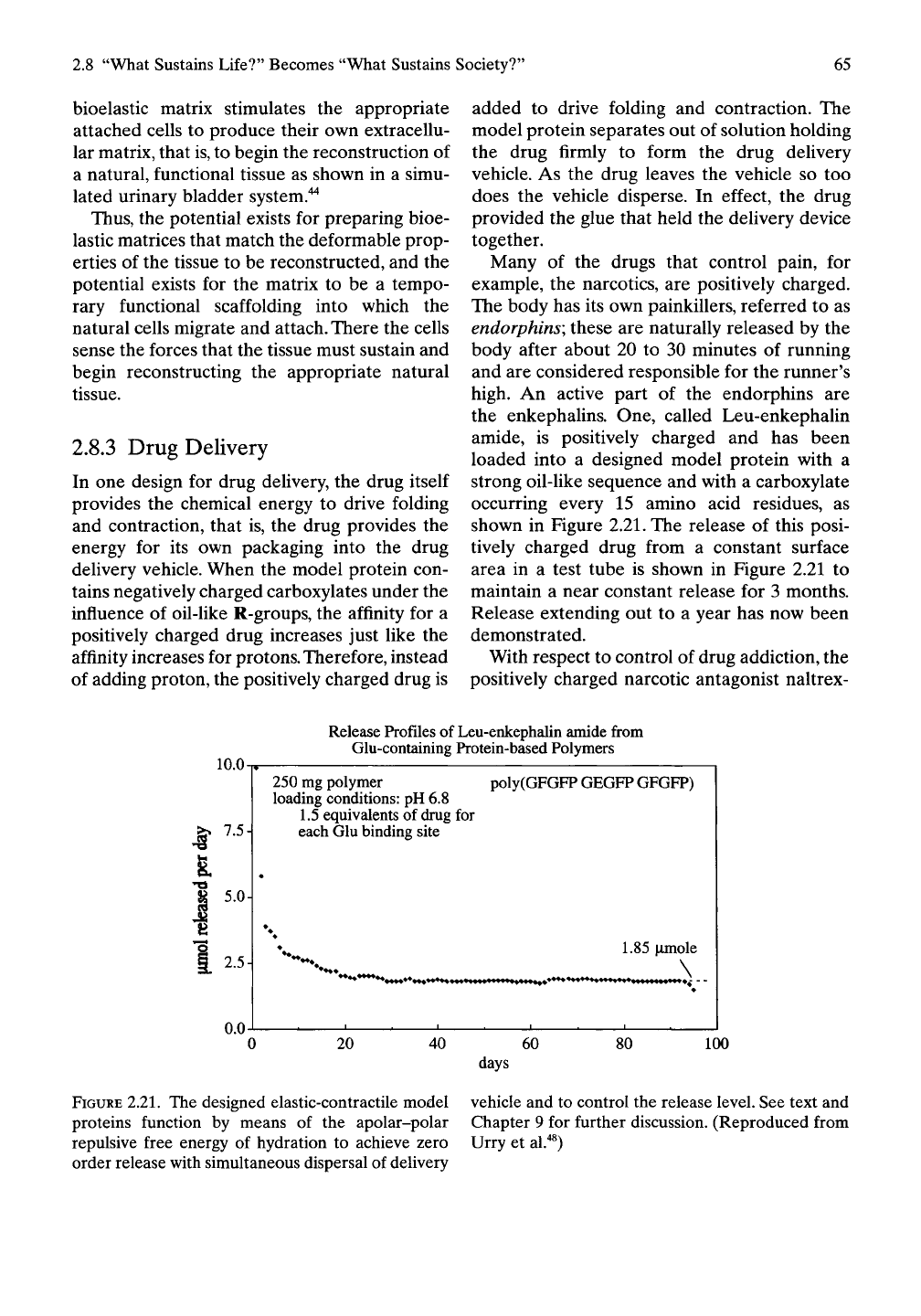
shown in Figure 2.21. The release of this posi-
tively charged drug from a constant surface
area in a test tube is shown in Figure 2.21 to
maintain a near constant release for 3 months.
Release extending out to a year has now been
demonstrated.
With respect to control of drug addiction, the
positively charged narcotic antagonist naltrex-
10.0
5.0
0.0
Release Profiles of Leu-enkephalin amide from
Glu-containing Protein-based Polymers
250 mg polymer
loading conditions: pH 6.8
1.5 equivalents of
drug
for
each Glu binding site
poly(GFGFP GEGFP GFGFP)
*•••
•••••^
1.85 [imole
20 40
60 80 100
days
FIGURE
2.21.
The designed elastic-contractile model vehicle and to control the release level. See text and
proteins function by means of the apolar-polar Chapter 9 for further discussion. (Reproduced from
repulsive free energy of hydration to achieve zero Urry et al."*^)
order release with simultaneous dispersal of delivery
66
2.
What Sustains Life? An Overview
one,
when in the blood, blocks the action of a
millionfold higher dose of heroin. An addict
with naltrexone in the bloodstream receives
no high from heroin. Therefore, a controlled
release of naltrexone from an implant in the
body to the bloodstream of an addict, after
withdrawal, would guard against return to
dependency.
2.8.4 Smart Plastics
A smart plastic would harmlessly disintegrate
once its useful Life were completed. Plastics
made of plastic-contractile model proteins with
controllable inverse temperature transitions
can be designed as smart plastics. A smart
protein-based plastic, having fulfilled its role,
would swell and become a fragile, edible
gelatin-like substance. Rather than foretell
death for the fishes, a smart protein-based
plastic could provide food for the fishes, once
its useful Life as a plastic were complete.
The natural amino acids asparagine and glu-
tamine provide chemical clocks for the disinte-
gration of protein-based plastics; they have the
R-groups -CH2CONH2 and -CH2CH2CONH2,
respectively. The carboxamide functional
group, -CONH2, hydrolyzes to negatively
charged carboxylates, -COO", at predesigned
rates.
Half of the carboxamides will convert to
carboxylates in as short a time as a few days or
in as long a time as 10 years. The time can be
set by the choice of the amino acid residues
immediately preceding and following in the
sequence and by the general oil-like character
of the model protein. The desired half-life can
be designed into the sequence of the protein-
based plastic. As the carboxylates form from
carboxamides at the surface, the plastic swells
to a fragile, edible gel. Protein-based plastics
could become food for marine life.
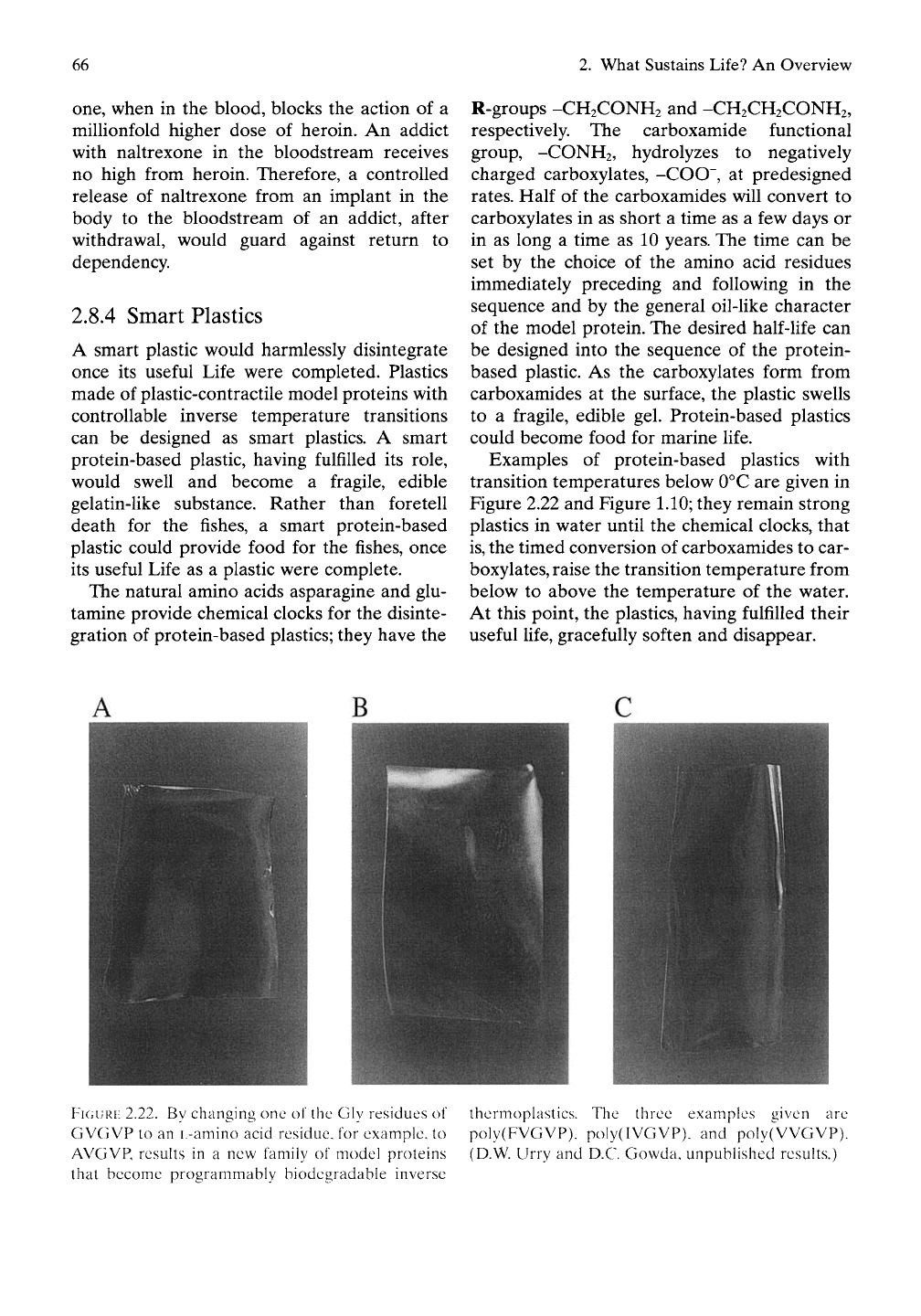
Examples of protein-based plastics with
transition temperatures below 0°C are given in
Figure 2.22 and Figure 1.10; they remain strong
plastics in water until the chemical clocks, that
is,
the timed conversion of carboxamides to car-
boxylates, raise the transition temperature from
below to above the temperature of the water.
At this point, the plastics, having fulfilled their
useful life, gracefully soften and disappear.
FIGURE
2.22. By changing one of the Gly residues of
GVGVP to an L-amino acid residue, for example, to
AVGVP, resuks in a new family of model proteins
that become programmably biodegradable inverse
thermoplastics. The three examples given are
poly(FVGVP), poly(IVGVP), and poly(VvGVP).
(D.W. Urry and D.C. Gowda, unpublished results.)

References 67
References
1.
D.W. Urry, "Elastic Biomolecular Machines: Syn-
thetic Chains of Amino Acids, Patterned After
Those in Connective Tissue, Can Transform Heat
and Chemical Energy into Motion." Sci. Am.
January 1995, 64-69.
2.
Vinegar is a solution primarily of acetic acid,
CH3COOH, and water. As shown by Pasteur in
1864,
vinegar is made from alcohol by certain
bacteria under aerobic conditions.
3.
C.B. Anfinsen, "Principles that Govern the
Folding of Protein Chains." Science, 181,
223-230,1973.
4.
D.W. Urry, "Protein Folding and the Movements
of Life." The World & /, (Natural Science, At The
Edge),
6, 301-309,1991.
5.
A. Szent-Gyorgyi, "Studies on Muscle." Acta
Physiol.
Scand.,
9 (suppl XXV), 1-116,1945.
6. D.W. Urry, "Molecular Machines: How Motion
and Other Functions of Living Organisms Can
Result from Reversible Chemical Changes."
Angew. Chem. (German) 105, 859-883, 1993;
Angew. Chem. Int. Ed. Engl., 32, 819-841,1993.
7.
D.W. Urry, "Physical Chemistry of Biological
Free Energy Transduction as Demonstrated by
Elastic Protein-based Polymers," invited Feature
Article, / Phys. Chem. B, 101, 11007-11028,
1997.
8. C.S. Roy, "The elastic properties of the arterial
wall." /. Physiol, 3,125-159,1880.
9. D.W. Urry and T.M. Parker, "Mechanics of
Elastin: Molecular Mechanism of Biological
Elasticity and its Relevance to Contraction." /.
Muscle Res. Cell Motil., 23,541-557,2002; Special
Issue, Mechanics of Elastic Biomolecules. H.
Granzier, M. Kellermayer, W Linke, Eds.
10.
L.B. Sandberg, N.T Soskel, and J.B. LesUe,
"Elastin Structure, Biosynthesis and Relation to
Disease States." A/:£ng/./.Me^.,304,566-579,1981.
11.
H. Yeh, N. Ornstein-Goldstein, Z. Indik, P
Sheppard, N. Anderson, J.C. Rosenbloom, G
Cicila, K. Yoon, and Rosenbloom, "Sequence
Variation of Bovine Elastin mRNA Due to
Alternative Splicing." J. Collagen Rel. Res., 1,
235-247,1987.
12.
It has now been possible to stretch a single
elastic protein-based polymer chain and to
obtain a uniformly increasing force versus exten-
sion curve.
^^'^"^
This was done with a surprisingly
simple device called an atomic force microscope,
the development of which resulted in the Nobel
prize for Paul Hansma. The performance of the
mechanical work of lifting a weight, shown in
Figure 2.4, utilized one-sixteenth of an inch thick
and one-fourth of an inch wide elastic bands. We
are now working to develop twisted filaments of
some three chains about a millionth of an inch
wide to perform similar energy conversions.
13.
D.W. Urry,T. Hugel, M. Seitz, H. Gaub, L. Sheiba,
J. Dea, J. Xu, and T. Parker, "Elastin: A Repre-
sentative Ideal Protein Elastomer." Philos. Trans.
R.
Soc.
Lond.
B, 357,169-184, 2002.
14.
D.W. Urry,T. Hugel, M. Seitz, H. Gaub, L. Sheiba,
J. Dea, J. Xu, L. Hayes, F. Prochazka, and T.
Parker, "Ideal Protein Elasticity: The Elastin
Model." In Elastomeric Proteins: Structures,
Biomechanical Properties and Biological Roles.
PR. Shewry, A.S. Tatham, and A.J. Bailey, Eds.
Cambridge University Press, The Royal Society;
Chapter Four, pages
54-93, 2003.
15.
E.O. Wilson, Consilience: The Unity of Knowl-
edge. Alfred E.
Knopf,
New York, 1998, p. 8.
16.
D.W. Urry, M.M. Long, and H. Sugano, "Cyclic
Analog of Elastin Polyhexapeptide Exhibits
an Inverse Temperature Transition Leading to
Crystallization." /. Biol. Chem., 253, 6301-6302,
1978.
17.
M.V. Stackelberg and H.R. Muller, "Zur Struk-
tur der Gashydrate." Naturwissenschaften, 38,
456-458,1951.
18.
D.W. Urry, S.Q. Peng,
J.
Xu, and D.T. McPherson,
"Characterization of Waters of Hydrophobic
Hydration by Microwave Dielectric Relax-
ation." /. Am. Chem. Soc, 119,1161-1162,1997.
19.
Entropy measures the change in order; a positive
change in entropy measures increased disorder,
and a negative change in entropy measures
increased order. For the transition where ice
melts to form water at 0°C (273°K on the
absolute scale where motion ceases at 0°K), the
increase in entropy is the experimentally deter-
mined heat absorbed during the transition
divided by the temperature for the transition,
that
is,
273°K (see Chapter 5 for a more complete
discussion).
20.
E. Schrodinger, What is Life? Cambridge
University Press, Cambridge, England, first
published in 1944, Canto edition with "Mind
and Matter" and Autobiographical Sketches,
Forward by R. Penrose, 1992.
21.
It should be recognized that a change in entropy,
AS,
is a readily determined experimental quan-
tity.
The measured heat of the transition, AH, the
heat in calories required to drive the folding rep-
resented in Figures 2.1 and 2.2, is simply divided
by the temperature,
Tt,
at which the folding tran-
sition occurs, that is, AH/Tt = AS.

68
2.
What Sustains Life? An Overview
22.
J.A.V. Butler, "The Energy and Entropy
of Hydration of Organic Compounds." Trans.
Faraday Soc, 33,229-238,1937.
23.
Commonly for elastic contractile model proteins
in aqueous solutions, the magnitude of the
decrease in the transition temperature,
Tt,
is pro-
portional to the negative entropy change for the
transition. The increased hydrophobicity that
lowers Tt translates into a greater exothermic
heat change, AHt, for the transition, that is,
AHt(after) - AHt(before) is a negative quantity.
The measure of the entropy change for the tran-
sition,
ASt,
is simply
AHt/Tj.
Within this measured
increase in entropy for the system of model
protein plus water, however, is a negative
entropy change due to the increase in order
resulting from model protein folding and assem-
bly. Thus, the energy sources that effect energy
conversion by this mechanism do so by means of
increasing negative entropy to the model protein
part of the system.
24.
For more details, see, for example, D. Voet, J.G.
Voet, and C.W Pratt, Fundamentals of Biochem-
istry. John Wiley & Sons, New York, 1999, pp.
529-561.
25.
The structure of the functional component of the
nicotinamides of photosynthesis and respiration
that undergoes oxidation and reduction is given
in Figure 3.6.
26.
For more details, see, for example, D. Voet, J.G.
Voet, and C.W. Pratt, Fundamentals of Biochem-
istry. John Wiley & Sons, New York, 1999, pp.
492-528.
27.
As is now taught in middle school, living organ-
isms oxidize glucose to produce the chemical
energy of ATP, the universal energy currency of
biology, whereas in our fireplaces the burning of
wood, which is the oxidation of cellulose, a
polymer of the same glucose molecule, provides
thermal energy for heating our homes.
28.
D.W. Urry, L.C. Hayes, and D. Channe Gowda,
"Electromechanical Transduction: Reduction-
driven Hydrophobic Folding Demonstrated in a
Model Protein to Perform Mechanical Work."
Biochem. Biophys. Res. Commun., 204, 230-237,
1994.
29.
A. Pattanaik, D. Channe Gowda, and D.W. Urry,
"Phosphorylation and Dephosphorylation Mod-
ulation of an Inverse Temperature Transition."
Biochem. Biophys. Res. Commun., 178, 539-545,
1991.
30.
D.W. Urry, D. Channe Gowda, S.Q. Peng, and
TM. Parker, "Non-linear Hydrophobic-induced
pKa Shifts: Implications for Efficiency of Con-
version to Chemical Energy." Chem. Phys. Lett.,
239,
67-74,1995. A millionfold increase in affin-
ity is what would be required for pumping
protons into the stomach.
31.
D.W. Urry, L.C. Hayes, D. Channe Gowda, S.Q.
Peng, and N. Jing, "Electrochemical Trans-
duction in Elastic Protein-based Polymers."
Biochem. Biophys. Res. Commun., 210,
1031-1039,1995.
32.
For more general details, see, for example, D.
Voet, J.G. Voet, and C.W. Pratt, Fundamentals of
Biochemistry. John Wiley & Sons, New York,
1999,
pp. 180-186. Muscle contraction is also
treated in some detail in Chapter 8.
33.
P.
Mitchell, "Keilin's Respiratory Chain Concept
and its Chemiosmotic Consequences." Science,
206,1148-1159,1979.
34.
I.Z. Steinberg, A. Oplatka, and A. Katchalsky,
"Mechanochemical Engines." Nature, 210,
568-571,1966.
35.
Interestingly, on the basis of the differences in
the
AGHA
values for the Asp-COOH and Asp-
COO"
in Table 5.3, protonation causes Asp to
become more oil-like and to seek out an oil-hke
site such as the lipid bilayer of the thylakoid and
inner mitochondrial membranes. The change in
AGHA
values times ten gives the calculated
energy input for the transport of ten protons to
drive one complete rotation of the Fo-motor,
which would be
38
kcal/mol-rotation. As a single
rotation of the Fo-motor can synthesize three
ATP molecules from ADP plus Pi, the chemical
energy output would be 24 kcal/mol-rotation,
which gives a maximum possible efficiency of
100x24/38 or 63%.
36.
D.W. Urry, "Free Energy Transduction in
Polypeptides and Proteins Based on Inverse
Temperature Transitions." Prog. Biophys. Mol.
Biol., 57,23-57,1992.
37.
D.M. Himmel, S. Gourinath, L. Reshetnikova, Y.
Shen, A.G. Szent-Gyorgyi, and C. Cohen, "Crys-
tallographic Findings on the Internally Uncou-
pled and Near Rigor States of Myosin: Further
Insights into the Mechanics of the Motor." Proc.
Natl.
Acad.
ScL U.S.A., 99,12645-12650,2002.
38.
It is a particular pleasure to point out in this
context that Andrew G. Szent-Gyorgyi is the son
of Albert Szent-Gyorgyi, the notable of section
2.2.1 who was perhaps the first person to see the
motion of Life outside of the living organism,
when he isolated actomyosin and added the
chemicals to cause contraction.^
39.
A.R. Fersht, "The Charging of tRNA," In Accu-
racy in Molecular Processes: Its Control and Rel-

References 69
evance to Living Systems, T.B.L. Kirkwood, R.F.
Rosenberger, and D.J. Galas, Eds., Chapman and
Hall, London, 1986, pp. 67-82.
40.
D.W. Urry and H. Eyring, "Stereochemistry
and Rate Theory in Protein Synthesis." Arch.
Biochem. Biophys., Suppl. 1, 52-62,1962.
41.
J. Bronowski, The Ascent of Man. Little, Brown
and Company, Boston/Toronto, 1973, p. 110.
42.
D.T. McPherson, J. Xu, and D.W. Urry, "Product
Purification by Reversible Phase Transition Fol-
lowing E. coli Expression of Genes Encoding up
to 251 Repeats of the Elastomeric Pentapeptide
GVGVP." Protein Expression Purification, 7,
51-57,1996.
43.
D.W. Urry, D.T. McPherson, J. Xu, H. Daniell, C.
Guda, D.C. Gowda,
N.
Jing,T.M.
Parker, "Protein-
based Polymeric Materials: Syntheses and Prop-
erties."
In The Polymeric Materials Encyclopedia:
Synthesis, Properties and Applications, CRC
Press,
Boca Raton, pp. 7263-7279,1996.
44.
D.W. Urry and A. Pattanaik, "Elastic Protein-
based Materials in Tissue Reconstruction." Ann.
N.Y.Acad ScL, 831, 32-46,1997.
45.
D.W. Urry, "Engineers of Creation," Chemistry
in Britain, 32, 39-42,1996.
46.
Dan W. Urry, "Elastic Molecular Machines in
Metabolism and Soft Tissue Restoration,"
TIBTECH,
17,249-257 (1999).
47.
N. Wang, IP Butler, and D.E. Ingber, "Mechan-
otransduction across the cell surface and
through the cytoskeleton." Science, 260,
1124-1127,1993.
48.
D.W. Urry, A. Pattanaik, M.A. Accavitti, C-X.
Luan, D.T. McPherson, J. Xu, D.C. Gowda, TM.
Parker, CM. Harris, and N. Jing, "Transductional
Elastic and Plastic Protein-based Polymers as
Potential Medical Devices," in Handbook of
Biodegradable Polymers, ed. by Domb, Kost, and
Wiseman, Harwood Academic Pubhshers, Chur,
Switzerland, pp. 367-386,1997.
The Pilgrimage: Highlights in Our
Understanding of the Products and
Components of Living Organisms
3.1 Initial Glimpses
3.1.1 The First Ionian Enlightenment:
Thales of Miletus
Historians point to a defined
period,
place,
and
person where began the ascent of man toward
an empowering understanding of
his
world.^ The
period was the 6th century
BC.
The place was
Miletus on the Aegean Sea in greater Greece,
and the person was Thales. In the 6th century
before the birth of Christ, Thales of Miletus
forged the first Ionian revolution and began dis-
peUing the illusions of knowledge embodied
in Greek mythology. No longer would myth
unchallenged provide an acceptable explana-
tion of natural phenomena. No longer would
Hesiod's accounts of the Greek Gods—Chaos,
Gaea (Earth), and Eros (Desire); and of Gaea's
giving
birth to the Heavens (Uranus), the Moun-
tains,
and the Sea (Pontus)—satisfy the desire
for understanding nature.
In its place Thales of Miletus launched the
sense of inquiry with the simple question,
"What is the world made of?" His simple
rejoinder "water" has been given explanation;
Thales of Miletus reasoned "that the principal
is water... getting the notion perhaps from
seeing that the nutriment of all things is moist
and kept alive by it... and from the fact that
the seeds of all things have a moist nature and
that water is the nature of moist things."^
As regards living things and as advanced in
this volume, water is the vital ingredient that
uniquely enables efficient function of biomo-
lecular machines. Water dissolves the more
polar parts of chain biomolecules and, in a
uniquely constrained way, transiently hydrates
"water-insoluble" oil-like parts of biomolecules
in a molecular interplay that powers protein-
based machines. Biomolecular machines
sustain Life, and, in an analogous manner,
man's machines sustain society. The require-
ments for sustaining both Life and society
necessitate access to adequate energy sources.
3.1.2 Staying the Prediction
of Malthus
In
1798,
Thomas Robert Malthus, in "An Essay
on the Principle of Population" made two
postulates: "First, That food is necessary to the
existence of man. Secondly, That the passion
between the sexes is necessary and will remain
nearly in its present state."^ Malthus then
expanded upon his postulates: "I say that the
power of population is indefinitely greater
than the power in the earth to produce subsis-
tence for man. Population, when unchecked,
increases in a geometrical ratio. Subsistence
increases only in an arithmetic ratio."
Malthus further argued, "The race of plants
and the race of animals shrink under this great
restrictive
law.
And the race of man cannot, by
any efforts of reason, escape from it. Among
plants and animals its effects are waste of
seed, sickness, and premature death. Among
mankind, misery and vice." How then has the
world for 200 years stayed the prediction of
Malthus?
70
3.1 Initial Glimpses
71
How does the earth sustain populations
greater and standards of living higher than con-
ceivable to Malthus? Machines and the ener-
gies that power them sustain Life and sustain
society. Technological developments hold back
the most disastrous devastations and derive
sustenance from the earth and sun to provide
standards of living beyond comprehension
200 years ago. By way of illustration, western
technological advancements, descendent from
that first Ionian revolution, find the oil, pump
the oil out of the earth's crust, refine the oil,
design the oil-powered machines, and thereby
provide the energy that power the machines
and increase the earth's capacity to sustain
society.
By acquiring sufficient energy resources to
fuel the molecular machines of biology (to
provide food sources) and to fuel the man-
made machines of society, technology has
stayed the Malthusian catastrophe. Popular
energy resources are not limitless, however, and
associated pollutions increasingly challenge
our biosphere. In this volume, we examine the
biomolecular machines of Life and then, in an
as yet very preliminary way, explore specific
means whereby efficient, environmentally
friendly biomolecular machines may help
sustain society.
3.1.3 A Process for Obtaining
Meaningful Answers
Beyond asking a meaningful question follows a
process for determining a meaningful answer.
That process has become known as the scien-
tific
method.
At a fundamental level the
Periodic Table of the Elements answers the
question posed 26 centuries earlier by Thales of
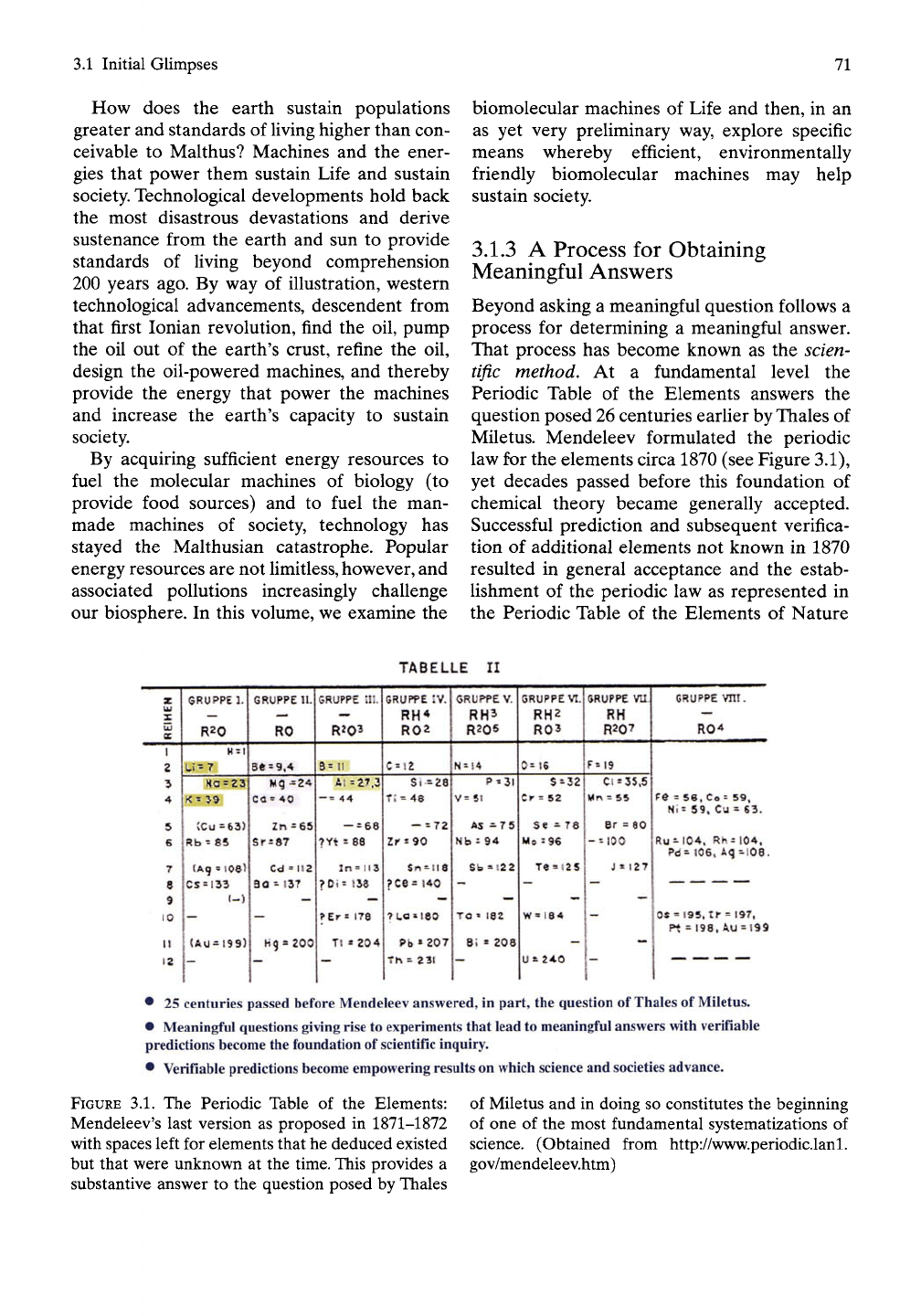
Miletus. Mendeleev formulated the periodic
law for the elements circa 1870 (see Figure 3.1),
yet decades passed before this foundation of
chemical theory became generally accepted.
Successful prediction and subsequent verifica-
tion of additional elements not known in 1870
resulted in general acceptance and the estab-
Ushment of the periodic law as represented in
the Periodic Table of the Elements of Nature
TA BELLE 11
X
X
i5
1
2
5
4
5
6
7
9
9
10
it
12
GRgPPCl.
—
R20
Hsi
mxm
(Cus63)
Rb-8S
(Agstoe)
CS=I33
C-)
—
(Au~i99)
I"-
GRUPPe 11.
—
RO
B»«9,4
MQ«24
C<1*40
Zn«65
Sr^ST
Cd«li2
90*137
—
—
H9
«
200
"~
GRUPPC III.
—
R20^
m^m
"mm::^
-=44
-*68
?Yt S88
ln=ll3
TOi= 138
—
?Er«l78
Tf «204
•"
GRUPPE W.
RH*
R02
C«>2
$1*28
T;*48
~»72
Zi'«90
Sn*tl8
?Ces|40
—
7
to*
180
Pb»207
Th»23l
GRUPPE V.
RH5
R205
N*14
P«3l
V=51
As a 75
Nbs94
Sb>l22
--
—
T0» 182
Bi * 208
""
GRUPPE VI.
RH2
R03
0»»6
S»32
Cr=52
SC *78
Mo>98
Te«l25
-
'-
W«i84
-
U»240
GRUPPE VU.
RH
R207
F«I9
Ci«35.5
Mn*59
Br
8
80
--I00
J«I27
-
—
-
-
,—
GRUPPE vm.
—
R04
Fe = 58.C«= 59,
Nis 59, Cu*63.
Ru*l04, Rha|04,
Pd>l06. Aqs.|08.
0$ = I95.tr = 197,
Pt = 198.
Au =
l99
mmmm
MIM
.M.
^mm
•
25
centuries passed before Mendeleev answered, in part, the question of
Thales
of Miletus.
• Meaningful questions giving rise
to
experiments that lead
to
meaningful answers with verifiable
predictions
become the
foundation of scientific inquiry.
• Verifiable predictions
become
empowering results
on
which
science
and societies advance.
FIGURE 3.1. The Periodic Table of the Elements:
Mendeleev's last version as proposed in 1871-1872
with spaces left for elements that he deduced existed
but that were unknown at the time. This provides a
substantive answer to the question posed by Thales
of Miletus and in doing so constitutes the beginning
of one of the most fundamental systematizations of
science. (Obtained from http://www.periodic.lanl.
gov/mendeleev.htm)
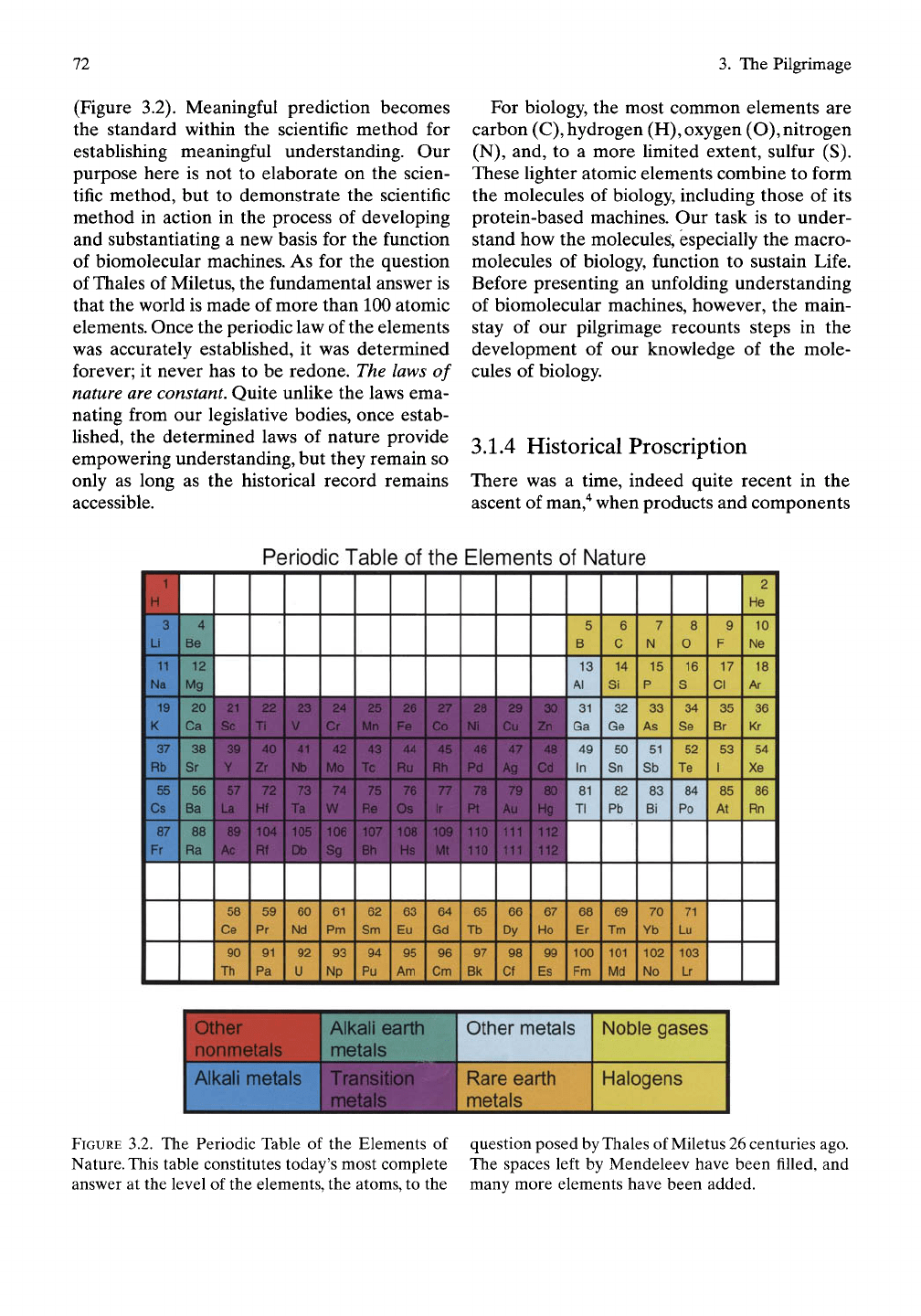
72
3.
The Pilgrimage
(Figure 3.2). Meaningful prediction becomes
the standard within the scientific method for
establishing meaningful understanding. Our
purpose here is not to elaborate on the scien-
tific method, but to demonstrate the scientific
method in action in the process of developing
and substantiating a new basis for the function
of biomolecular machines. As for the question
of Thales of Miletus, the fundamental answer is
that the world is made of more than 100 atomic
elements. Once the periodic law of the elements
was accurately established, it was determined
forever; it never has to be redone. The laws of
nature
are
constant.
Quite unlike the laws ema-
nating from our legislative bodies, once estab-
lished, the determined laws of nature provide
empowering understanding, but they remain so
only as long as the historical record remains
accessible.
For biology, the most common elements are
carbon
(C),
hydrogen
(H),
oxygen
(O),
nitrogen
(N),
and, to a more limited extent, sulfur (S).
These fighter atomic elements combine to form
the molecules of biology, including those of its
protein-based machines. Our task is to under-
stand how the molecules, especially the macro-
molecules of biology, function to sustain Life.
Before presenting an unfolding understanding
of biomolecular machines, however, the main-
stay of our pilgrimage recounts steps in the
development of our knowledge of the mole-
cules of biology.
3.1.4 Historical Proscription
There was a time, indeed quite recent in the
ascent of
man,"*
when products and components
Periodic Table of the Elements of Nature
Other metals
Rare earth
metals
Noble gases
Halogens
FIGURE
3.2. The Periodic Table of the Elements of
Nature. This table constitutes today's most complete
answer at the level of the elements, the atoms, to the
question posed by Thales of Miletus 26 centuries ago.
The spaces left by Mendeleev have been filled, and
many more elements have been added.

3.1 Initial Glimpses
73
of living organisms were considered beyond
man's competence to comprehend and beyond
man's potential to produce. This boundary
beyond which man's capacity was not to extend
prevailed long after the building of the great
gothic cathedrals. This barrier continued to
stand even after many of the profound advances
in science and medicine had withstood the test
of centuries—ev6n after the rousing advances
of Vesalius, Copernicus, Galileo, Harvey,
Malpighi, and Newton at renowned Universi-
ties like Padua, Bologna, and Cambridge.
In 1543, nearly three centuries before what
was to be the accidental rout of this particular
restraint to progress, two historic works were
pubUshed. In the first great book of modern
medical science, Humana Corpora Fabrica,
Vesalius ignored the constraints against human
dissection, accurately described human
anatomy, and thereby overturned 13 centuries
of Galenist dogma.^ In an antithetical view of
the cosmos entitled On the Revolutions of the
Celestial Spheres, Copernicus removed the
earth from the center of the universe.
Exactly two centuries before eventual bio-
logical enUghtenment, in 1628, Harvey^ pub-
Ushed De Motus
Cordis
showing the heart to be
not the seat of emotion but rather a mechani-
cal pump. Harvey further predicted the exis-
tence of capillaries to connect the outward flow
from the heart through the arteries to the
return flow through the veins, as subsequently
confirmed microscopically by Malpighi in
1661.
The Copernican view of the heavens had
been championed by Galileo, refined by
Kepler, and remarkably extended to a set of
axioms by Newton with the three laws of
mechanics, the resulting formulation of a uni-
versal law of gravitation, and the theory of the
tides.
Newton, who is viewed as the father of
modern physics, presented much of this in 1687
in his book
Principia,
often considered "one of
the most important works of science ever
written," thus capping the Scientific Revolution
of the seventeenth century.
Despite all this, more than another century
was yet to pass before a product or component
of a living organism was to be within man's
capacity to produce and to comprehend.
3.1.5 Accidental Synthesis
of Urea
The turning point, the first synthesis of a
product of living organisms, occurred in 1828
when Frederick Wohler,^ a chemist working in
Berlin, accidentally synthesized urea. Wohler
heated ammonium cyanate, NH4OCN, a well-
known inorganic compound comprised of
the NH/ and OCN~ ions. He recognized the
rearrangement product of heating as urea,
H2NCONH2, the natural non-ionic urinary
excretory product of mammals, now known to
be the primary nitrogen-containing product of
protein metabohsm.
This first organic^ synthesis began an extra-
ordinary Odyssey, emerging highUghts of
which continue to be greeted regularly with
excitement by the press, principally because
of their potential significance to health care,
to the economy, and to the environment.
This proscribed yet singular, accidental synthe-
sis of urea has transformed into the crescendo
that is the Biotechnological Revolution of
today.
Now man has the capacity to make, by chem-
ical synthesis at the laboratory bench, the major
products and components of living organisms.
Man has come to understand much of the
physical and chemical functioning of living
organisms. With this understanding, he can
design and prepare model proteins to emulate
the set of energy conversions that living organ-
isms require for survival. Furthermore, as a
hallmark of the Biotechnological Revolution,
man can transform living organisms into
chemical factories to work in society's
behalf,
for
example,
to produce natural as well as newly
designed products of use to society.
From the latter comes the fated promise to
replace the finite petroleum reserves and
to prepare products in an environmentally
friendly manner to sustain an increasingly
complex society. It is now possible, by genetic
engineering, to produce proteins that evolution
has never called upon nature to prepare and to
produce protein-based materials for addressing
primary problems required for sustaining our
society.
74
3.
The Pilgrimage
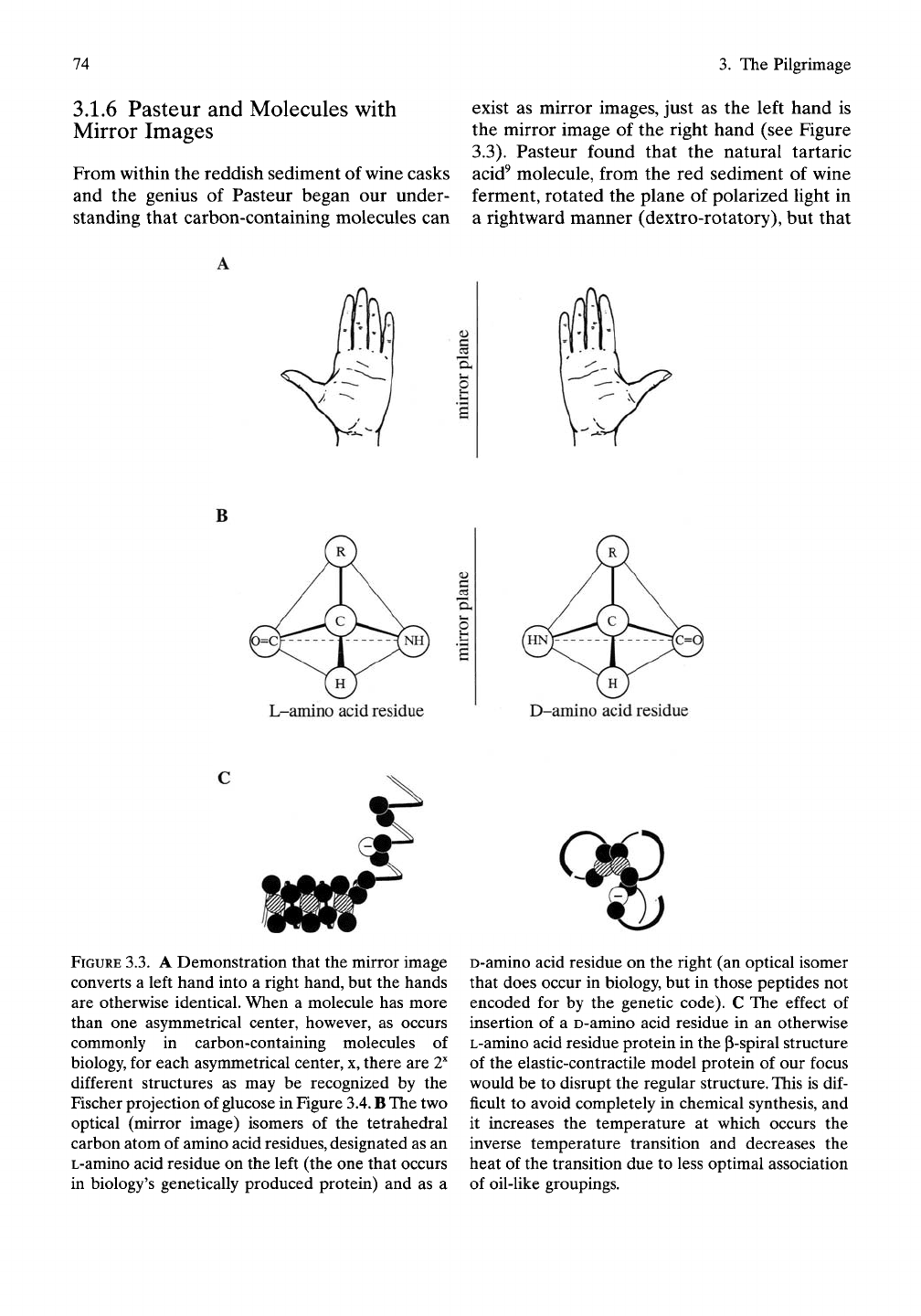
3.1.6 Pasteur and Molecules with
Mirror Images
From within the reddish sediment of wine casks
and the genius of Pasteur began our under-
standing that carbon-containing molecules can
exist as mirror images, just as the left hand is
the mirror image of the right hand (see Figure
3.3). Pasteur found that the natural tartaric
acid^ molecule, from the red sediment of wine
ferment, rotated the plane of polarized light in
a rightward manner (dextro-rotatory), but that
B
L-amino acid residue
D-amino acid residue
FIGURE
3.3.
A Demonstration that the mirror image
converts a left hand into a right hand, but the hands
are otherwise identical. When a molecule has more
than one asymmetrical center, however, as occurs
commonly in carbon-containing molecules of
biology, for each asymmetrical center, x, there are
T"
different structures as may be recognized by the
Fischer projection of glucose in Figure
3.4.
B The
two
optical (mirror image) isomers of the tetrahedral
carbon atom of amino acid residues, designated as an
L-amino acid residue on the left (the one that occurs
in biology's genetically produced protein) and as a
D-amino acid residue on the right (an optical isomer
that does occur in biology, but in those peptides not
encoded for by the genetic code). C The effect of
insertion of a D-amino acid residue in an otherwise
L-amino acid residue protein in the p-spiral structure
of the elastic-contractile model protein of our focus
would be to disrupt the regular structure. This is
dif-
ficult to avoid completely in chemical synthesis, and
it increases the temperature at which occurs the
inverse temperature transition and decreases the
heat of the transition due to less optimal association
of oil-like groupings.
