Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.

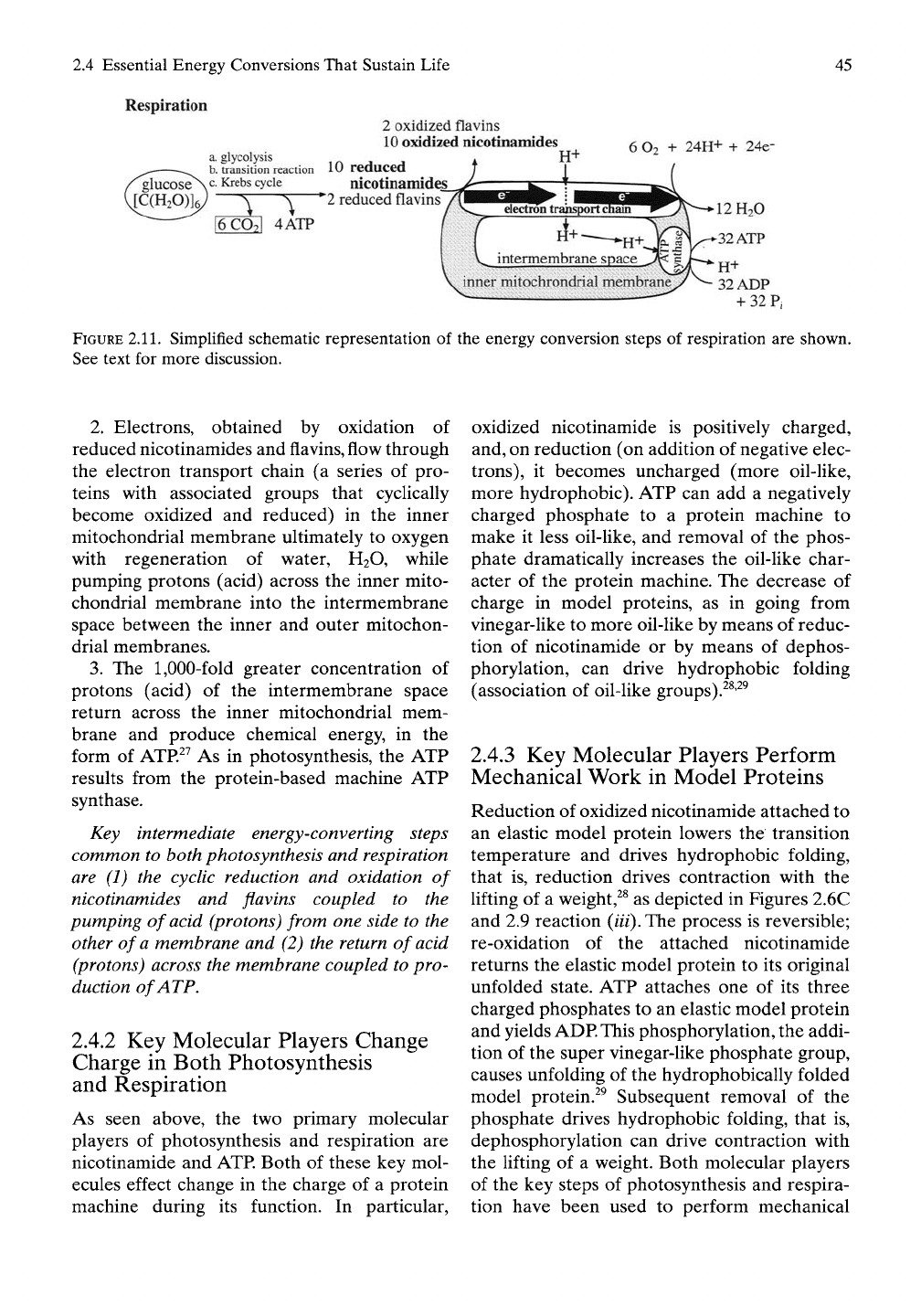
2.4 Essential Energy Conversions That Sustain Life
45
Respiration
2 oxidized flavins
10
oxidized nicotinamides
a. glycolysis ir^ j j
b.
transition reaction 10 redUCea
6
O2
+ 24H+ + 24e-
gluCOSe
>.
c.
Krebs cycle
I6CO2I 4ATP
nicotinamide^
*'2
reduced flavins
FIGURE
2.11.
Simplified schematic representation of the energy conversion steps of respiration are shown.
See text for more discussion.
2.
Electrons, obtained by oxidation of
reduced nicotinamides and flavins, flow through
the electron transport chain (a series of pro-
teins with associated groups that cyclically
become oxidized and reduced) in the inner
mitochondrial membrane ultimately to oxygen
with regeneration of water, H2O, while
pumping protons (acid) across the inner mito-
chondrial membrane into the intermembrane
space between the inner and outer mitochon-
drial membranes.
3.
The
1,000-fold
greater concentration of
protons (acid) of the intermembrane space
return across the inner mitochondrial mem-
brane and produce chemical energy, in the
form of ATP.^^ As in photosynthesis, the ATP
results from the protein-based machine ATP
synthase.
Key intermediate energy-converting steps
common to both photosynthesis and respiration
are (1) the cyclic reduction and oxidation of
nicotinamides and flavins coupled to the
pumping of acid (protons) from one side to the
other of
a
membrane and (2) the return of acid
(protons) across the membrane coupled to pro-
duction of ATP.
2.4.2 Key Molecular Players Change
Charge in Both Photosynthesis
and Respiration
As seen above, the two primary molecular
players of photosynthesis and respiration are
nicotinamide and ATP. Both of these key mol-
ecules effect change in the charge of a protein
machine during its function. In particular.
oxidized nicotinamide is positively charged,
and, on reduction (on addition of negative elec-
trons),
it becomes uncharged (more oil-like,
more hydrophobic). ATP can add a negatively
charged phosphate to a protein machine to
make it less oil-like, and removal of the phos-
phate dramatically increases the oil-like char-
acter of the protein machine. The decrease of
charge in model proteins, as in going from
vinegar-Uke to more oil-Uke by means of reduc-
tion of nicotinamide or by means of dephos-
phorylation, can drive hydrophobic folding
(association of oil-like groups).^^'^^
2.4.3 Key Molecular Players Perform
Mechanical Work in Model Proteins
Reduction of oxidized nicotinamide attached to
an elastic model protein lowers the transition
temperature and drives hydrophobic folding,
that is, reduction drives contraction with the
lifting of a weight,^^ as depicted in Figures 2.6C
and 2.9 reaction
(Hi).
The process is reversible;
re-oxidation of the attached nicotinamide
returns the elastic model protein to its original
unfolded state. ATP attaches one of its three
charged phosphates to an elastic model protein
and yields ADP This phosphorylation, the addi-
tion of the super vinegar-Hke phosphate group,
causes unfolding of the hydrophobically folded
model protein.^^ Subsequent removal of the
phosphate drives hydrophobic folding, that is,
dephosphorylation can drive contraction with
the lifting of a weight. Both molecular players
of the key steps of photosynthesis and respira-
tion have been used to perform mechanical

46
2.
What Sustains Life? An Overview
work with properly designed model proteins.
The cydic reduction/oxidation and the cydic
dephosphorylation/phosphorylation achieve
one contraction/relaxation cyde with the con-
sumption of two electrons and a proton and
with consumption of one phosphate of the ATP
molecule, respectively.
2.4.4 Relationship of Key Molecular
Players to Protons
Further direct analogy to the key intermediate
steps of photosynthesis and respiration com-
bines reduction of positively charged groups (to
drive hydrophobic folding) with performing
chemical work of changing the concentration of
protons, of pumping protons. It further com-
bines protonation of negatively charged groups
(to drive hydrophobic folding) with performing
the chemical work of energizing a phosphate
plus ADP to form ATP.
The justification for this view of ours is in the
demonstration that making a model protein
more oil-like can increase the affinity of car-
boxylates for protons up to a millionfold,^°
whereas an increase of only some 3,000-fold is
required to pump protons for the relevant steps
of photosynthesis and respiration.
As noted above in Figures 2.6C and 2.9
reaction (///), the reduction of a nicotinamide
attached to a model protein lowers the folding
temperature by increasing hydrophobic hydra-
tion and, therefore, drives folding at body
temperature.^^ Importantly, however, when
the model protein also contains a negatively
charged carboxylate, -COO", increasing the
hydrophobicity by reduction of the nicoti-
namide forces the functional vinegar-like, neg-
atively charged carboxylate group to become
uncharged and more oil-like by the uptake of a
positive proton, ¥t (see Figure 2.12B).^^ Also
directly, the association of proton, H^, with a
negatively charged R-group, for example,
-CH2-CH2-COO", on a model protein lowers
the folding temperature and drives hydropho-
bic folding at body temperature (See Figure
2.6B). Furthermore, protonation of a negatively
charged -COO" group increases hydrophobic-
ity and is expected, in our view, to energize an
attached phosphate. Pi, such that it would have
the capacity to phosphorylate ADP to produce
ATP (see Figure 2.12A). ATP
is,
again, the basic
source of energy that produces motion and per-
forms other biological functions. This combin-
ing of two different functional groups within
the same molecular machine becomes central
to the use of designed elastic-contractile model
proteins to provide insight into the mechanism
for fundamental processes of photosynthesis
and respiration. The point to retain is that the
studies on designed elastic-contractile model
protein demonstrate the correct energy consid-
erations to perform the indicated energy con-
versions, but different structures may be used
to harvest properly output energies other than
mechanical.
2.4.5 Immediate Energy Sources for
the Motion of Muscle Contraction
The source of energy for muscle contraction is
ATP.
During one cycle of contraction and relax-
ation, ATP is bound, spUt into the products of
ADP and Pi (or PO/"), and the products are
sequentially released.^^ The triggering mecha-
nism for contraction, however, is the release of
calcium ion (Ca^^) from nearby stores into the
muscle cell, where the calcium ions bind to car-
boxylates of the muscle proteins.
This sudden increase in Ca^^ concentration
also constitutes an energy requirement for con-
traction. In fact, calcium pumps in the cell mem-
brane use ATP to pump calcium ion out of the
cell into nearby storage compartments to be
held in readiness for release through trans-
membrane channels that are electrically trig-
gered to open by a nerve impulse. Thus, the
energy to drive contraction of the voluntary (or
striated) muscle of the discus thrower comes
directly from ATP and from an increase in
calcium ion concentration, with the latter also
being achieved through expenditure of ATP.
What can be demonstrated with model
proteins functioning as contractile molecular
machines is that two of the most effective
means of lowering the temperature of an
inverse temperature transition to drive con-
traction are positively charged calcium ions
(Ca^^) binding at paired negatively charged
carboxylates (COO~) to decrease net charge
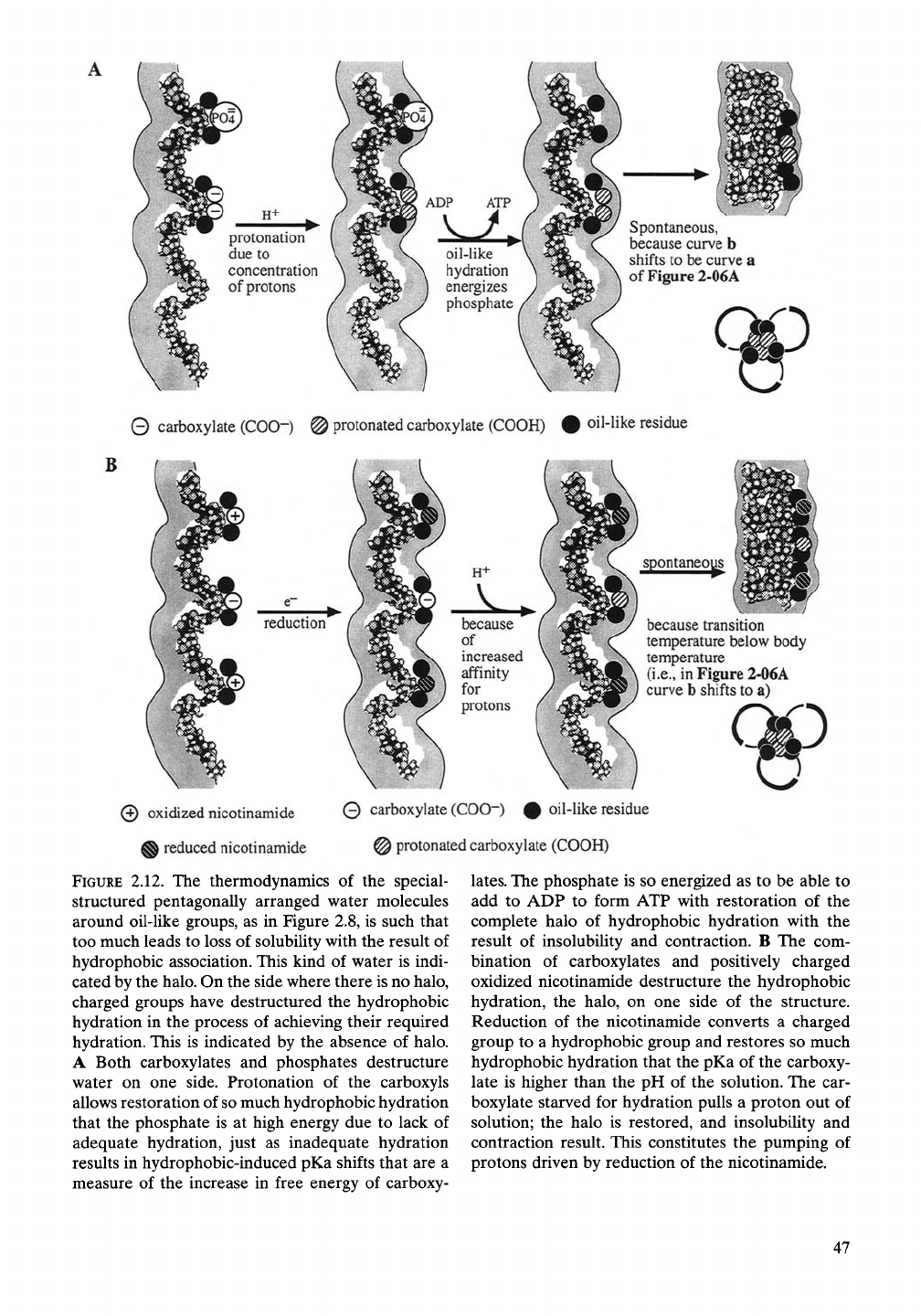
H+
protonation
due to
concentration
of protons
ADP ATP
^^
oil-like
hydration
energizes
phosphate
Spontaneous,
because curve b
shifts to be curve a
of Figure
2-06A
0 carboxylate (COO"-) @ protonated carboxylate (COOH) # oil-like residue
B
H+
^
because
of
increased
affinity
for
protons
spontaneous
because transition
temperature below body
temperature
(i.e.,
in Figure
2-06A
curve b shifts to a)
© oxidized nicotinamide 0 carboxylate (COO-) # oil-like residue
A reduced nicotinamide @ protonated carboxylate (COOH)
FIGURE
2.12. The thermodynamics of the special-
structured pentagonally arranged water molecules
around oil-like groups, as in Figure 2.8, is such that
too much leads to loss of solubility with the result of
hydrophobic association. This kind of water is indi-
cated by the halo. On the side where there is no halo,
charged groups have destructured the hydrophobic
hydration in the process of achieving their required
hydration. This is indicated by the absence of halo.
A Both carboxylates and phosphates destructure
water on one side. Protonation of the carboxyls
allows restoration of
so
much hydrophobic hydration
that the phosphate is at high energy due to lack of
adequate hydration, just as inadequate hydration
results in hydrophobic-induced pKa shifts that are a
measure of the increase in free energy of carboxy-
lates.
The phosphate is so energized as to be able to
add to ADP to form ATP with restoration of the
complete halo of hydrophobic hydration with the
result of insolubility and contraction. B The com-
bination of carboxylates and positively charged
oxidized nicotinamide destructure the hydrophobic
hydration, the halo, on one side of the structure.
Reduction of the nicotinamide converts a charged
group to a hydrophobic group and restores so much
hydrophobic hydration that the pKa of the carboxy-
late is higher than the pH of the solution. The car-
boxylate starved for hydration pulls a proton out of
solution; the halo is restored, and insolubility and
contraction result. This constitutes the pumping of
protons driven by reduction of the nicotinamide.
47

48
2.
What Sustains Life? An Overview
(see Figures 5.15 and 5.27) and dephosphoryla-
tion to remove charge (see Table 5.2).TTiese are
the most immediate energy-converting events
of muscle contraction.
2.5 Synthetic Model Protein
Machines Emulate Energy
Conversions of Photosynthesis,
Respiration, and Motion
2.5.1 Model Protein Machines in the
Coupling of Functions
Seen through the looking glass of energy con-
version, a living organism constitutes the
successful integration of many proteins func-
tioning as molecular machines. By definition, a
machine converts energy from one form to
another, and an engine is the particular
machine that performs useful mechanical work,
for example, lifting a weight or throwing an
object. Overall, therefore, the discus thrower
functions as a complex molecular engine having
converted the chemical energy derived from
the intake of food into the useful mechanical
work of throwing the discus. In doing so, the
discus thrower has successfully utilized many
coordinated molecular machines, proteins, of
different energy-converting functions. In addi-
tion to the coordination of different machines,
there is also the important coupUng of functions
within a single molecular machine.
Here we describe the coupling of functions.
A function involves any chemical entity that
can exist in either of two states, and by the con-
siUent mechanism the two states differently
affect hydrophobic hydration to a significant
extent. Two functions become coupled when
the more polar state of each decreases
hydrophobic hydration while the more
hydrophobic state of each increases the poten-
tial for hydrophobic hydration. The two func-
tions can be a chemical couple such as
-C007-C00H and a redox couple such as
the interconvertible states of oxidized nicoti-
namide/reduced nicotinamide.
With both functional groups in the same
model protein in their more polar states, that is,
ionized and oxidized, an electrical or electro-
chemical energy input to produce the reduced
state of the redox couple by making the model
protein more hydrophobic brings about the
chemical energy output of the increase in affin-
ity of a carboxylate for its proton. The bases of
the coupling of functions are the insights of the
consilient mechanism arising from the coher-
ence of the simpler contractile processes of
Figure 2.6. In fact, the common form of the
graphs of Figure
2.6,
independent of the energy
source, underscores the unifying element of
controlling hydrophobic hydration as shown
in Figure 2.9. Accordingly, de novo-designed
elastic-contractile model proteins experimen-
tally demonstrate the coupling of functions.^^
The biomolecular machines of living organ-
isms convert the chemical energies of food and
molecular oxygen, O2, through many different
energy-conversion steps, to the mechanical
work of motion to maintain Life and to the
construction of the macromolecules and cel-
lular structures that form living cells. The key
steps,
however, are the coupling of oxidation
to proton release and reduction to proton uptake,
and the coupling of proton transmembrane
flow to phosphorylation of ADP to form ATP.
In the following we use the molecular struc-
ture of the elastic-contractile model protein, as
exemplified in the simplified structural repre-
sentations in Figures 2.5,2.6, and
2.9,
to demon-
strate a principle that is transferable to protein
machines of different configurations, such as
linear and rotary motors. The fundamental
principle arises out of the competition for
hydration between apolar (oil-like) and polar
(e.g., charged) groups, given the technical term
of an apolar-polar repulsive free energy of
hydration and represented as AGap.^ Chapter 5
details the experimental foundation and analy-
sis giving rise to AGap, which may also be
thought of as a water-mediated repulsion
between hydrophobic and charged species.
Chapters 7 and 8 demonstrate the relevance of
AGap to molecular machines of biology.
2.5.2 Synthetic Model Protein
Machines Pump Protons on Reduction
Oxidized nicotinamide is more vinegar-like due
to its positive charge; this is indicated in Figure
2.9 by reaction (///) and in Figure 2.12B by the

2.5 Synthetic Model Protein Machines Emulate Energy Conversions of Photosynthesis
49
R-group with an open circle containing a plus
sign in an elastic-contractile model protein that
also contains carboxylates indicated by an open
circle with a negative sign. Like the carboxylate
in Figure 2.9 of reaction (/), charge destructures
the pentagonally structured waters surrounding
oil-like groups. Reduction of (addition of
negative electrons to) oxidized nicotinamide
makes it more oil-like and results in a more
complete shell of hydrophobic hydration sur-
rounding the second structure of Figure 2.12B.
When oxidized nicotinamide, attached to a
model protein with an inverse temperature
transition above body temperature, is reduced,
more low entropy water forms; the inverse tem-
perature transition drops below body tempera-
ture and increases the apolar-polar repulsion
between oil-like groups and carboxylates. The
consequence is a shift in the pKa of the car-
boxylate, resulting in protonation, followed by
completion of the shell of hydrophobic hydra-
tion and hydrophobic folding and contraction
represented in Figure 2.12B^^ (see also Figure
2.9 and the graphical representations in Figure
2.6).
The result of reduction is the uptake, that
is,
the pumping, of protons. Thus, reduction
pumps protons, i.e., causes the uptake of
protons, and, ahhough the structure of the
elastic-contractile model protein is inadequate to
localize a concentration buildup of protons, the
same physical principle applies as demonstrated
with the protein-based machines of the electron
transport chain (see Figures 8.12 through 8.25
of Chapter 8 and related text).
If reduction of the oxidized nicotinamide
were to occur in an appropriately structured
and carboxylate-containing protein within a
membrane, however, the pickup of proton by
the carboxylate could occur from one side of a
membrane, thereby lowering the concentration
on that side, and on oxidation the proton
release could occur to a confined space on the
other side, thereby increasing the proton con-
centration within the confined space on the
other side. Our proposal is that the cyclic reduc-
tion and oxidation of redox couples within the
thylakoid membrane of chloroplasts and within
the inner mitochondrial membrane achieves
proton pumping by means of
AGap,
the aqueous
mediated repulsion between oil-like and
charged groups that disrupts association of oil-
like groups to open access on one side and then
the other of these membranes (again, see
Figures 8.12 to 8.25 of Chapter 8 and related
text).
The resulting proton concentration gradi-
ent, as described by Mitchell's chemiosmotic
hypothesis,^^ provides the energy for phospho-
rylation of ADP to form ATP as noted below
and discussed more extensively in the text asso-
ciated with Figures 8.26 to 8.36 of Chapter 8.
2.5.3 Synthetic Elastic-contractile
Model Protein Machines to
Energize Phosphates
Both reduction of a positively charged nico-
tinamide and the protonation of a negatively
charged carboxylate increase the oil-Hke nature
of a protein. Both remove vinegar-like charge.
Phosphate, however, is super vinegar; one phos-
phate attached to a model protein is equivalent
to three or four carboxylates in its capacity to
disrupt hydrophobic association by destructur-
ing hydrophobic hydration. When the model
protein, functioning as a biomolecular machine,
is made more vinegar-like by binding of phos-
phate, the affinity of carboxylates for protons
is less (so too is affinity of a negative for a
positive charge as in ion-pair formation). The
1,000-fold
higher concentration of proton on
one side of the membrane could, however, still
drive protonation from that side of the
membrane.
Now, making the model protein more oil-hke
by protonation of several carboxylates allows
much more pentagonally structured water to
form, as schematically depicted in the Figure
2.12A. This, in our view, energizes the highly
charged phosphate, as it competes for its own
hydration by destructuring the waters hydrat-
ing the oil-like groups in an effort to obtain
the hydration that it requires. With a properly
designed active site, the phosphate could
escape the energetically charged situation by
adding to an ADP molecule to form ATP
when faced off against a sufficiently oil-like
surface.
This scenario, however, would not provide
for the most efficient energy conversion
because the phosphate, so activated, could have
a significant probability of hydrolytic cleavage
with release of heat. Too often the result could

50
2.
What Sustains Life? An Overview
be the production of thermal energy instead of
the desired chemical energy. As was noted in
Chapter 1 and as will be treated in some detail
in Chapter 8, biology has evolved a remarkably
efficient rotary motor that spatially separates
the protonation of carboxylates from the acti-
vation of phosphate. Nonetheless, from the
perspective presented in Chapter 8, the same
physical process, a counterintuitive competition
for hydration between oil-like domains and
vinegar-like species, obtains at both sites. First,
however. Chapter 5 develops an extensive
experimental and analytical basis for the water-
mediated repulsion between oil-like and
vinegar-like groups, the apolar-polar repulsive
free energy of hydration, AGap.
2.5.4 Synthetic Model Protein
Machines Pump Iron
One of the more dramatic ways to increase the
oil-like character of a model protein function-
ing as a biomolecular machine is to neutralize
the charge on a pair of carboxylates by ion
pairing to a calcium ion with its double positive
charge. Ion pairing of calcium ion with car-
boxylate ion lowers the transition from above
to below body temperature, as shown in lower-
ing the cusp of insolubility of Figure 1.1 and as
is apparent in Figure 2.6A. As in Figures 5.15
and 5.27, this allows the waters of oil-like
hydration to form with the consequence of low-
ering the transition temperature so much that
it forces hydrophobic folding and drives con-
traction. Interestingly, the trigger for muscle
contraction is calcium ion binding to a pair of
carboxylates.
The very most dramatic way to increase the
oil-like nature of a model protein is the removal
of an attached phosphate. This is demonstrated
in Figure 2.12A. Calcium ion binding to a pair
of carboxylates is second only to protonation of
a carboxylate in driving hydrophobic associa-
tion (See Figures 5.27 and 5.34). Thus, the com-
bination of calcium ion binding to a pair of
carboxylates followed by phosphate removal,
as occurs in muscle contraction, provides
perhaps the most potent means of bringing
about hydrophobic association and the associ-
ated contraction. It is our view that hydropho-
bic association caused through lowering the
cusp of insolubility, as represented in Figure 1.1,
provides the primary driving force for muscle
contraction, as will be shown in part below
in Figure 2.17 for the hydrophobic association
that occurs in the cross-bridge of the myosin II
motor in its rigor-like state and that is dissoci-
ated in the ATP bound state.
2.6 Progression to Biology's
Machines from Model
Protein Machines
2.6.1 Equivalence of Energy
Conversion to Biology's Proteins yet
Structural Limitations of Elastic-
contractile Model Proteins
Designed elastic-contractile model proteins
served to elucidate the consiUent mechanism
for energy conversion. With proper design,
they remarkably perform mechanical work
using five different energy inputs. Furthermore,
as shown by Steinberg and coworkers,^"^ there
are designs whereby a continuous band of
cross-linked elastic-contractile protein can
provide a continuous driving force for rotary
engines. The rotary engine contains two sepa-
rated baths, one that drives contraction and the
second that effects relaxation. In all, elastic-
contractile model proteins functioning by the
consilient mechanism are capable of demon-
strating more than 15 classes of pairwise energy
conversions.
It is important to realize that the principles
so derived are independent of a specific mole-
cular structure. The requirements of the mole-
cular constructs are that they can exist in water,
exhibit adequate elasticity or flexibility, and
contain oil-like groups in variable spatial rela-
tionship to vinegar-like groups with the latter
being capable of occurring in states of different
degrees of vinegar-like character.
Much fundamental science came from the
study of elastic-contractile model proteins. The
elastic-contractile model proteins provided
the molecular system for realizing the correct
description of elasticity.^ Furthermore, compe-
tition for hydration between oil-like and polar.

2.6 Progression to Biology's Machines from Model Protein Machines
51
for example, charged, groups came conclusively
from numerous designed elastic-contractile
model proteins and their characterization us-
ing a number of different physical methods.
Chapter 5 presents this in some detail.
Despite these extraordinary virtues, elastic-
contractile model proteins are structurally
limited and thereby are themselves unable to
take full advantage of the principles to which
they gave rise. Biological protein-based
machines, however, provide the fertile ground
of examples wherein the consilient mechanism
can be given generality. A progression of
gedanken images from the elastic-contractile
model proteins, based on a pentameric repeat
with translational symmetry, demonstrate
approaches to the very diverse structures of
biology's protein-based machines. The remain-
der of this section begins a succession of steps,
and Chapters 7 and 8 carry the argument
directly to a number of the protein machines of
biology.
2.6.2 ATP Synthase Common to Most
Living Systems
As impUed by Figure 2.10, which shows details
of photosynthesis, ATP synthase plays the
central role for the production of ATP in pho-
tosynthetic plants, which ATP is used in the syn-
thesis of carbohydrate and all other organic
molecules of plants and as the energy source to
power the protein-based machines of plants. As
similarly indicated in Figure 2.11 for details of
respiration, the same ATP synthase provides
nearly 90% of the ATP produced in the oxida-
tion of glucose to carbon dioxide and water.
Because of the central role of ATP in the syn-
thesis of biomolecules and in powering
biology's machines, it is not unreasonable to
consider ATP synthase to be the most impor-
tant protein-based machine of biology.
As introduced in Chapter
1,
ATP synthase is
in reality a pair of mechanically coupled rotary
motors; the Fo-motor driven by the proton
gradient provides the mechanical output of a
rotating shaft, and the Fi-motor driven by the
rotating shaft produces ATP from ADP and Pi
(inorganic phosphate, P04~^).
2.6.2.1 The Fo-motor of ATP Synthase
The Fo-motor presents a simplistic utilization
of the consiUent mechanism. The repulsion,
between a vinegar-like carboxylate of a cleat of
a wheel and the sea of oil of a cell membrane,
becomes an attraction on protonation,^^ and
the wheel rotates one cleat into the oily
membrane as a second cleat rotates into a
suf-
ficiently polar/aqueous position for a carboxyl
to release its proton to the other side of
the membrane. The direction of rotation of
the wheel and importantly of the axle (the
y-rotor) that extends from the wheel depends
on the frequency with which protons enter
and leave from each side of the membrane.
This straightforward demonstration of the
consilient mechanism will be considered in
more detail in relation to Figures 8.26 through
8.30.
2.6.2.2 The Fj-motor: ATP Synthesis
by Changing Repulsion Between the
Oil-like Surface of the Rotor and the
Charged Nucleotides at the Origin of
Water-filled Clefts
The same repulsive interaction, AGap, between
an oil-like surface and charges associated with
the nucleotides occurs in the synthesis of ATP
from ADP and Pi by the Fi-motor—the second,
the extramembrane, component of ATP syn-
thase. As noted above, the intramembrane
component functions to rotate an axle-like
projection, called the y-rotor, from the center
of an intramembrane rotary motor driven by
protons.
In the extramembrane component the y-
rotor forms the stem and core of an orange-
shaped structure comprised of six sections,
three a-subunits and three p-subunits, arranged
as threefold symmetrical (aP) pairs, designated
as
(ap)3.
The key element of the consilient
mechanism appUed to ATP synthase is that the
y-rotor exhibits three faces of very different
hydrophobicity. In our view, rotation of the
y-rotor by the Fo-motor causes the very
hydrophobic side of the rotor to be spatially
opposed, through a water-filled cleft, to the cat-
alytic site containing the most charged state.
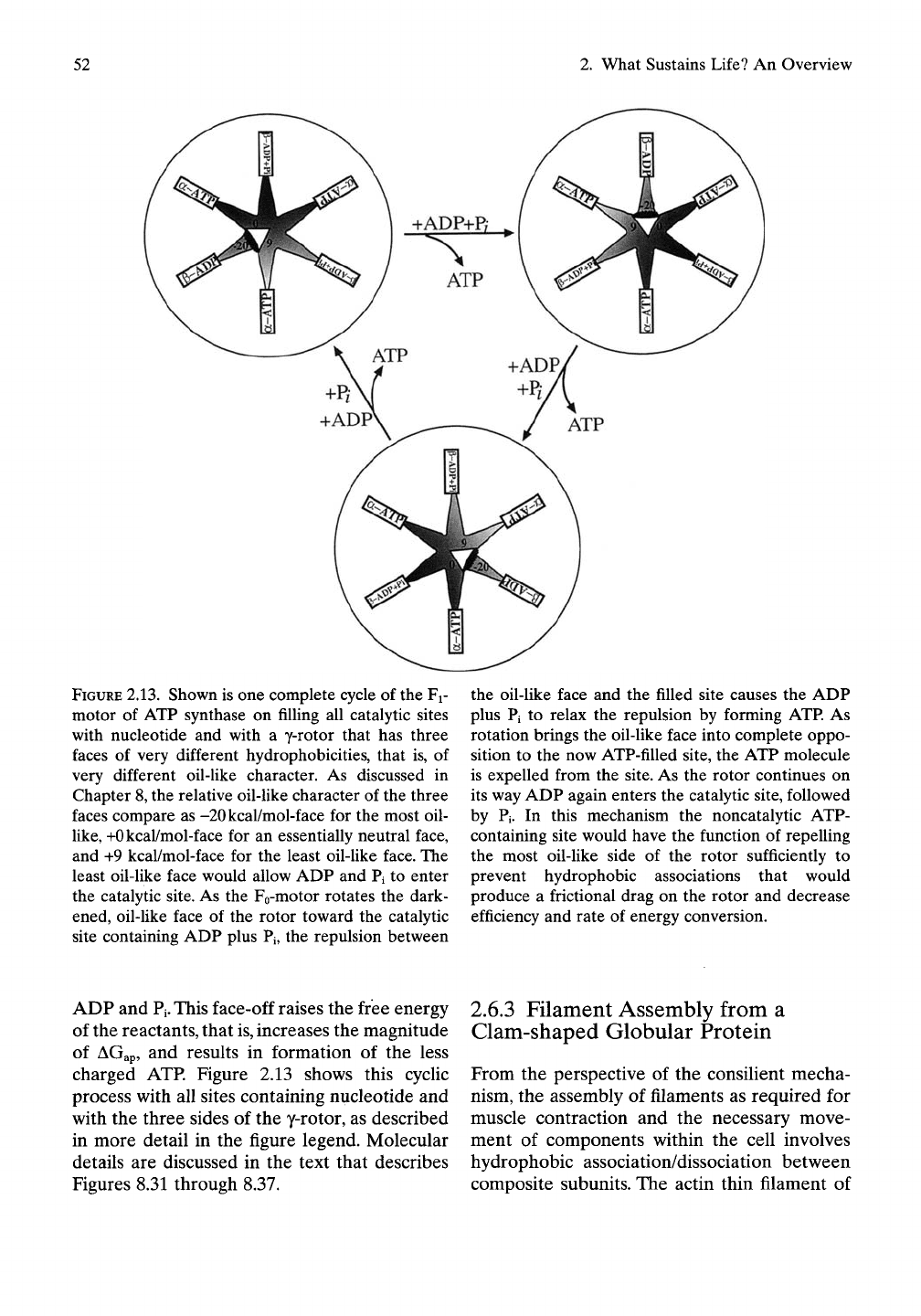
52
2.
What Sustains Life? An Overview
FIGURE
2.13.
Shown is one complete cycle of the Fi-
motor of ATP synthase on filling all catalytic sites
with nucleotide and with a y-rotor that has three
faces of very different hydrophobicities, that is, of
very different oil-like character. As discussed in
Chapter 8, the relative oil-like character of the three
faces compare as -20kcal/mol-face for the most oil-
like,
+Okcal/mol-face for an essentially neutral face,
and +9 kcal/mol-face for the least oil-like face. The
least oil-like face would allow ADP and Pi to enter
the catalytic site. As the Fo-motor rotates the dark-
ened, oil-like face of the rotor toward the catalytic
site containing ADP plus Pi, the repulsion between
the oil-like face and the filled site causes the ADP
plus Pi to relax the repulsion by forming ATP. As
rotation brings the oil-like face into complete oppo-
sition to the now ATP-fiUed site, the ATP molecule
is expelled from the site. As the rotor continues on
its way ADP again enters the catalytic site, followed
by Pi. In this mechanism the noncatalytic ATP-
containing site would have the function of repelling
the most oil-like side of the rotor sufficiently to
prevent hydrophobic associations that would
produce a frictional drag on the rotor and decrease
efficiency and rate of energy conversion.
ADP and
Pi.
This face-off raises the free energy
of the reactants, that
is,
increases the magnitude
of AGap, and results in formation of the less
charged ATP. Figure 2.13 shows this cyclic
process with all sites containing nucleotide and
with the three sides of the y-rotor, as described
in more detail in the figure legend. Molecular
details are discussed in the text that describes
Figures 8.31 through 8.37.
2.6.3 Filament Assembly from a
Clam-shaped Globular Protein
From the perspective of the consilient mecha-
nism, the assembly of filaments as required for
muscle contraction and the necessary move-
ment of components within the cell involves
hydrophobic association/dissociation between
composite subunits. The actin thin filament of

2.6 Progression to Biology's Machines from Model Protein Machines
53
muscle contraction and the microtubules of
intracellular transport present two relevant
examples. Both pose the same challenge to the
consilient mechanism. Assembly of the actin
filament requires ATP and assembly of micro-
tubules requires structurally and energetically
equivalent GTP (guanosine triphosphate).
Thus,
how could binding a triphosphate to a soluble
subunit unit bring about hydrophobic assembly,
when, by the consiUent mechanism, the tri-
phosphate should be disrupting hydrophobic
association? The issue is approached below by
considering dimerization of a hydrophobically
closed clam-shaped monomer.
2.6.3.1 Disrupting Intramolecular
Hydrophobic Association to Form
Intermolecular Hydrophobic Association
Phosphorylation initiated dimerization by
hydrophobic association is illustrated in Figure
2.14. In Figure 2.14A, a closed clam-shaped
globular protein, representing globular G-actin,
is held closed by hydrophobic association; but
phosphorylation near the mouth destroys a sig-
nificant amount of the hydrophobic hydration
and allows the clam-shaped globular protein to
open^^in analogy to the unfolding of our elastic
model protein on phosphorylation.^^ The resid-
ual hydrophobic hydration of the open state,
however, becomes the driving force for associ-
ation, that is, for the dimerization shown in
Figure 2.14B.
2.6.3.2 Treadmilling in Formation and
Maintenance of Actin and Microtubular
Tracts for Motion
Addition of a third dimension to the two-
dimensional representation in Figure 2.14B
provides an additional hydrophobic face for fil-
ament growth. Growth of the actin filament
occurs at one end by the conversion of
monomeric globular G-actin to filamentous F-
actin on ATP binding. In this case ATP binding
equates to the phosphorylation in Figure 2.14B.
Dissociation to form a G-actin occurs on
hydrolysis, giving ADP and Pi at the opposite
end of the filament. Release of
Pi,
leaving ADP
allows for recovery of the monomeric form with
ADP release.
Thus,
there is a treadmilling with
ATP-dependent growth at the positive end and
hydrolysis-dependent dissociation at the nega-
tive end.
In the case of microtubules the unit that adds
or leaves is an ap heterodimer called tubulin.
2.6.4 Deriving Contraction from a
Hydrophobically Closed Clam-shaped
Globular Protein
2.6.4.1 Adapting the Clam-shaped
Globular Protein Motif to Approach a
Sliding Filament Element of Contraction
In Figure 2.15, the clam-shaped globular
protein motif is adapted to demonstrate one
of many possible ways that the control of
hydrophobic folding could achieve motion by
means of a sliding filament/cross-bridge model
for muscle contraction. In this case the increase
in energy of the attached and energized phos-
phate allows that water become the receptor
molecule for the high-energy phosphate rather
than a molecule of ADP.
We hasten to clarify the purpose of Figure
2.15,
which is to exemplify the compliant nature
of a mechanism based on the repulsion
between oil-like and vinegar-like groups. It
shows the ease of a conceptual progression
from the more obvious phosphorylation control
of opening and closing of a clam-shaped
globular protein to a more involved swinging
cross-bridge/sliding filament process.
2.6.5 A First Step in Putting
an ATPase Motor to Work,
Controlling Association with a
Complementary Surface
The families of proteins that consume ATP
while functioning as protein-based machines
are called ATPases. Muscle contraction, just
noted
above,
is
a member of the family of linear
(contractile) protein motors that also includes
ATPases that walk along protein tubules and
transport elements from one part of the cell to
another. Another class of protein motors that
uses ATP rotary and nonrotary ion pumps
transports ions from one side to the other of the
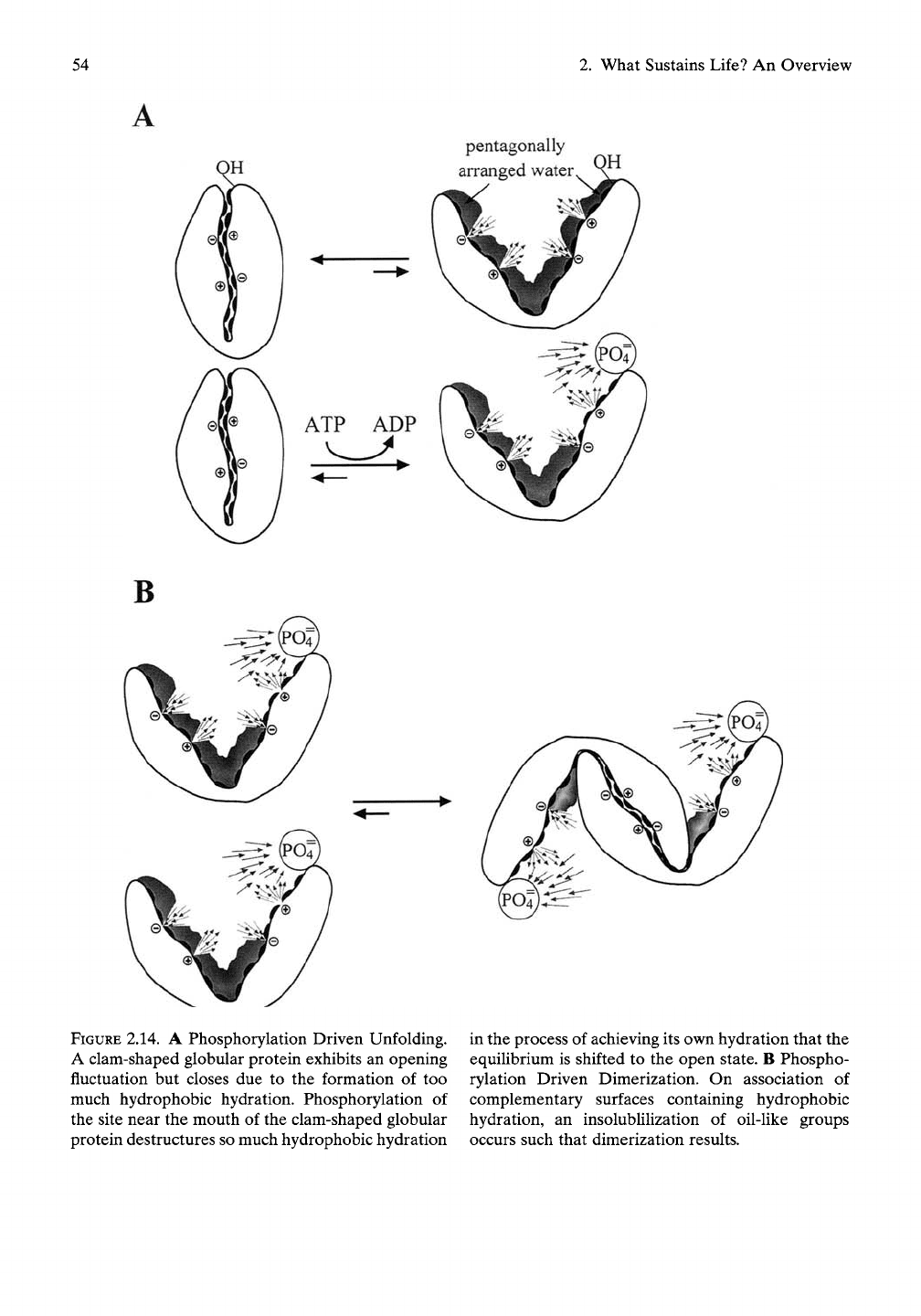
54
2.
What Sustains Life? An Overview
pentagonally
arranged water ^
B
FIGURE 2.14. A Phosphorylation Driven Unfolding.
A clam-shaped globular protein exhibits an opening
fluctuation but closes due to the formation of too
much hydrophobic hydration. Phosphorylation of
the site near the mouth of the clam-shaped globular
protein destructures so much hydrophobic hydration
in the process of achieving its own hydration that the
equilibrium is shifted to the open state. B Phospho-
rylation Driven Dimerization. On association of
complementary surfaces containing hydrophobic
hydration, an insolublilization of oil-like groups
occurs such that dimerization results.
