Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.

2.3 Energy Sources Cause Proteins to Fold, Assemble, and Function
35
than did the aortic wall of Roy's study. As dis-
cussed below, now more than a century after
Roy's observations, the molecular process has
been described and used not only with heating
(thermal energy) but also with the many ener-
gies that biology utilizes in performing the
work required to maintain viable organisms.^
2.3.3 Proteins Modeled After Elastin
Convert Heat to Motion
The underlying mechanism for conversion of
heat to motion derives from the interesting
property exhibited by certain proteins in water
whereby these chain molecules increase order
on increasing the temperature. Heating causes
the oil-hke groups, dispersed along the chain, to
come together, forcing the folding and assem-
bly of chains, as shown in Figure 2.1C,D. Thus,
an extended elastic model protein chain, con-
ceptually anchored at one end and attached to
a weight at the other, would fold and shorten to
move or lift the weight as the temperature is
raised over a particular range, say, as with
Roy's study, from room temperature to body
temperature.
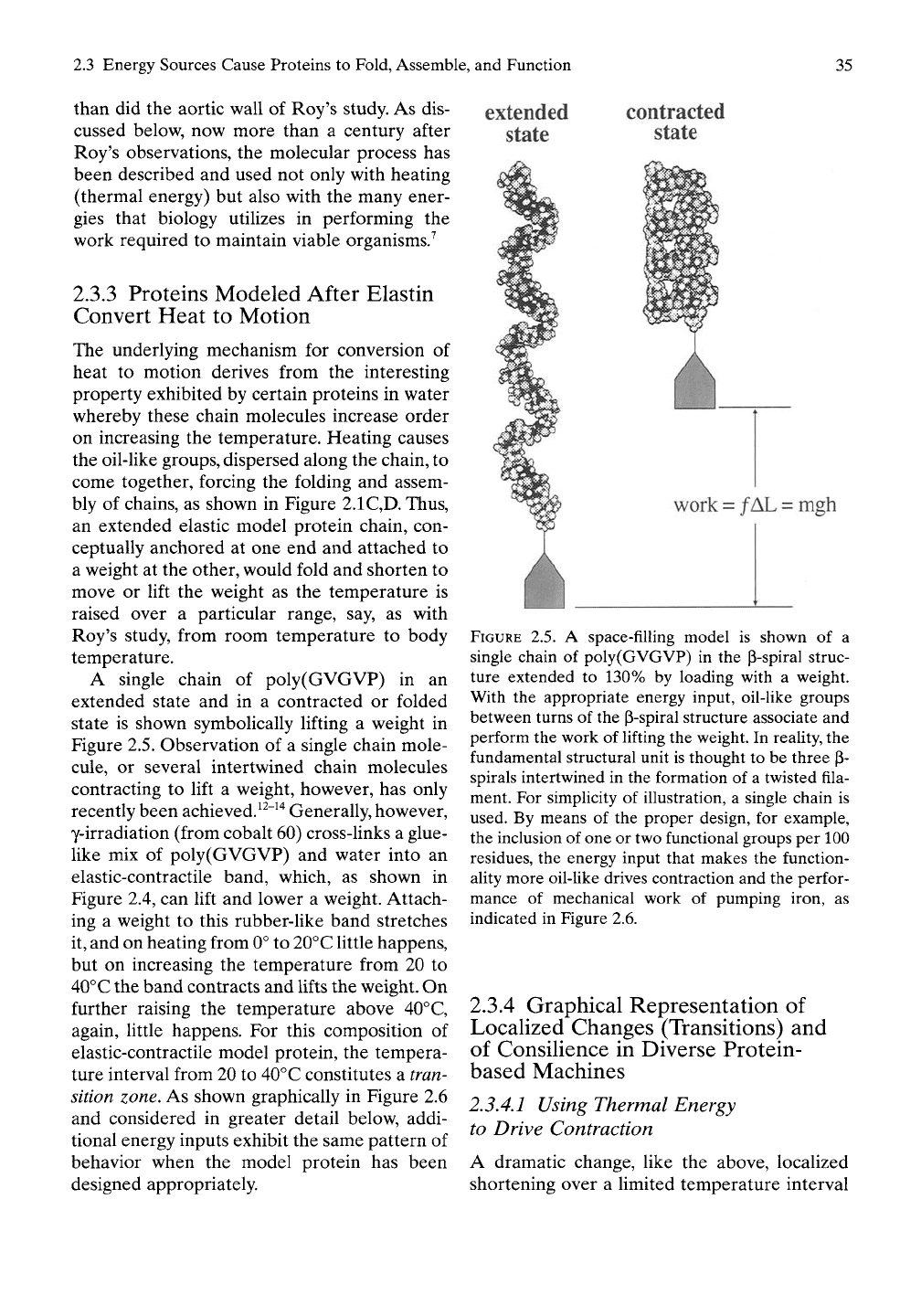
A single chain of poly(GVGVP) in an
extended state and in a contracted or folded
state is shown symbolically lifting a weight in
Figure 2.5. Observation of a single chain mole-
cule,
or several intertwined chain molecules
contracting to lift a weight, however, has only
recently been achieved.^^"^"^ Generally, however,
y-irradiation (from cobalt 60) cross-links a glue-
Uke mix of poly(GVGVP) and water into an
elastic-contractile band, which, as shown in
Figure 2.4, can lift and lower a weight. Attach-
ing a weight to this rubber-like band stretches
it, and on heating from 0° to 20°C little happens,
but on increasing the temperature from 20 to
40°C the band contracts and lifts the weight On
further raising the temperature above 40°C,
again, little happens. For this composition of
elastic-contractile model protein, the tempera-
ture interval from 20 to 40°C constitutes a tran-
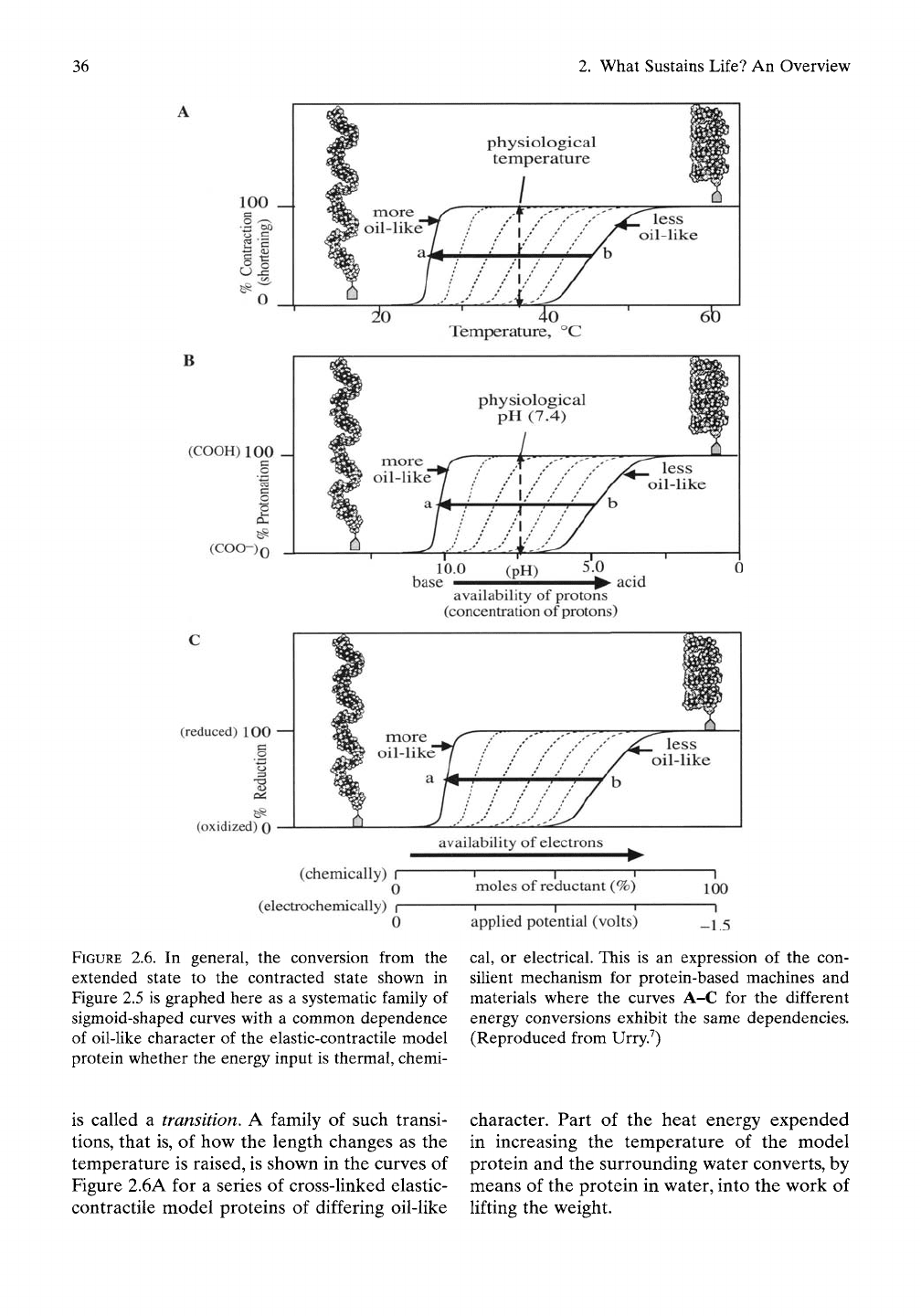
sition zone. As shown graphically in Figure 2.6
and considered in greater detail below, addi-
tional energy inputs exhibit the same pattern of
behavior when the model protein has been
designed appropriately.
extended
state
contracted
state
work = /AL = mgh
FIGURE 2.5. A space-filling model is shown of a
single chain of poly(GVGVP) in the p-spiral struc-
ture extended to 130% by loading with a weight.
With the appropriate energy input, oil-like groups
between turns of the p-spiral structure associate and
perform the work of lifting the weight. In reality, the
fundamental structural unit is thought to be three P-
spirals intertwined in the formation of a twisted fila-
ment. For simplicity of illustration, a single chain is
used. By means of the proper design, for example,
the inclusion of one or two functional groups per 100
residues, the energy input that makes the function-
aUty more oil-like drives contraction and the perfor-
mance of mechanical work of pumping iron, as
indicated in Figure 2.6.
2.3.4 Graphical Representation of
Localized Changes (Transitions) and
of Consilience in Diverse Protein-
based Machines
2,3.4.1 Using Thermal Energy
to Drive Contraction
A dramatic change, like the above, localized
shortening over a limited temperature interval
36
2.
What Sustains Life?
An
Overview
100
-4
•it
Ml
51
0
B
(COOH)
100 J
(COO-)o
(reduced)
100 —
I
(oxidized)
0 •
physiological
temperature
I
more
/^ 7" ]7f
^
oil-like^
/ I
aL
/ / / / .( /\>
/
/
y-
do
^ '
^"^^'^^'^^
Temperature,
°C
less
oil-like
65^
physiological
pH
(7.4)
^^^^ -W^'^'T'
i' /" /"y''
y^less
oil-likeY
/ / I / / /
/^ii!^e
aii^
/ ,' j' / / /h
/
/* /
-f^-*^—"^^^
^y-^ "^
r
10.0
(pH) 5.0
base
•
acid
availability
of
protons
(concentration of protons)
more
oil-like
222?^
less
oil-like
(chemically)
r"
0
(electrochemically) (—
0
FIGURE
2.6. In
general,
the
conversion from
the
extended state
to the
contracted state shown
in
Figure
2.5 is
graphed here
as a
systematic family
of
sigmoid-shaped curves with
a
common dependence
of oil-Uke character
of the
elastic-contractile model
protein whether
the
energy input
is
thermal, chemi-
availability
of
electrons
^
1
1 r
moles
of
reductant
(%)
100
-T
1 1 1
applied potential (volts)
_i 5
cal,
or
electrical. This
is an
expression
of the
con-
silient mechanism
for
protein-based machines
and
materials where
the
curves
A-C for the
different
energy conversions exhibit
the
same dependencies.
(Reproduced from Urry.^)
is called
a
transition,
A
family
of
such transi-
tions,
that
is, of how the
length changes
as the
temperature
is
raised,
is
shown
in the
curves
of
Figure
2.6A for a
series
of
cross-linked elastic-
contractile model proteins
of
differing oil-like
character. Part
of the
heat energy expended
in increasing
the
temperature
of the
model
protein
and the
surrounding water converts,
by
means
of the
protein
in
water, into
the
work
of
lifting
the
weight.

2.3 Energy Sources Cause Proteins to Fold, Assemble, and Function
37
This model protein chain intersperses oil-
like,
hydrophobic (water-fearing) R-groups and
neutral R-groups along a chain of hydrophilic
(water-loving) backbone peptide units (see Fig.
2.1
A).
At low temperature the protein dissolves
in water, but, as the temperature is raised to
the critical transition temperature for a given
sequence of residues, the oil-like R-groups
separate out of the water by folding to form
contacts within and between chain molecules.
Accordingly, the contraction on heating occurs
as oily units along the chain separate from water
and force the chain to
fold;
the oily groups come
together, minimize their contact with water, and
result in association within and between chain
molecules. In short, the oil-like groups simply
become insoluble.
The sigmoid-shaped curves of Figure 2.6A
represent the shortening of contraction that
occurs on raising the temperature through the
relevant temperature interval for the particular
extent of oil-like character of the model protein.
Elastic-contractile model proteins of more oil-
hke composition contract at lower tempera-
tures and over narrower temperature intervals.
2.3.4.2 Using Chemical Energy
to Drive Contraction
The sigmoid-shaped curves of Figure 2.6B for
model proteins containing negatively charged
vinegar-like groups demonstrate contraction
on adding positively charged protons (acid, H"^)
over the appropriate concentration range of
acid. The plot is percentage of protonation
versus decreasing pH (i.e., increasing concen-
tration of protons). Again, the negatively
charged groups within model proteins of more
oil-like composition neutralize at lower con-
centrations of acid and over a narrower con-
centration range, that is, with a larger Hill
coefficient indicating a more efficient energy
conversion. This is considered in Chapter 1,
Figures 1.2 and 1.3 and more extensively in
Chapter 5.
2.3.4.3 Using Electrical Energy
to Drive Contraction
The sigmoid-shaped curves of Figure 2.6C for
model proteins containing positively charged
groups demonstrate contraction on adding neg-
atively charged electrons (e") to again form a
more oil-like group. The positively charged
groups within model proteins of more oil-like
composition neutralize at lower availability of
electrons (a lower apphed potential) and over
a narrower change in applied potential. Again
for the more oil-like compositions, there result
larger equivalent Hill coefficients indicating a
more efficient energy conversion in analogy to
the positive cooperativity discussion in Chapter
1 and relevant to Figures 1.2 and 1.3, but
treated mathematically in Chapter 5, sections
5.7 and 5.8.
Whether the energy input is heating with
an increase in temperature or is at constant
temperature with the chemical energy input of
adding positively charged protons to negatively
charged groups or with the electrical energy
input of adding negatively charged electrons
to positively charged groups, the dependence
on the oil-Hke character of the model protein is
the same. Chapter 5 presents these correlations
with experimental data using a series of model
proteins of systematically increased oil-like
character.^
The correlations of Figure 2.6 represent one
profound expression of the consilient mecha-
nism for protein function in energy conversion;
that is, the data of Figure 2.6 represent a
''common groundwork of explanation''^^ for
protein function.
2.3.5 Heating Reversibly Increases
Protein Order, an Inverse
Temperature Transition
In general, heating at the melting point of water
causes a decrease in order, as occurs for the
transition where crystalline water, ice (in which
every atom exists in a fixed position), melts to
form less-ordered liquid water in which mole-
cules constantly shift associations from one
cluster of molecules to another. Heating at the
boiling point of water also causes a decrease in
order in the transition in which water molecules
in the liquid state, with a regular relationship
dictated by direct contact between water mole-
cules,
become water vapor with only rare colli-
sions between water molecules.
38
2.
What Sustains Life? An Overview
Quite the inverse occurs for w^ater-dissolved
protein of interest here; that
is,
by the consiHent
mechanism, heating from below to above the
folding transition increases the order of the
model protein. Because heating increases
protein order, the transition is called an inverse
temperature transition.
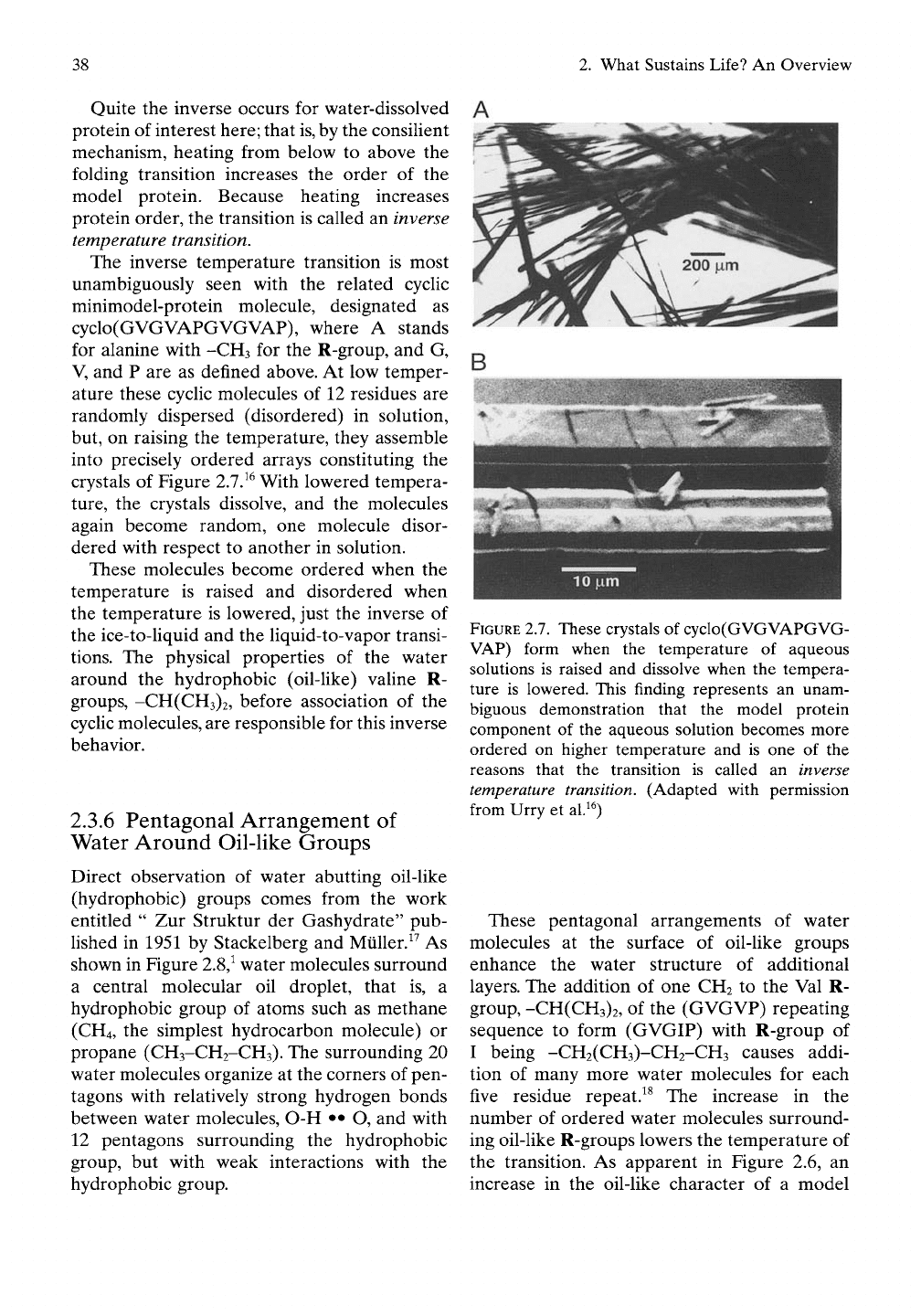
The inverse temperature transition is most
unambiguously seen with the related cyclic
minimodel-protein molecule, designated as
cyclo(GVGVAPGVGVAP), where A stands
for alanine with -CH3 for the R-group, and G,
V, and P are as defined above. At low temper-
ature these cycHc molecules of 12 residues are
randomly dispersed (disordered) in solution,
but, on raising the temperature, they assemble
into precisely ordered arrays constituting the
crystals of Figure 2.1}^ With lowered tempera-
ture,
the crystals dissolve, and the molecules
again become random, one molecule disor-
dered with respect to another in solution.
These molecules become ordered when the
temperature is raised and disordered when
the temperature is lowered, just the inverse of
the ice-to-Hquid and the Uquid-to-vapor transi-
tions.
The physical properties of the water
around the hydrophobic (oil-like) vaUne R-
groups, -CH(CH3)2, before association of the
cyclic molecules, are responsible for this inverse
behavior.
2.3.6 Pentagonal Arrangement of
Water Around Oil-like Groups
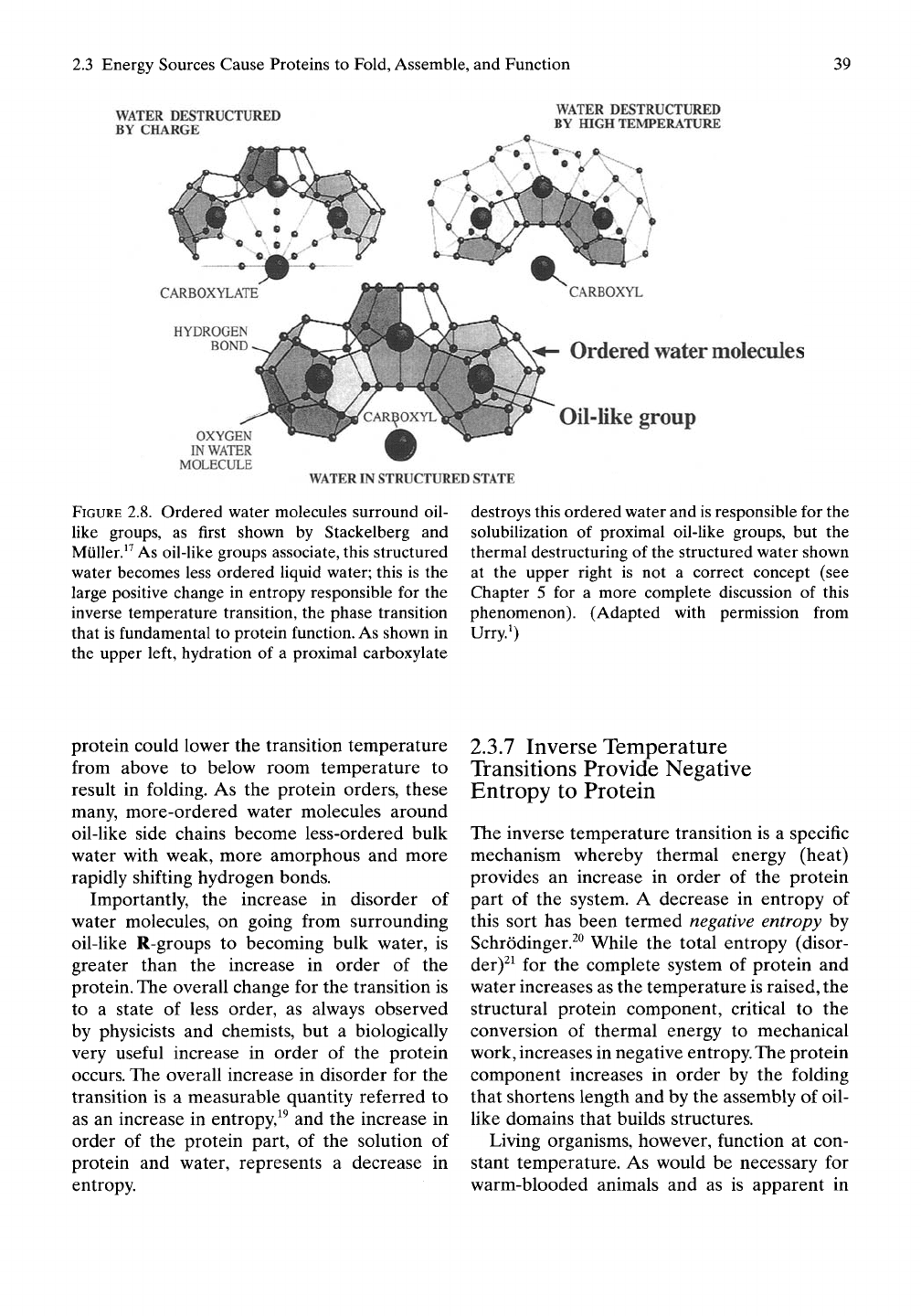
Direct observation of water abutting oil-like
(hydrophobic) groups comes from the work
entitled " Zur Struktur der Gashydrate" pub-
Hshed in 1951 by Stackelberg and Miiller.^^ As
shown in Figure 2.8,^ water molecules surround
a central molecular oil droplet, that is, a
hydrophobic group of atoms such as methane
(CH4,
the simplest hydrocarbon molecule) or
propane (CH3-CH2-CH3). The surrounding 20
water molecules organize at the corners of pen-
tagons with relatively strong hydrogen bonds
between water molecules, O-H •• O, and with
12 pentagons surrounding the hydrophobic
group, but with weak interactions with the
hydrophobic group.
FIGURE
2.7.
These crystals of cyclo(GVGVAPGVG-
VAP) form when the temperature of aqueous
solutions is raised and dissolve when the tempera-
ture is lowered. This finding represents an unam-
biguous demonstration that the model protein
component of the aqueous solution becomes more
ordered on higher temperature and is one of the
reasons that the transition is called an inverse
temperature transition. (Adapted with permission
from Urry et al.^^)
These pentagonal arrangements of water
molecules at the surface of oil-like groups
enhance the water structure of additional
layers. The addition of one CH2 to the Val R-
group, -CH(CH3)2, of the (GVGVP) repeating
sequence to form (GVGIP) with R-group of
I being -CH2(CH3)-CH2-CH3 causes addi-
tion of many more water molecules for each
five residue repeat.^^ The increase in the
number of ordered water molecules surround-
ing oil-like R-groups lowers the temperature of
the transition. As apparent in Figure 2.6, an
increase in the oil-like character of a model
2.3 Energy Sources Cause Proteins to Fold, Assemble, and Function
39
WATER DESTRUCTURED
BY CHARGE
WATER DESTRUCTURED
BY HIGH TEMPERATURE
OXYGEN
IN WATER
MOLECULE
Ordered
water
molecules
Oil-like group
WATER IN STRUCTURED STATE
FIGURE 2.8. Ordered water molecules surround oil-
like groups, as first shown by Stackelberg and
Muller.^^ As oil-like groups associate, this structured
water becomes less ordered liquid water; this is the
large positive change in entropy responsible for the
inverse temperature transition, the phase transition
that is fundamental to protein function. As shown in
the upper left, hydration of a proximal carboxylate
destroys this ordered water and is responsible for the
solubilization of proximal oil-like groups, but the
thermal destructuring of the structured water shown
at the upper right is not a correct concept (see
Chapter 5 for a more complete discussion of this
phenomenon). (Adapted with permission from
Urry.^
protein could lower the transition temperature
from above to below room temperature to
result in folding. As the protein orders, these
many, more-ordered water molecules around
oil-like side chains become less-ordered bulk
water with weak, more amorphous and more
rapidly shifting hydrogen bonds.
Importantly, the increase in disorder of
water molecules, on going from surrounding
oil-like R-groups to becoming bulk water, is
greater than the increase in order of the
protein. The overall change for the transition is
to a state of less order, as always observed
by physicists and chemists, but a biologically
very useful increase in order of the protein
occurs. The overall increase in disorder for the
transition is a measurable quantity referred to
as an increase in entropy,^^ and the increase in
order of the protein part, of the solution of
protein and water, represents a decrease in
entropy.
2.3.7 Inverse Temperature
Transitions Provide Negative
Entropy to Protein
The inverse temperature transition is a specific
mechanism whereby thermal energy (heat)
provides an increase in order of the protein
part of the system. A decrease in entropy of
this sort has been termed negative entropy by
Schrodinger.^^ While the total entropy (disor-
der)^^
for the complete system of protein and
water increases as the temperature is raised, tiie
structural protein component, critical to the
conversion of thermal energy to mechanical
work, increases in negative entropy. The protein
component increases in order by the folding
that shortens length and by the assembly of oil-
like domains that builds structures.
Living organisms, however, function at con-
stant temperature. As would be necessary for
warm-blooded animals and as is apparent in

40
2.
What Sustains Life? An Overview
Figure 2.6B,C, inverse temperature transitions
can convert energy at constant temperature.
Under these circumstances the energy inputs
change the temperature at which the inverse
temperature transition occurs, as depicted in
Figure
1.1.
This requires that the model proteins
be designed with a composition such that
the energy inputs change the transition tem-
perature from above to below body tempera-
ture.
In general, to lower the transition
temperature, the energy input makes a vinegar-
like R-group, or otherwise attached group,
more oil-like.
2.3.8 Lowering the Temperature at
Which Ordering Occurs Rather Than
Increasing the Temperature
2.3.8.1 Increasing Oil-like Character
Lowers Transition Temperature
Significantly, when the protein chain contains
more hydrophobic (oil-like) groups, the transi-
tion for folding and assembly (contraction)
occurs at a lower temperature, as in curve a of
Figure 2.6A, and over a narrower temperature
interval. Similarly, as shown in curve b of Figure
2.6A, a chain with less oil-Hke R-groups begins
its folding at a higher temperature and requires
a wider temperature interval. The dependence
of the transition temperature on the degree of
oil-like nature of the protein is pivotal to our
view of how living organisms make use of
available energy, that is, to the consilient mech-
anism of energy conversion and the negative
entropy flow that sustains Life at constant
temperature.
An example of a change to a less hydropho-
bic model protein would be the replacement of
one of the valine (V) residues having the oil-
like R-group, -CH(CH3)2, with an alanine (A)
residue having the smaller oil-like R-group,
-CH3,
as in poly(GAGVP). When y-radiation
cross-linked to form a rubber-like band,
poly(GAGVP) contracts on raising the tem-
perature, but it does so with a higher transition
temperature as shown in curve b of Figure
2.6A. In general, any energy input that makes
the protein of curve b more oil-like lowers the
transition temperature, as in curve a, and drives
contraction when the temperature is fixed at an
intermediate value.
Therefore, instead of changing the tempera-
ture,
one changes the temperature interval over
which folding occurs. Depending on the com-
position of the designed model protein, differ-
ent energy inputs increase the oil-like character
of the protein and lower the temperature range
over which folding occurs. Initially the temper-
ature range for folding may be above body tem-
perature, say 40° to 55°C, and an energy input
lowers temperature range for folding to below
body temperature, for example, to the interval
of 25° to 37°C, and drives folding to move or lift
a weight. In fact, any change that lowers the
temperature for hydrophobic folding from
above to below body temperature causes the
chain, at a constant body temperature, to go
from unfolded to folded. In this case, the
movable cusp of insolubility of Figure 1.1 moves
to lower temperatures.
2.3.8.2 Increasing Oil-like Character by
Chemical Energy
An important example occurs when the protein
chain contains charged, vinegar-Uke R-groups,
for example, -CH2COO". These charged groups
raise the temperature interval for the inverse
temperature transition. An increase in the
chemical energy, due to adding acid (H^),
results in protonation of the vinegar-like car-
boxylate, COO", to form the uncharged, more
oil-like -CH2COOH R-group. As shown in
Figure 2.6B, the stepwise conversion of
-CH2COO- groups to -CH2COOH groups
stepwise lowers the temperature of the inverse
temperature transition and has the effect in
Figure 2.6A of shifting the thermally driven
contraction from curve b to curve a. Protona-
tion folds the protein! Accordingly, the energy
input of increasing the amount of acid, thereby,
drives contraction. Energy inputs—such as
changes in the concentration of certain chemi-
cals that make the protein more oil-like—lower
the temperature range over which the folding
occurs. Such energy inputs drive contraction.
They result in the performance of mechanical
work.

2.3 Energy Sources Cause Proteins to Fold, Assemble, and Function
41
2.3.8.3 Increasing Oil-like Character by
Electrochemical Energy
As represented in Figure 2.6C, reduction of
a more polar oxidized group attached to the
model protein drives contraction. For more oil-
like model proteins, the affinity for electrons is
greater, as in curve a, and reduction requires
less electrical energy due to the steeper curve.
More oil-like model proteins require less elec-
trical energy to drive contraction. Furthermore,
reduction of a group attached to a model
protein increases the oil-like character of
the model protein, because reduction lowers
the temperature at which contraction occurs, as
shown in Figure 2.6A.
2.3.8.4 The ATt-mechanism of
Energy Conversion
A shorthand way of identifying this process for
energy conversion becomes helpful. If the tem-
perature at which the onset of the inverse tem-
perature transition occurs is called Tt and the
magnitude of the change in transition temper-
ature is indicated by a delta,
A,
then the process
of converting energy by changing the transition
temperature can be called the ATt-mechanism.
The insights of the ATt-mechanism did not
originate from studying a particular energy
conversion of an existing biological process,
such as muscle contraction. As described above,
the understanding resulted from observing
hydrophobic folding of elastic model proteins
with increases in temperature. Then it was
found, with the proper model protein design,
that many energy inputs changed the transition
temperature, that is, caused a ATt.
Now we emphasize that the most effective
chemical means for changing the temperature
range for folding involves attachment of phos-
phate, for example, phosphorylation by ATP, or
the binding of ATP
itself.
2.3.8.5 Increasing Hydration Around Oil-
like Groups Lowers T^ That Is, Lowers the
Cusp of Insolubility
The preceding discussion considered only phe-
nomenology, but it is possible to state the mech-
anism whereby the contractions due to oil-like
associations occur. Insight into the mechanism
was apparent as early as 1937 in the work of
Butler,^^ and it resides in the thermodynamics
of the solubility of oil-like groups in water, as
discussed in more detail in Chapter 5. The
Gibbs free energy, AG, governs solubility, that
is,
AG(solubiUty) = AH -
TAS.
AH is the heat of
the transition (i.e., the area of a cusp of insolu-
bility in Fig. 1.1) and is negative for favorable
reactions that release heat, and AS, the change
in entropy, is positive for an increase in disor-
der and negative for an increase in order.
In
brief,
the addition of oil-like groups in
water results in the release of heat (AH is neg-
ative).
Somewhat surprisingly, the dissolution
of oil-like groups in water is a favorable exo-
thermic reaction. Solubility of oil-like groups in
water, however, is limited, because the second
term of the Gibbs free energy for solubility,
-TAS,
is positive for hydration of oil-like
groups. The water that forms around oil-like
groups, as in Figure 2.8, is structured water,
obviously more ordered than bulk water. In this
case,
the (-TAS) term is positive, because for-
mation of structured (more ordered) water
around oil-like groups (hydrophobic hydra-
tion) gives an inherently negative AS. Thus, as
more oil-like hydration develops, an unfavor-
able positive (-TAS) term begins to become
larger than the inherently negative AH term;
the AG(solubility) becomes positive, and solu-
bility is \o^i. Accordingly, association of oil-like
groups (insolubility) results when too much
structured water develops around oil-like
groups. This constitutes a central insight into
protein function in energy conversion. In
summary, the development of too much
hydrophobic hydration causes insolubility, as
verified experimentally^^ and as discussed in
Chapter 5.
The above analysis of the processes of Figure
2.6B,C allows for the simplistic representation
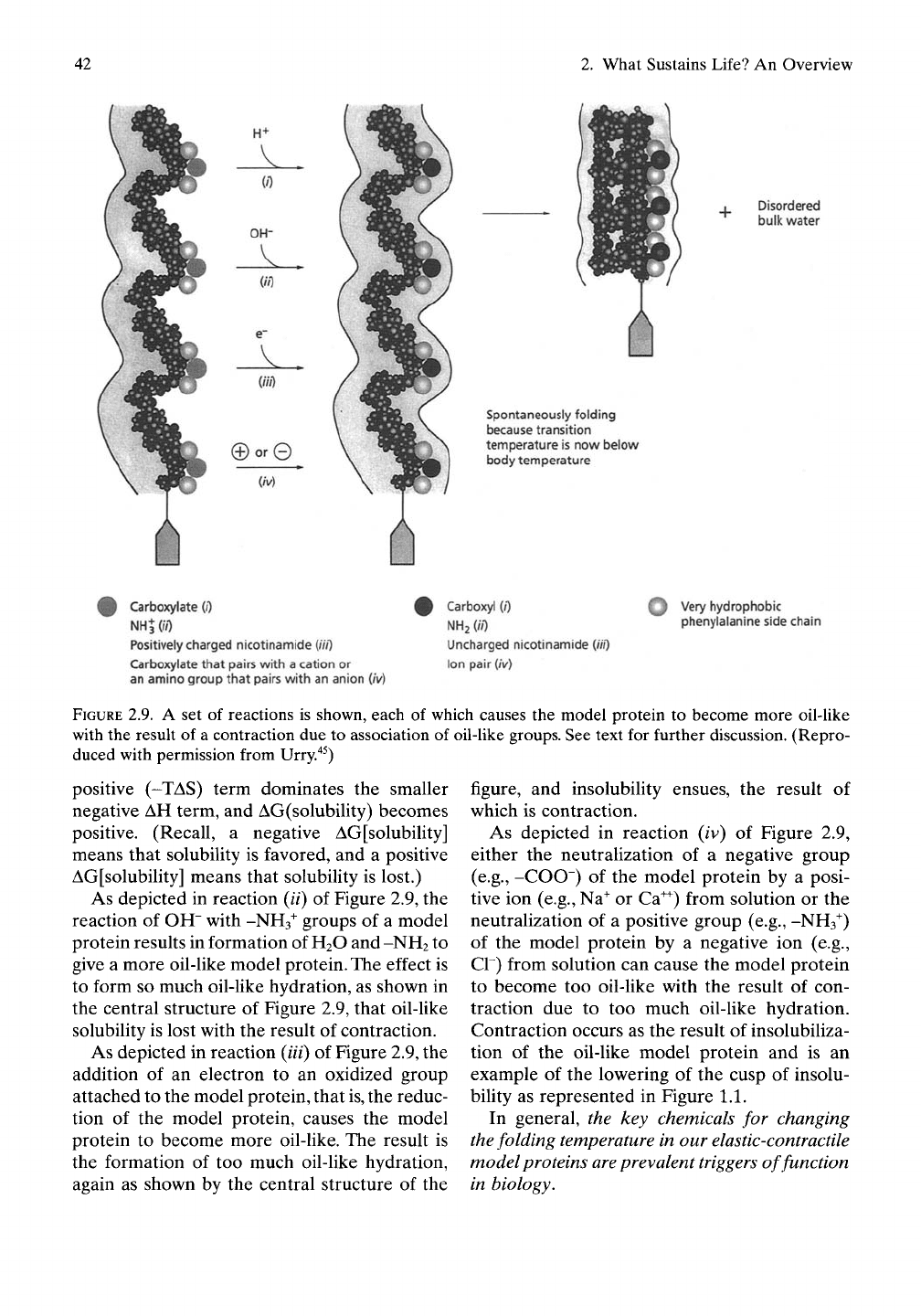
shown in Figure 2.9. As depicted in reaction
(/) of Figure 2.9, addition of proton, ff,
to a carboxylate, -COO", makes the model
protein more oil-like and allows formation of
more structured water around the model
protein, and the more oil-like model protein
becomes
insoluble.
The result is contraction due
to association of oil-like groups, because the
42
2.
What Sustains Life? An Overview
V^
(0
OH-
(//)
A^
iiii)
®orQ
iiv)
Disordered
bulk water
Spontaneously folding
because transition
temperature is now below
body temperature
Carboxylate (/)
NHJOV)
Positively charged nicotinamide
{Hi)
Carboxylate that pairs with a cation or
an amino group that pairs with an anion (/V)
Carboxyl (/")
NH2 (//)
Uncharged nicotinamide (//V)
Ion pair (/V)
Q Very hydrophobic
phenylalanine side chain
FIGURE 2.9. A set of reactions is shown, each of which causes the model protein to become more oil-Hke
with the result of a contraction due to association of oil-like groups. See text for further discussion. (Repro-
duced with permission from Urry.'^^)
positive (-TAS) term dominates the smaller
negative AH term, and AG(solubility) becomes
positive. (Recall, a negative AG [solubility]
means that solubility is favored, and a positive
AG [solubility] means that solubility is lost.)
As depicted in reaction (//) of Figure 2.9, the
reaction of OH" with -NHs^ groups of a model
protein results in formation of
H2O
and -NH2 to
give a more oil-like model protein. The effect is
to form so much oil-like hydration, as shov^n in
the central structure of Figure 2.9, that oil-like
solubility is lost with the result of contraction.
As depicted in reaction (///) of Figure 2.9, the
addition of an electron to an oxidized group
attached to the model protein, that
is,
the reduc-
tion of the model protein, causes the model
protein to become more oil-like. The result is
the formation of too much oil-like hydration,
again as shown by the central structure of the
figure, and insolubility ensues, the result of
which is contraction.
As depicted in reaction (iv) of Figure 2.9,
either the neutralization of a negative group
(e.g., -COO") of the model protein by a posi-
tive ion (e.g., Na^ or Ca^) from solution or the
neutralization of a positive group (e.g., -NHs"^)
of the model protein by a negative ion (e.g.,
Cr) from solution can cause the model protein
to become too oil-like with the result of con-
traction due to too much oil-like hydration.
Contraction occurs as the result of insolubiliza-
tion of the oil-like model protein and is an
example of the lowering of the cusp of insolu-
bility as represented in Figure 1.1.
In general, the key chemicals for changing
the folding temperature in our elastic-contractile
model proteins are prevalent triggers of function
in biology.

2.4 Essential Energy Conversions That Sustain Life
43
2.3.9 Inverse Temperature Transitions
Extract Order (Negative Entropy)
from Energy Sources!
Inverse temperature transitions provide the
mechanism whereby order, that is, negative
entropy, can be extracted from an energy
source to sustain Life. This becomes a basis
whereby the existing order of Life can be main-
tained. It requires only that the proteins of
Uving organisms be of a composition that
responds by a change in the transition temper-
ature when acted on by an available energy
source.^^ In our view, the resulting hydrophobic
association of oil-like domains of proteins con-
stitutes the negative entropy flow that sustains the
order of living organisms.
Holding such a view requires substantial
experimental verification. In our case the
required evidence resides in the capacity to
design model proteins that utilize inverse
temperature transitions for converting energy
from one form to another. This has been done
for the conversion of thermal, pressure, chemi-
cal,
electrical, and electromagnetic (e.g., light)
energies into the mechanical work of lifting
a weight simply by the mechanical aspect of
association of oil-like domains of the model
protein.
Quoting Schrodinger, "The living organism
seems to be a macroscopic system which in part
of its behaviour approaches to that purely
mechanical (as contrasted with thermodynami-
cal) conduct to which all systems tend, as the
temperature approaches the absolute zero and
the molecular disorder is removed''^^
While not approaching absolute zero of
temperature, but rather due to raising the tem-
perature, the central effect of the inverse
temperature transition is, nonetheless, mechan-
ical in origin with the analogous result of cre-
ating ordered structures. Even when energy
conversion by this mechanism does not involve
the performance of mechanical work, the
model protein can go from dissociated oil-like
domains to association of oil-like domains in
the process of converting one form of energy to
another. For example, as shown in more detail
below, when the model protein contains both an
attached positively charged oxidized group and
an attached negatively charged carboxylate, the
reduction of (the addition of negative electrons
to) the positively charged, oxidized group
lowers the transition temperature to below
body temperature and causes the protein to
fold. Significantly, in the process the energy
input of reduction forces the uptake of a proton
by a carboxylate, COO", at a distance along the
protein chain from the reduced group. In this
case,
electrochemical energy converts to the
chemical work of pumping protons, that is, of
picking up protons during the mechanical (con-
tractile) change of hydrophobic association.
2.4 Essential Energy
Conversions That Sustain Life
2.4.1 Photosynthesis and Respiration:
Summations of the Energy
Conversions of Life
The overall statements for the energy conver-
sions of biology have been known for more
than a century. They are embodied in the
expressions for photosynthesis and respiration,
as currently written and balanced for the for-
mation and utilization of a carbohydrate such
as glucose with six carbon (C) atoms and the
equivalent of a molecule of water, H2O, for
each carbon atom, consequently, the name
carbohydrate.
Photosynthesis:
carbon dioxide
-\-
water
-I-
light energy
m^
6(C02) 6(H20)
carbohydrate + oxygen
[C(H20)]6 6(02)
Respiration:
carbohydrate -t- oxygen
m^
water -t-
[C(H20)]6 6(02) 6(H20)
carbon dioxide
-1-
chemical energy
6(C02)
From the standpoint of atomic balance, respi-
ration is essentially the reverse of photo-
synthesis, but there exists the fundamental
distinction that the energy obtained from res-
44
2.
What Sustains Life? An Overview
piration is not light energy but rather the chem-
ical energy utilized to construct and maintain
living organisms. The combined effect of photo-
synthesis and respiration is to convert light
energy (more than 50 photons to produce a
glucose molecule) to chemical energy (some 36
ATPs per glucose molecule) that sustains Life.
Again, we note that ATP, adenosine triphos-
phate, is biology's common denomination for
readily accessible chemical energy. We may also
note that an estimate of the efficiency of energy
conversion from sunlight to ATP of direct use
to animals is but a few percent (see Chapter 4).
The above equations for photosynthesis and
respiration, exactly balanced with respect to
CO2,
H2O, [C(H20)]6, and O2, mask an extraor-
dinarily intricate set of reactions where balance
tends to be masked by blurring detail. The
objective here, however, is to dissect out suffi-
cient detail to expose the primary energy-
converting steps common to both processes and
to demonstrate that model proteins, utilizing
inverse temperature transitions, emulate key
elements of those energy-converting steps.^"^
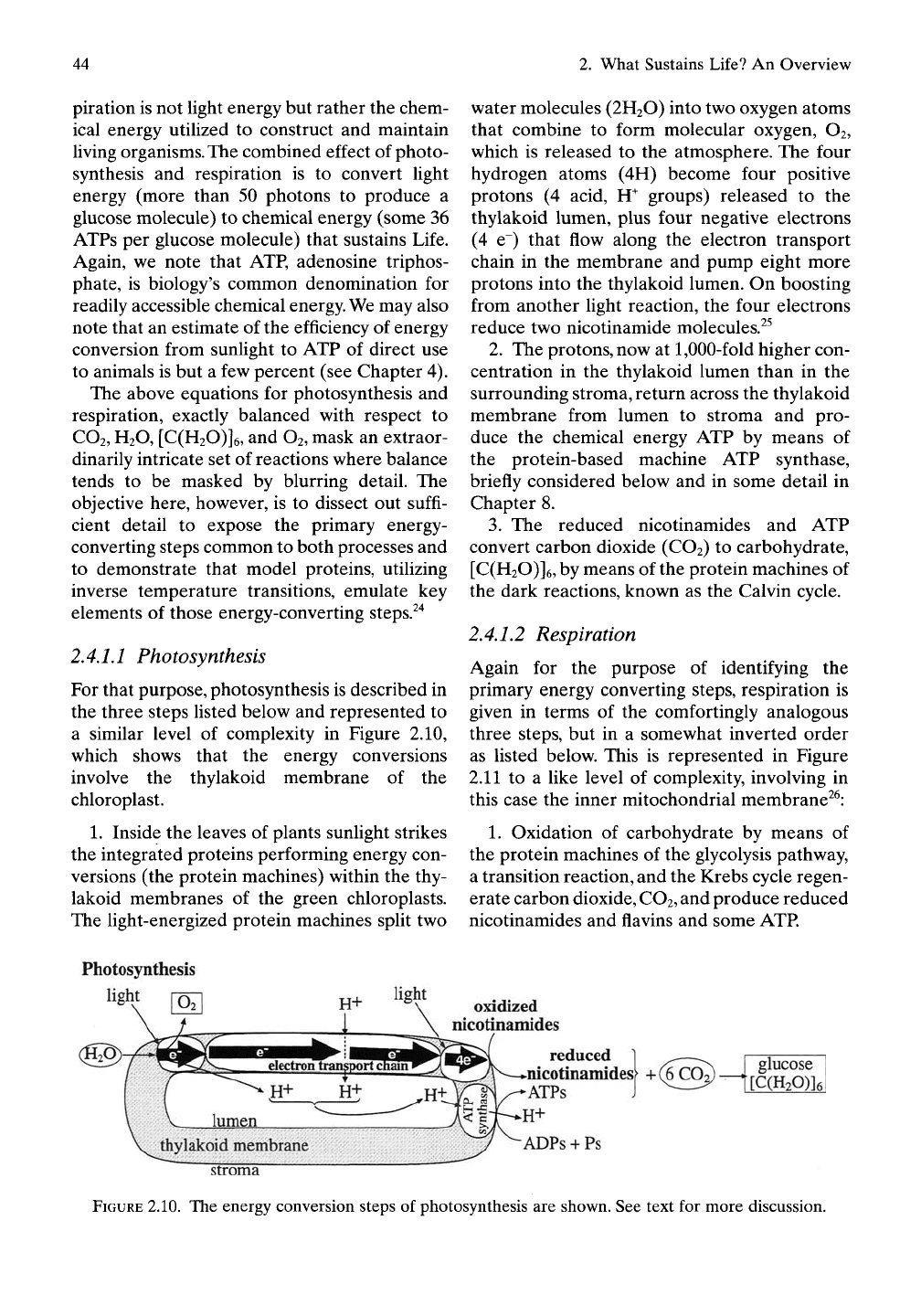
2.4,1.1 Photosynthesis
For that purpose, photosynthesis is described in
the three steps listed below and represented to
a similar level of complexity in Figure 2.10,
which shows that the energy conversions
involve the thylakoid membrane of the
chloroplast.
1.
Inside the leaves of plants sunlight strikes
the integrated proteins performing energy con-
versions (the protein machines) within the thy-
lakoid membranes of the green chloroplasts.
The light-energized protein machines split two
water molecules (2H2O) into two oxygen atoms
that combine to form molecular oxygen, O2,
which is released to the atmosphere. The four
hydrogen atoms (4H) become four positive
protons (4 acid, H^ groups) released to the
thylakoid lumen, plus four negative electrons
(4 e~) that flow along the electron transport
chain in the membrane and pump eight more
protons into the thylakoid lumen. On boosting
from another light reaction, the four electrons
reduce two nicotinamide molecules.^^
2.
The protons, now at
1,000-fold
higher con-
centration in the thylakoid lumen than in the
surrounding stroma, return across the thylakoid
membrane from lumen to stroma and pro-
duce the chemical energy ATP by means of
the protein-based machine ATP synthase,
briefly considered below and in some detail in
Chapter 8.
3.
The reduced nicotinamides and ATP
convert carbon dioxide (CO2) to carbohydrate,
[C(H20)]6, by means of the protein machines of
the dark reactions, known as the Calvin cycle.
2.4.1.2 Respiration
Again for the purpose of identifying the
primary energy converting steps, respiration is
given in terms of the comfortingly analogous
three steps, but in a somewhat inverted order
as Usted below. This is represented in Figure
2.11 to a like level of complexity, involving in
this case the inner mitochondrial membrane^^:
1.
Oxidation of carbohydrate by means of
the protein machines of the glycolysis pathway,
a transition reaction, and the Krebs cycle regen-
erate carbon dioxide,
CO2,
and produce reduced
nicotinamides and flavins and some ATP.
Photosynthesis
light
oxidized
nicotinamides
reduced 1 ^—^
nicotinamidesf +(6COo)-
ATPs J ^^—^
glucose
[C(H20)]6
H+
ADPs + Ps
stroma
FIGURE 2.10. The energy conversion steps of photosynthesis are shown. See text for more discussion.
