Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.

5.7 What Is the Physical Basis for the ConsiUent Mechanism of Energy Conversion?
185
These interpretations were reinforced by the
observation in the same time period of stretch-
induced pKa shifts (discussed immediately
below) by insights of the T^based hydropho-
bicity scale (discussed above) and then by
hydrophobic-induced pKa shifts (as discussed
further below).
5,7.8.2 Stretch-inducedpKa Shifts
In 1937, using series of simple organic mole-
cules,
Butler^^ first observed that exposure of
CH2 groups to water resulted in an exothermic
reaction with formation of a unique water
around such oil-like groups. In
1970,
Weis-Fogh
and Andersen^^ reported the exothermic reac-
tion on stretching elastin and interpreted it in
terms of the exposure of hydrophobic groups
with formation of hydrophobic hydration.
Hoeve and Flory^^ further affirmed this in their
1974 publication. Accordingly, stretching of
elastin-like protein forces exposure of
hydrophobic groups to the surrounding water
with the result of an exothermic reaction of
hydrophobic hydration.
Consideration of the effects of stretching
begins with hydrophobically associated and
cross-linked elastomeric matrix composed of
the same protein-based polymer as used in the
calorimetry studies with poly[0.8(GVGVP),
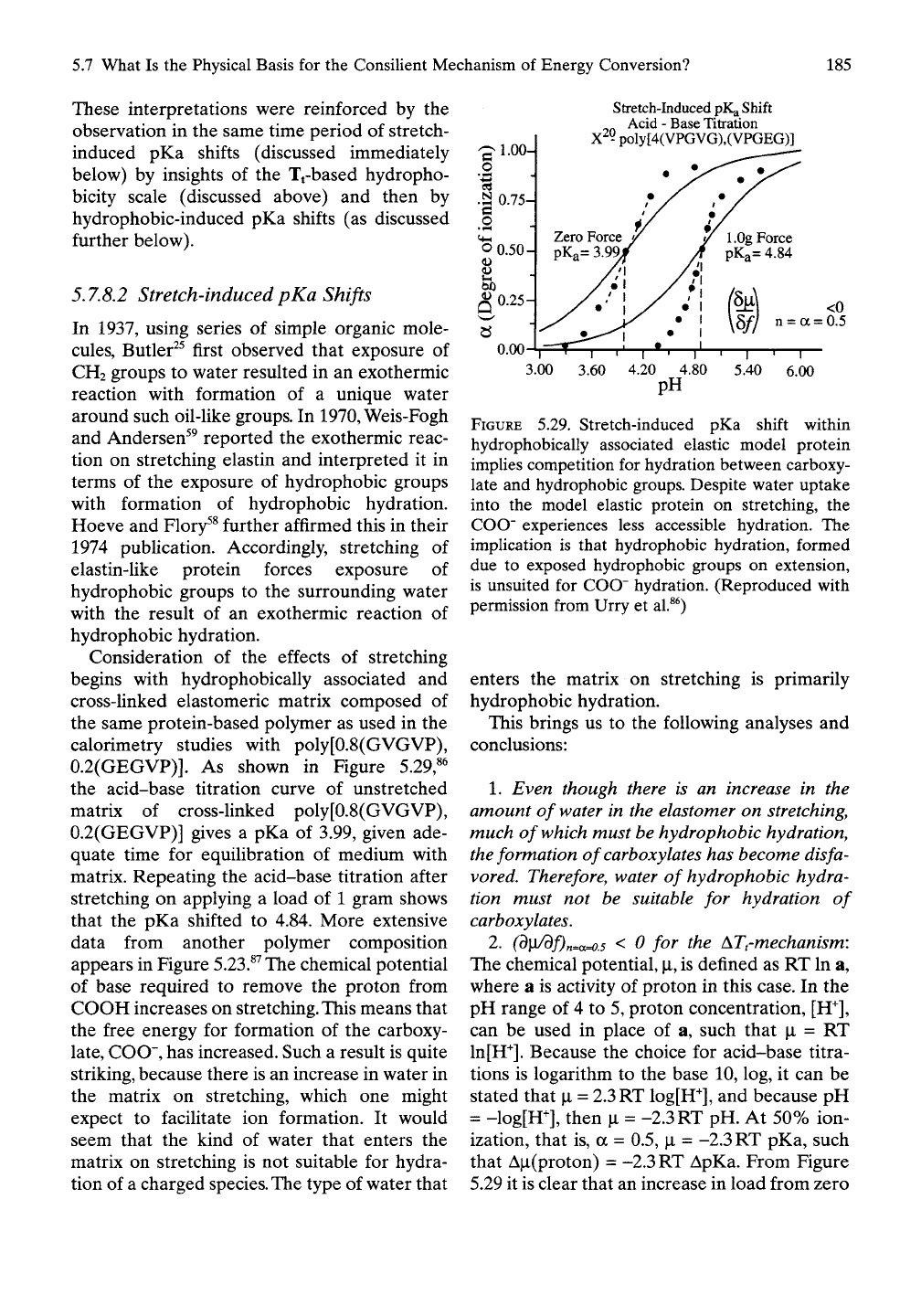
0.2(GEGVP)]. As shown in Figure 5.29,^^
the acid-base titration curve of unstretched
matrix of cross-linked poly[0.8(GVGVP),
0.2(GEGVP)] gives a pKa of 3.99, given ade-
quate time for equilibration of medium with
matrix. Repeating the acid-base titration after
stretching on applying a load of 1 gram shows
that the pKa shifted to 4.84. More extensive
data from another polymer composition
appears in Figure
5.23.^^
The chemical potential
of base required to remove the proton from
COOH increases on stretching. This means that
the free energy for formation of the carboxy-
late,
COO", has increased. Such a result is quite
striking, because there is an increase in water in
the matrix on stretching, which one might
expect to facilitate ion formation. It would
seem that the kind of water that enters the
matrix on stretching is not suitable for hydra-
tion of a charged
species.
The type of water that
Stretch-Induced
pKg
Shift
Acid
- Base
Titration
poly[4(VPGVG),(VPGEG)]
3.00 3.60 4.20 4.80
pH
5.40 6.00
FIGURE 5.29. Stretch-induced pKa shift within
hydrophobically associated elastic model protein
implies competition for hydration between carboxy-
late and hydrophobic groups. Despite water uptake
into the model elastic protein on stretching, the
COO"
experiences less accessible hydration. The
implication is that hydrophobic hydration, formed
due to exposed hydrophobic groups on extension,
is unsuited for COO" hydration. (Reproduced with
permission from Urry et al.^^)
enters the matrix on stretching is primarily
hydrophobic hydration.
This brings us to the following analyses and
conclusions:
1.
Even though there is an increase in the
amount of water in the elastomer on stretching,
much of which must be hydrophobic hydration,
the formation of carboxylates has become disfa-
vored.
Therefore, water of hydrophobic hydra-
tion must not be suitable for hydration of
carboxylates.
2.
(dli/df)n=aM).5
< 0 for the ATt-mechanism:
The chemical potential,
|Li,
is defined as RT In a,
where a is activity of proton in this case. In the
pH range of 4 to 5, proton concentration, [H^],
can be used in place of a, such that \i = RT
ln[H^].
Because the choice for acid-base titra-
tions is logarithm to the base 10, log, it can be
stated that |i = 2.3 RT log[H^, and because pH
= -log[H^, then
|LI
= -2.3 RT pH. At 50% ion-
ization, that is, a = 0.5,
|LI
= -2.3 RT pKa, such
that A|i(proton) = -2.3 RT ApKa. From Figure
5.29 it is clear that an increase in load from zero

186
5.
Consilient Mechanisms for Diverse Protein-based Machines
to 1.0 gram represents an increase in force;
therefore, Af is positive. On the other hand, the
pH at the pKa increased from 3.99 to 4.84;
therefore, A|x(proton) is a negative quantity.
Thus,
it can be written that (3|Li/3f)n=a=o.5 < 0, that
is,
stretching results in an uptake of protons,
where n = a = 0.5 indicates evaluation at the
degree of ionization of 0.5 which is the pKa, the
point of 50% ionization.
3.
(d[i/df)n=a=o.5
> 0 for the charge-charge
repulsion mechanism: Studying polyelectrolytes
with the example of poly(methacrylic acid),
[-CH-CCHsCOOH-Jn, Katchalsky et al.'^ in
1960 presented the argument that stretching
resulted in the release of protons, that is,
(9|i/3f)n=a=o.5 > 0. The description of the process
for cross-linked poly(methacryUc acid), with a
carboxyl function on every other backbone
atom, follows: (a) At 60% ionization,
charge-charge repulsion gives complete exten-
sion into stiff rods, (b) At 0 to 10% ionization,
charge-charge repulsion is no longer present
and the chain randomizes to give full contrac-
tion, (c) When the chemical potential of proton
in the bathing medium allows 20% ionization,
contraction will have occurred to the extent
allowed by charge-charge repulsion. On
stretching of the matrix at this point, there is an
increase in distance between charges, and for
the unchanged proton chemical potential in the
bathing medium there can be a release of
protons into the medium until the chemical
potential of the fiber again matches that of the
medium, (d) Therefore, stretching results in a
release of protons for the charge-charge repul-
sion mechanism, which is to say that
(3|a/Df)n=a=o.5 > 0 for the charge-charge repulsion
mechanism, whereas for the ATt-mechanism it
is exactly the opposite, (3|i/3f)n=a=o.5 < 0. (e) Con-
clusion: A mechanism other than charge-charge
repulsion is responsible for the stretch-induced
pKa shift exhibited by Tf-based molecular
machines.
4.
Relationship of cooperativity to tspKa'.
Furthermore, the increase in Gibbs free energy
of hydroxyl ion required to remove the proton
from COOH, indicated by the pKa shift of 0.85,
is AG2,i = A|x(hydroxyl ion) = 2.3 RT ApKa =
1.16kcal/mole. In association with the pKa
shift, there is an increase in steepness of the
acid-base titration curve on stretching, indicat-
ing an increase in positive cooperativity. For
small pKa shifts this may be estimated using the
Wyman equation^^ AG = RT(1 - l/n)/a(l - a),
where n (the Hill coefficient)^^ is the slope of
the curve at the pKa and a is the degree of ion-
ization, which at the pKa is 0.5. Under zero
load, n was 1.07; on loading to 1 gram, n became
2.21.
For the zero load case, AGi is 0.15
kcal/mole, whereas for the 1 gram load, AG2 is
1.30, such that the difference, AGi,2, is 1.15
kcal/mole. Thus, both the pKa shift and the
increase in positive cooperativity represent
dif-
ferent expressions of the same energy of inter-
action. Conclusion: Positive cooperativity is
another expression of the competition for hydra-
tion between apolar and polar groups that gave
rise to the stretch-induced pKa shift.
5.
Nonlinear stretch-induced pKa shift: The
stress/strain curve of the cross-linked pro-
tein-based polymer X''-poly[0.82(GVGIP),
0.18(GEGIP)] is shown in the inset of Figure
5.23A to be linear such that the work done on
the elastomeric band is directly proportional to
the apphed force. On the other hand, the pKa
shifts resulting from linear increases in load are
very nonlinear (Figure 5.23B).^^ This carries
significant implication with regard to the effi-
ciency of the conversion of mechanical energy
into the chemical work of pumping protons.
In addition, the acid-base titration curve
becomes steeper, that is, exhibits an increase in
positive cooperativity as the elastomeric band
is further extended (Figure 5.23B). This effect
was explicitly calculated above. Because the
change in chemical potential times the change
in number of moles involved to go from the
relaxed state to the contracted state is the
required chemical energy, the positive co-
operativity means less chemical energy will be
required to perform a given amount of mechan-
ical work. Not only is there a different mecha-
nism from that of the charge-charge repulsion
mechanism, but the new mechanism is more
efficient, as was briefly discussed in Chapter 2
and shown in Figure
2.16.
This will be discussed
more extensively below.

5.7 What Is the Physical Basis for the Consihent Mechanism of Energy Conversion?
187
5.7.8.3 Hydrophobic-inducedpKa Shifts
Four studies based on different model protein
systems and experimental variables have been
used to vary hydrophobicity systematically and
thereby to demonstrate the basis of hydropho-
bic-induced pKa shifts. The four studies are (1)
dilution of a recurrent ionizable residue in a
poly(5-mer) from 1 ionizable group every 5
residues to 1.2 per 100 residues by stepwise
replacement of a polar residue with an apolar
(hydrophobic) Val (V) residue, (2) maintenance
of the composition of a poly(30-mer) but
rearranging the proximity of more hydro-
phobic residues to the ionizable residue in a
poly(30-mer), (3) increasing systematically the
hydrophobic residues within a poly(30-mer)
while holding the ionizable residue of the
poly(30-mer) constant at 1 per 30 residues, and
(as discussed in section 5.7.8.2) (4) stretching
incrementally a hydrophobically folded cross-
linked elastic matrix containing ionizable
residues (in this case the polymer composition
was kept constant but the hydrophobicity was
increased by application of an extending force
that increasingly exposed hydrophobic groups
to water in sufficient proximity to the ionizable
function.
5.7.8.3.1 Increasing Hydrophobicity by
Stepwise Replacement of the Functional
Group with a More Hydrophobic Residue
The model system, poly[/v(GVGIP),
/x(GXGIP)],
is used where /x and f are mole
fractions and X is defined as follows for each of
three studies: (1) X = Glu(E),/x = 1.0 to 0.06;
(2) X = Asp(D), /x = 1.0 to 0.06; and (3) X =
Lys(K),/x = 1.0 to 0.06. In each of these studies,
the starting polymer is poly(GXGIP), and then
the residue X is stepwise replaced by the more
hydrophobic valine (Val, V) until the X residue
is very dilute in the polymer, 1.2 residues per
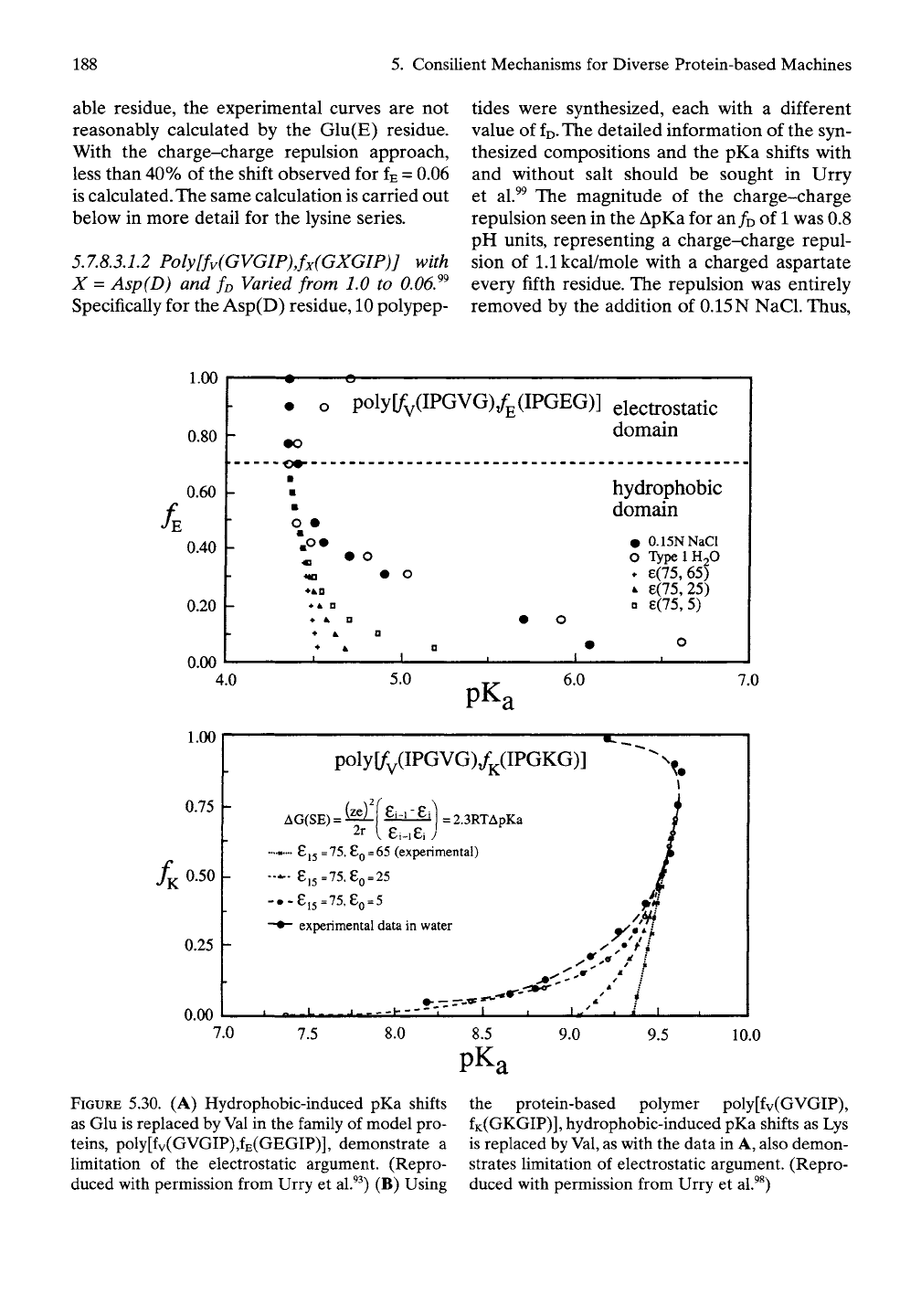
100 residues. As shown in Figure 5.30A,B
for starting compositions of poly(GEGIP)^^
and poly(GKGIP),^^ respectively, the charge
density is high enough such that charge-charge
repulsion is observed in the absence of
salt.
This
charge-charge repulsion is entirely removed by
0.15 N NaCl for X = E and D (data not shown)'^
and significantly relieved for X = K, and the
essentially normal pKa is obtained for these
ionizable functions. In the absence of salt, the
charge-charge repulsion disappears for
/E
=
/D
= 0.7 and for
/K
= 0.75. The remarkable feature
of these studies is that, as the dilution of
charged species continues, the pKa shift
resumes with a vengeance, instead of decreas-
ing as it must if charge-charge repulsion were
relevant. The largest pKa shifts are found at the
highest dilutions examined with 1.2 ionizable
residues per 100 residues of polymer.
5.7.8.3.1.1 Poly[fv(GVGIP)Jx(GXGlP)] with
X = Glu(E) and fx Varied from 1.0 to
0.06.^^
Specifically for the Glu(E) residue, 10 polypep-
tides were synthesized, each with a different
value of ffi. The value for fn = 1 in pure water
is 4.7, and on addition of 0.15 N NaCl the
value drops to 4.35. The magnitude of the
charge-charge repulsion is about 0.49kcal/mole
as estimated from the ApKa of 0.35. A pKa of
4.35 is the lowest value obtained in the absence
of salt at fE= 0.7. On further dilution, that is, for
ffi < 0.7, the increase in pKa resumes until at fe
= 0.06 the pKa becomes 6.6, which relaxes to 6.1
on the addition of physiological saUne.Thus, the
mechanism operative in these Tt-type protein-
based polymers, which is not charge-charge
repulsion, has brought to bear a repulsive free
energy of interaction of over 3kcal/mole. As
will be discussed later, this new mechanism can
give rise to repulsive free energies of interac-
tion of 8 to lOkcal/mole.
Calculated curves using the charge-charge
repulsion argument are included in Figure
5.30A, and the means of calculating is given
in the following section on poly[/v(GVGIP),
/K(GKGIP)].The minimal experimental dielec-
tric constant for poly(GVGIP) at 36° is obtain-
able from Table 5.6, where the high-frequency
component (£°° of 23) and the
5
MHz compo-
nent (Ae of 42) sum to give a minimal value of
65.
With this value, only a few tenths of a pH
unit shift is calculated (see Figure 5.30A). Even
when taking the unrealistic value of 5 for the
dielectric constant in the absence of any ioniz-
188
5.
Consilient Mechanisms for Diverse Protein-based Machines
able residue, the experimental curves are not
reasonably calculated by the Glu(E) residue.
With the charge-charge repulsion approach,
less than 40% of the shift observed for fE = 0.06
is calculated. The same calculation is carried out
below in more detail for the lysine series.
5.7.8.3.1.2 Poly[fv(GVGIP),fx(GXGIP)J with
X = Asp(D) and fo Varied from 1.0 to
0.06.^^
Specifically for the Asp(D) residue, 10 polypep-
tides were synthesized, each with a different
value of
fo.
The detailed information of the syn-
thesized compositions and the pKa shifts with
and without salt should be sought in Urry
et al.^^ The magnitude of the charge-charge
repulsion seen in the ApKa for an/o of
1
was 0.8
pH units, representing a charge-charge repul-
sion of l.lkcal/mole with a charged aspartate
every fifth residue. The repulsion was entirely
removed by the addition of 0.15 N NaCl. Thus,
1.00
0.80
/p
0.60
0.40
0.20
0.00
4.0
1.00
0.75 h
/K
0.50 h
0.25 h
0.00
7.0
• o poly[f^(IPGVG)4(IPGEG)] electrostatic
domain
•o
o •
m
4«a • O
• 4 0
« 4 O
hydrophobic
domain
• O.lSNNaCl
O Type
1
H2O
• 6(75,65)
4 e(75,25)
D e(75,5)
5.0
PK
6.0
7.0
a
1 poly[^^(IPGVG)4(IPGKG)]
1 AG(SE) = ^ £kll£i = 2.3RTApKa
•...-.• 6j5 =75.
£Q
=65 (experimental)
I ---e,5 =75.80=25
1 -.-8,5=75.60=5
"•- experimental data in water
"cn:^ 1
/
/
J
*
i_i 1 1 1
7.5
8.0
8.5
pK
9.0
9.5
10.0
a
FIGURE
5.30. (A) Hydrophobic-induced pKa shifts
as Glu is replaced by
Val
in the family of model pro-
teins,
poly[fv(GVGIP),fE(GEGIP)], demonstrate a
limitation of the electrostatic argument. (Repro-
duced with permission from Urry et al.^^) (B) Using
the protein-based polymer poly[fv(GVGIP),
fK(GKGIP)],
hydrophobic-induced pKa shifts as Lys
is replaced by
Val,
as with the data in A, also demon-
strates limitation of electrostatic argument. (Repro-
duced with permission from Urry et al.^^)
5.7 What Is the Physical Basis for the Consilient Mechanism of Energy Conversion?
189
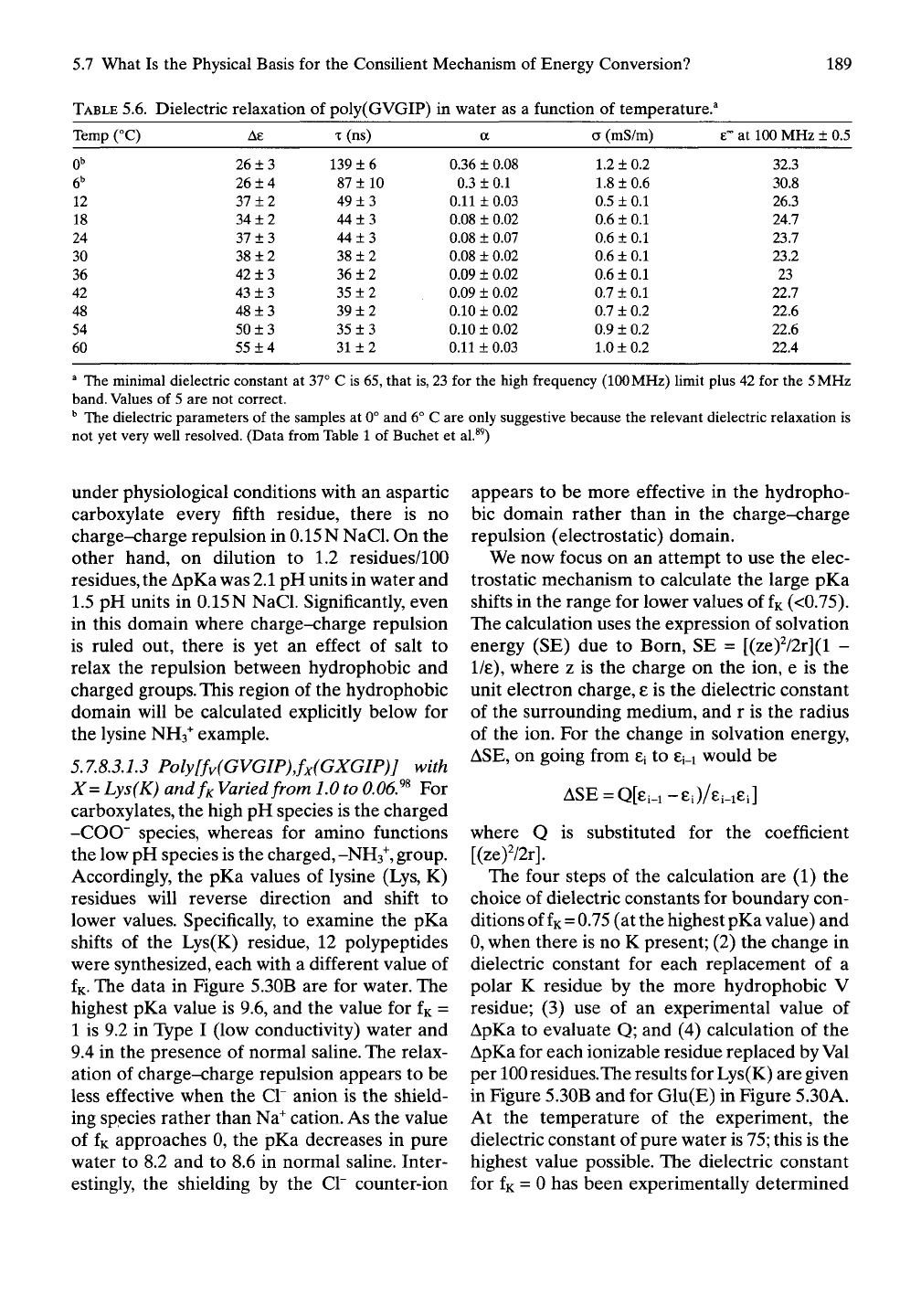
TABLE
5.6. Dielectric relaxation of poly(GVGIP) in water as a function of temperature.^
Temp (°C)
Ae
T
(ns) a (mS/m)
6°-
at
100
MHz ± 0.5
0^
6"
12
18
24
30
36
42
48
54
60
26
±3
26
±4
37
±2
34 ±2
37
±3
38
±2
42
±3
43
±3
48
±3
50
±3
55
±4
139
±6
87 ±10
49 ±3
44 ±3
44
±3
38
±2
36 ±2
35
±2
39
±2
35
±3
31
±2
0.36 ± 0.08
0.3 ± 0.1
0.11 ± 0.03
0.08 ± 0.02
0.08 ± 0.07
0.08 ± 0.02
0.09 ± 0.02
0.09 ± 0.02
0.10 ± 0.02
0.10 ± 0.02
0.11 ± 0.03
1.2 ± 0.2
1.8 ± 0.6
0.5 ± 0.1
0.6 ± 0.1
0.6 ± 0.1
0.6 ± 0.1
0.6 ± 0.1
0.7 ± 0.1
0.7 ± 0.2
0.9 ± 0.2
1.0 ± 0.2
32.3
30.8
26.3
24.7
23.7
23.2
23
22.7
22.6
22.6
22.4
^ The minimal dielectric constant at 37° C is 65, that is, 23 for the high frequency (100 MHz) limit plus 42 for the
5
MHz
band. Values of 5 are not correct.
^ The dielectric parameters of the samples at 0° and 6° C are only suggestive because the relevant dielectric relaxation is
not yet very well resolved. (Data from Table 1 of Buchet et al.^^)
under physiological conditions with an aspartic
carboxylate every fifth residue, there is no
charge-charge repulsion in 0.15N NaCl. On the
other hand, on dilution to 1.2 residues/100
residues,
the ApKa
was
2.1
pH units in water and
1.5 pH units in
0.15
N
NaCl. Significantly, even
in this domain where charge-charge repulsion
is ruled out, there is yet an effect of salt to
relax the repulsion between hydrophobic and
charged
groups.
This region of the hydrophobic
domain will be calculated explicitly below for
the lysine NHa^ example.
5.7.8.3.1.3
Poly[fv(GVGIP)Jx(GXGIP)] with
X= Lys(K) andfK Varied from
1.0
to
0.06.^^
For
carboxylates, the high pH species is the charged
-COO"
species, whereas for amino functions
the low pH species is the charged,
-NHs^,
group.
Accordingly, the pKa values of lysine (Lys, K)
residues will reverse direction and shift to
lower values. Specifically, to examine the pKa
shifts of the Lys(K) residue, 12 polypeptides
were synthesized, each with a different value of
fx.
The data in Figure
5.SOB
are for water. The
highest pKa value is 9.6, and the value for fx =
1 is 9.2 in Type I (low conductivity) water and
9.4 in the presence of normal
saline.
The relax-
ation of charge-charge repulsion appears to be
less effective when the CI" anion is the shield-
ing species rather than
Na"^
cation. As the value
of fx approaches 0, the pKa decreases in pure
water to 8.2 and to 8.6 in normal saline. Inter-
estingly, the shielding by the CI" counter-ion
appears to be more effective in the hydropho-
bic domain rather than in the charge-charge
repulsion (electrostatic) domain.
We now focus on an attempt to use the elec-
trostatic mechanism to calculate the large pKa
shifts in the range for lower values of fx (<0.75).
The calculation uses the expression of solvation
energy (SE) due to Born, SE = [(ze)V2r](l -
1/e), where z is the charge on the ion, e is the
unit electron charge,
8
is the dielectric constant
of the surrounding medium, and r is the radius
of the ion. For the change in solvation energy,
ASE, on going from
Ei
to 8i_i would be
ASE = Q[8i_i-ei)/ei_iEi]
where Q is substituted for the coefficient
[(zcf/2r].
The four steps of the calculation are (1) the
choice of dielectric constants for boundary con-
ditions of fx=0.75 (at the highest pKa value) and
0, when there is no K present; (2) the change in
dielectric constant for each replacement of a
polar K residue by the more hydrophobic V
residue; (3) use of an experimental value of
ApKa to evaluate Q; and (4) calculation of the
ApKa for each ionizable residue replaced by Val
per 100 residues.The results for
Lys(K)
are given
in Figure
5.30B
and for Glu(E) in Figure
5.30A.
At the temperature of the experiment, the
dielectric constant of pure water is
75;
this is the
highest value possible. The dielectric constant
for fK = 0 has been experimentally determined

190
5.
Consilient Mechanisms
for
Diverse Protein-based Machines
to
be 65 (See the
36°
C
data
in
Table 5.6).^^ The
use
of
these values
for the
boundary conditions
results
in a
maximal ApKa
of
only 0.3
pH
units,
whereas
the
true ApKa
is
five times larger. Only
when
a
fictitious dielectric constant
of
5
is
used
for the phase-separated state can the electrosta-
tic calculations begin
to
approach
the
experi-
mentally observed shifts.
One might argue that
the
lysine side chain
is
buried
in a
local dielectric constant
of 5
even
though
the
macroscopic dielectric constant
is
65.
Tliis
is not
reasonable because
the
barrier
to backbone mobility
of
poly(GVGIP)
is
only
l.lkcal/mole.^^
A
barrier many times greater
would
be
required
to
hold
a
lysine side chain
at
a local dielectric constant
of
5
for a
viable elec-
trostatic argument.
In
fact
the
magnitude
of the
pKa shifts
can be as
large
as 8 to
lOkcal/mole
for
an Asp
residue
in a
more hydrophobic
Tt-type protein-based polymer. ^^^
Conclusion: Neither charge-charge repulsion
nor being buried
in a
medium
of low
dielectric
constant provides
a
satisfactory explanation
for
the increasing
pKa
shift attending dilution
of
ionizable side chains
in a
moderately hydropho-
bic protein-based polymer
5.7.8.3.2 Carboxylate-Containing
Polytricosapeptides
of
Constant Composition
but with Rearrangement
of
More
Hydrophobic Residues: The Power
of Sequence!
5.7.8.3.2.1 Effect
of
Different Pentamer
Sequence Arrangements
of the
Same 30-mer
Composition. Chemically synthesized polytri-
cosapeptides, poly (30-mers), were prepared
with compositions
of 1
aspartic acid residue
(Asp,
D) and 5
more-hydrophobic phenylala-
nine (Phe,
F)
residues replacing valine (Val,
V)
residues
per
repeat
of 30
residues,
but
with
dif-
ferent relative locations
of D and F
residues.
These compositions
are
written:
Polymer
XI:
Poly(GFGFP GVGVP
GDG
VP GFGFP GFGVP GVGVP)
Polymer
V:
Poly(GVGVP GVGFP
GDG
FP GVGVP GVGFP GFGFP)
The
pKa for
Polymer
V was
found
to be 6.7,
whereas that
for
Polymer
XI
under identical
conditions
was
determined
to be 10 (see
Figure
5.32, below)."^^
The
normal
pKa for
aspartic
in
this polymer without
any F
residues having
replaced
V
residues
(see
Polymer
I
in Table
5.5)
is 3.8.
The
polymers were designed with
the (5-
spiral structure
as the
basis. (This structure
was
developed
in
section
5.2.)
Given
the
p-spiral
structure as the design
basis,
Polymer XI had
the
more-hydrophobic
F
residues more distal from
the
D
residue, whereas
in
Polymer
V the
more-
hydrophobic
F
residues were more proximal
to
the
D
residue,
as
shown
in
Figure 5.32, below).
Accordingly, without changing
the
mean com-
position
of the
polytricosapeptide,
but by
designing structures with different proximities
to
the
ionizable function,
the pKa
shift changed
from 6.7
-
3.8 = 2.9
to
10
-
3.8
=
6.2.'^ The Varied
hydrophobic proximity
of
the functional groups,
resulting from
the
primary structure, causes
dif-
ferences
in
hydrophobic-induced
pKa
shifts.
5.7.8.3.2.2 Comparison
of
a Fixed Sequence
of
Six Pentamers with
a
Random
Mix of
the Same
Pentamers.^^^
The
importance
of
sequence
control
is
also understood through
two
addi-
tional comparisons.
The
polytricosapeptide
Polymers
X and XII in
Table 5.5, with specified
sequences, exhibited
pKa
values
of
8.1
and 7.8,
respectively, whereas
a
random mixture
of
the same combination
of
pentamers, namely,
poly[(GEGFP),2(GVGVP),2(GVGFP),
(GFGFP)],
gave
a pKa of 5.2.
Similarly,
the
specified sequence poly(GEGFP GVGVP
GVGVP GVGVP GFGFP GFGFP) gave
a
pKa
of 7.7,
whereas
the
same pentamers
in random mixture, namely, poly[(GEGFP,
3(GVGVP),2(GFGFP)] gave
a pKa of
4.7
(see
Table
1 in
Urry
et
al.).^^^
The pKa
shift with
specified sequence
is
four times larger than
for
random polymers
of the
same mean pentamer
composition.
In
terms
of
energies,
the
average
pKa shift
of the
polymers composed
of
random
pentamers,
0.65,
represents
a
repulsive free
energy
of
less than Ikcal/mole, whereas
the
average
pKa
shift
of the
fixed sequence
protein-based polymers
(3.6)
represents
a
repulsive free energy
of
about
5
kcal/mole.
For
energy conversions required
by
living organ-
isms,
sequence matters.
In
part, this
is why
biology puts
so
much energy into synthesis
of
protein,
as
discussed
in
Chapter
4.
5.7 What Is the Physical Basis for the ConsiUent Mechanism of Energy Conversion?
191
5.7.8.3.3 Increasing Hydrophobicity While
the Composition of the Functional Group
Remains Constant
In the polytricosapeptides of this study the
aspartic acid residue in one series and the glu-
tamic acid residue in a second series are kept
constant at 1 per 30 residues, and the number
of valine residues replaced by the more
hydrophobic phenylalanine residues systemati-
cally increases from 0 to 2 to 3 to 4 and to 5, as
foUow^s:
Polymer
Polymer
Polymer
Polymer
Polymer
Polymer
Polymer
Polymer
Polymer
Polymer
I:
II:
III:
IV:
V:
VI:
VII:
VIII:
IX:
X:
Poly(GVGVP GVGVP
Poly(GVGVP GVGFP
Poly(GVGVP GVGVP
Poly(GVGVP GVGFP
Poly(GVGVP GVGFP
Poly(GVGVP GVGVP
Poly(GVGVP GVGFP
Poly(GVGVP GVGVP
Poly(GVGVP GVGFP
Poly(GVGVP GVGFP
GDGVP GVGVP GVGVP GVGVP) D/OF
GDGFP GVGVP GVGVP GVGVP) D/2F
GDGVP GVGVP GVGFP GFGFP) D/3F
GDGFP GVGVP GVGFP GVGFP) D/4F
GDGFP GVGVP GVGFP GFGFP) D/5F
GEGVP GVGVP GVGVP GVGVP) E/OF
GEGFP GVGVP GVGVP GVGVP) E/2F
GEGVP GVGVP GVGFP GFGFP) E/3F
GEGFP GVGVP GVGFP GVGFP) E/4F
GEGFP GVGVP GVGFP GFGFP) E/5F
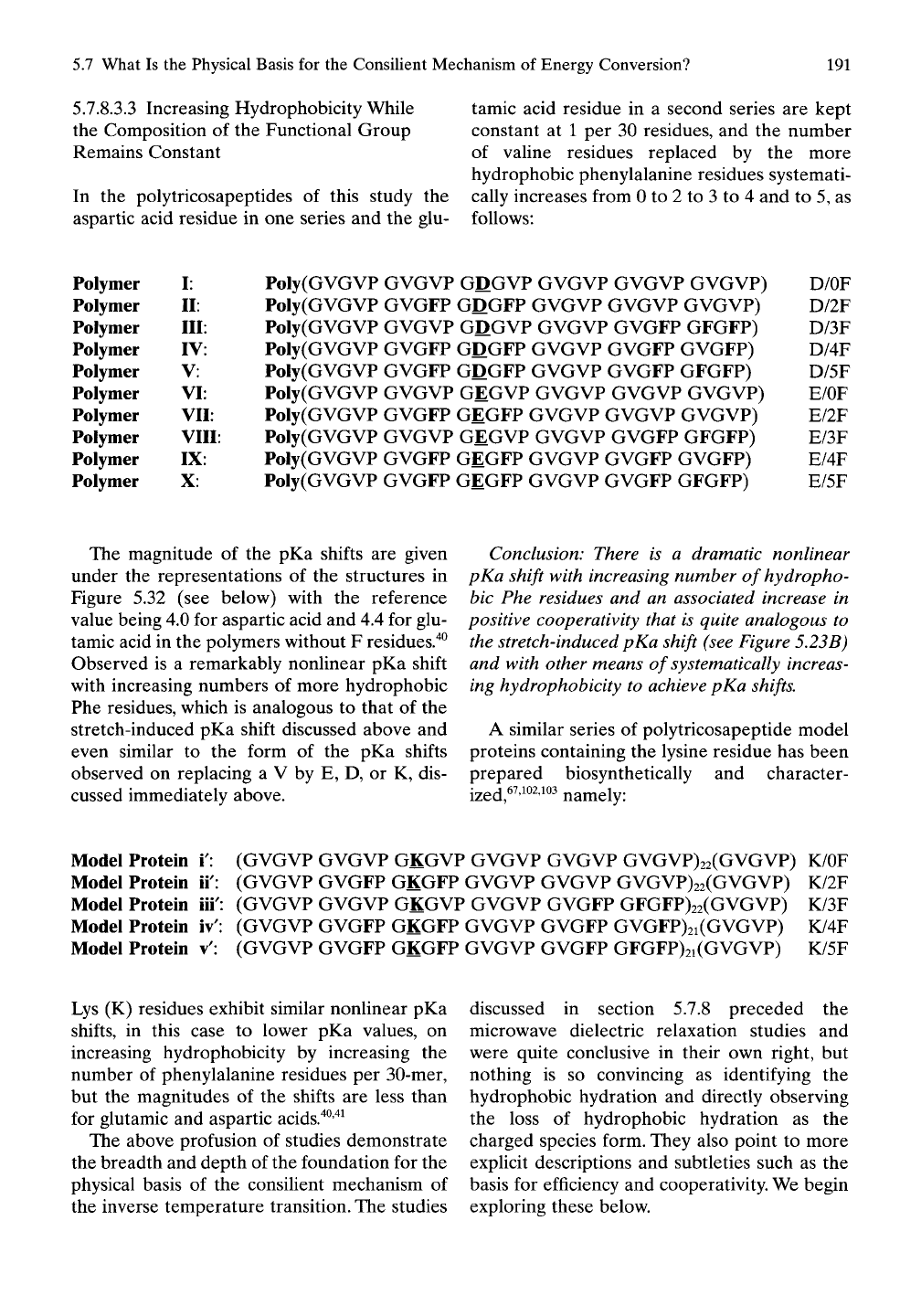
The magnitude of the pKa shifts are given
under the representations of the structures in
Figure 5.32 (see below) with the reference
value being 4.0 for aspartic acid and 4.4 for glu-
tamic acid in the polymers without F residues."^^
Observed is a remarkably nonhnear pKa shift
with increasing numbers of more hydrophobic
Phe residues, which is analogous to that of the
stretch-induced pKa shift discussed above and
even similar to the form of the pKa shifts
observed on replacing a V by E, D, or K, dis-
cussed immediately above.
Conclusion: There is a dramatic nonlinear
pKa shift with increasing number of hydropho-
bic Phe residues and an associated increase in
positive cooperativity that is quite analogous to
the stretch-induced pKa shift (see Figure 5.23B)
and with other means of systematically increas-
ing hydrophobicity to achieve pKa shifts.
A similar series of polytricosapeptide model
proteins containing the lysine residue has been
prepared biosynthetically and character-
i^^d
67,102,103
namely:
Model Protein i': (GVGVP GVGVP GKGVP GVGVP GVGVP GVGVP)22(GVGVP) K/OF
Model Protein ii': (GVGVP GVGFP GKGFP GVGVP GVGVP GVGVP)22(GVGVP) K/2F
Model Protein iii': (GVGVP GVGVP GKGVP GVGVP GVGFP GFGFP)22(GVGVP) K/3F
Model Protein iv': (GVGVP GVGFP GKGFP GVGVP GVGFP GVGFP)2i(GVGVP) K/4F
Model Protein v': (GVGVP GVGFP GKGFP GVGVP GVGFP GFGFP)2i(GVGVP) K/5F
Lys (K) residues exhibit similar nonhnear pKa
shifts,
in this case to lower pKa values, on
increasing hydrophobicity by increasing the
number of phenylalanine residues per 30-mer,
but the magnitudes of the shifts are less than
for glutamic and aspartic acids."^^"^^
TTie above profusion of studies demonstrate
the breadth and depth of the foundation for the
physical basis of the consilient mechanism of
the inverse temperature transition. The studies
discussed in section 5.7.8 preceded the
microwave dielectric relaxation studies and
were quite conclusive in their own right, but
nothing is so convincing as identifying the
hydrophobic hydration and directly observing
the loss of hydrophobic hydration as the
charged species form. They also point to more
explicit descriptions and subtleties such as the
basis for efficiency and cooperativity. We begin
exploring these below.

192
5.
Consilient Mechanisms for Diverse Protein-based Machines
5.7.9 Primary Source of pKa Shifts in
Model Proteins i Through v:
Competition for Hydration Between
Hydrophobic and Charged Groups
In section 5.1.3.4, we derived expressions for
the Gibbs free energy of hydrophobic associa-
tion, AGHA- In this section 5.7, hydrophobic-
induced pKa shifts were shown to arise from a
competition for hydration between the polar
(ionized) and apolar (hydrophobic) groups.
Accordingly, the pKa shifts provide an oppor-
tunity to express the Gibbs free energy repre-
sented by this competition for hydration, AGap
and to compare it to the
AGHA
determined
under similar circumstances and to Monod's
"second secret of life" embodied in allostery-
based positive cooperativity.^^'* In our case,
the allostery involves the two conformational
states,
hydrophobically associated and hydro-
phobically dissociated.
5.7.9.7
Generally Considered Physical
Bases for pKa Shifts in Polymers
The three generally considered sources for pKa
shifts are (1) the electrostatic-based charge-
charge repulsion, (2) the ionizable group being
forced into an environment of low dielectric
constant where it cannot ionize, and (3) the per-
spective developed by the systematic series of
model proteins considered here of a competi-
tion for hydration between hydrophobic and
charged groups constrained by sequence to
coexist along a chain molecule such as a protein.
5.7.9.1.1 Electrostatic Charge-Charge
Repulsion
As seen for the series poly[/v(GVGIP),
/E(GEGIP)] in Figure 5.30, when fn = 1, a small
charge-charge repulsion occurs with four
residues separating the ionizable carboxylate-
containing side chain of the Glu (E) residues.
The addition of 0.15 N NaCl removes that
repulsion. When f^ = 0.17, as in Model Proteins
i through v of Table 5.5, with 29 residues sepa-
rating charged residues, charge-charge repul-
sion is no longer a tenable explanation. This is
verified by the observed cooperativity, which is
negative for charge-charge repulsion, as shown
in Figure 5.35 below for poly(methacrylic acid)
with a carboxyl on every other backbone atom.
On the other hand. Model Proteins i through v
exhibit a positive cooperativity that increases
with increased replacement of less hydrophobic
Val (V) residues with more hydrophobic Phe
(F) residues.
5.7.9.1.2 Local Environment of Low
Dielectric Constant
The classic argument for pKa shifts in proteins
is the claim that the energy of a folded state is
so favorable that it can force the ionizable
species into the energetically unfavorable cir-
cumstance of a local environment of low die-
lectric constant. This issue was considered in
some detail and rejected by Urry et al.^^ in
connection with the series poly[/v(GVGIP),
/K(GKGIP)] for which the data are given in
Figure 5.30.
5.7.9.1.3 Competition for Hydration Between
Charged and Hydrophobic Groups
The conclusion that this is the mechanism
for the hydrophobic-induced pKa shifts of
the model proteins of consideration here was
extensively developed earlier in this section 5.7.
In what follows, this mechanism is explored
further.
5.7.9.2 Derivation of
AGap,
the Change
in Gibbs Free Energy Due to the
Apolar-Polar Repulsive Free Energy
of Hydration
Derivation of AGap, the change in Gibbs free
energy due to an apolar-polar repulsive free
energy of hydration, begins with the expression
for chemical potential of acid (proton, H^),
|IH
=
RTln(a), where a is the activity of proton. Under
the normal low concentrations for acid, the
activity coefficient is 1, and a can be replaced by
the concentration of the acid, [ff], to give
|IH
=
RTln[H^].
Because acid-base titrations are
plotted using a log scale,
|LIH
= 2.3RTlog[H^] and
as pH = -log[H'^], the chemical potential of
proton becomes,
|IH
= -2.3RTpH. The chemical
potential for the value at which the pH = pKa is
simply |IH = -2.3RTpKa. Now the change in
5.7 What Is the Physical Basis for the Consilient Mechanism of Energy Conversion?
193
chemical potential, A|LI, resulting from the
hydrophobic-induced pKa shift can be written
as A|i2,i = ^2 - 1^1 = -2.3RT(pKa2 - pKai) =
-2.3RTApKa. Finally, we note that the ApKa
arises from the competition for hydration
between charged (polar) and hydrophobic
(apolar) groups, that
is,
competition resulting in
repulsion arises on formation of the carboxylate,
-COO",
which parallels formation of hydroxyl
ion. As we would like to express the change in
Gibbs free energy per mole resulting from the
apolar-polar repulsion as each species reaches
out for water unperturbed by the other, this has
the effect of sign reversal, such that we can write
A|IH
= -A|LioH = -AGap, that is.
AGap = 2.3RT ApKa
(5.13)
where the subscript ap stands for apolar-polar
repulsion. It will be of interest to see how this
quantity relates to
AGRA
and to positive coop-
erativity and charge-charge repulsion in the
treatment of acid-base titration data that con-
sider both interactions in section 5.8. Before
doing so, however, we note another experi-
mental demonstration of AGap, but in this case
there occurs delineation between the compo-
nent that results from sequence without the
capacity to form a conformation to reUeve
apolar-polar repulsion and that component
resulting from lowering repulsion by hydropho-
bic association and charge neutralization.
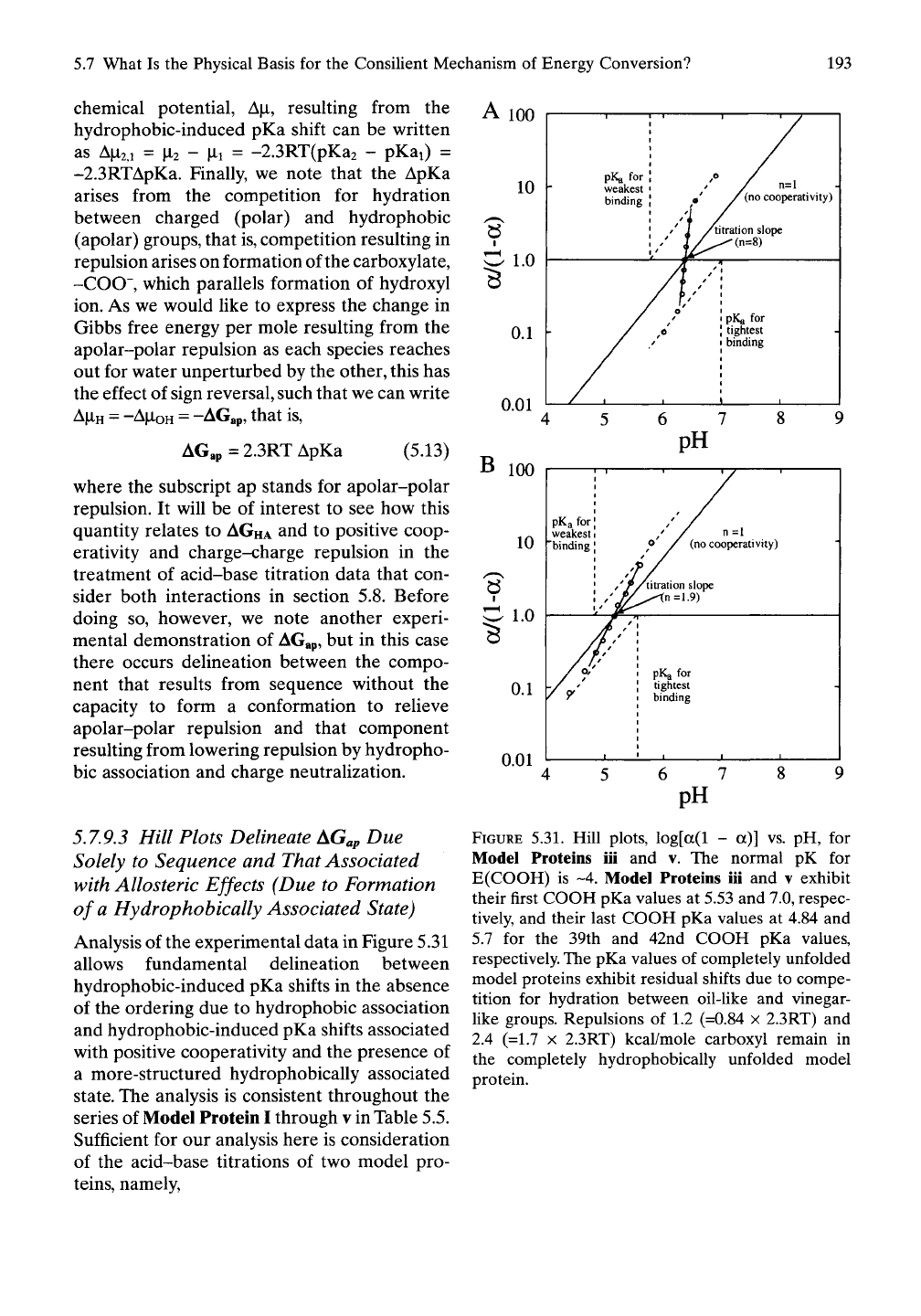
5.7.9.3 Hill Plots Delineate AGap Due
Solely to Sequence and That Associated
with Allosteric Effects (Due to Formation
of a Hydrophobically Associated State)
Analysis of the experimental data in Figure 5.31
allows fundamental delineation between
hydrophobic-induced pKa shifts in the absence
of the ordering due to hydrophobic association
and hydrophobic-induced pKa shifts associated
with positive cooperativity and the presence of
a more-structured hydrophobically associated
state.
The analysis is consistent throughout the
series of Model Protein I through v in Table 5.5.
Sufficient for our analysis here is consideration
of the acid-base titrations of two model pro-
teins,
namely,
A 100
10
1.0
0.1
0.01
B 100
10
1.0
0.1
0.01
1 ' '
1 pKa for
r weakest
binding
1—1 1 1—7
/' / n=l -
a' /
(no
cooperativity)
/ I /titration slope
/ 1 1
pKa for
tightest J
binding
1 1
6 7
PH
pKa for
1
weakest
binding
X
O/'
/ P''
T 1 ry— —1
/'/ n=l J
o / (no cooperativity)
'/ /
/'//titration slope
/i^O-1n=1.9)
1 pKg for
] tightest -J
1 binding
1 1 1 1 1 1
pH
FIGURE 5.31. Hill plots, log[a(l - a)] vs. pH, for
Model Proteins iii and v. The normal pK for
E(COOH) is ~4. Model Proteins iii and v exhibit
their first COOH pKa values at 5.53 and 7.0, respec-
tively, and their last COOH pKa values at 4.84 and
5.7 for the 39th and 42nd COOH pKa values,
respectively. The pKa values of completely unfolded
model proteins exhibit residual shifts due to compe-
tition for hydration between oil-like and vinegar-
like groups. Repulsions of 1.2 (=0.84 x 2.3RT) and
2.4 (=1.7 X 2.3RT) kcal/mole carboxyl remain in
the completely hydrophobically unfolded model
protein.

194
5.
Consilient Mechanisms for Diverse Protein-based Machines
2 Phc/30 mcr
(proximal)
3 Phc/30 mcr
(proximal)
4 Phc/30 mcr
(proximal)
5 Phc/30 mcr
(proximal)
5 Phc/30 mcr
(distal)
Asp
C31u
Pblymcrll
ApKa=0.4
PblymcrVn
ApKa=0.3
Pblymerm Polymer IV
ApKa=0.7 ApKa=1.4
Polymer VEI Polymer IX
ApKa=0.6 ApKa=l.l
Q Aspartic Acid (D) or Glutamic Acid (E)
Polymer V
ApKa=6.1
Polymer X
ApKa=3.8
^ Phcn^dalatiinc (F)
Polymer XI
ApKa=2.9
PblymcrXn
ApKa=3.5
FIGURE 5.32. As indicated below the schematic (3-
spiral representations of the structures, stepwise
increases in hydrophobicity as Phe replaces Val
result in suprahnear increases in carboxyl pKa values
in studies of chemically synthesized polymers in
analogy to the hydrophobic-induced pKa shifts in
Figures 5.29, 5.30, and 5.31. (Reproduced with per-
mission from Urry et
al.'^^).
Model Protein iii: (GVGVP GVGVP GEGVP GVGVP GVGFP GFGFP)39(GVGVP) E/3F
Model Protein v: (GVGVP GVGFP GEGFP GVGVP GVGFP GFGFP)32(GVGVP) E/5F
The Hill plots in Figure 5.31 provide remark-
able insight into the hydrophobic-induced pKa
shifts.
Starting in the hydrophobically associ-
ated state for Model Protein v, on decreasing
acid concentration, that is, raising the pH, the
pKa of the first carboxyl to form carboxylate is
7.0. In Figure 5.31 A, this is the pKa for the most
tightly bound proton. As more carboxylates
form, further ionization becomes easier, and
finally the last carboxyl to ionize does so with a
pKa of 5.7. Remarkably, the last carboxyl to
ionize does so with a pKa shifted 1.7 pH units
from that of the unperturbed Glu pKa of about
4.
Even in the completely unfolded state there
remains an apolar-polar repulsion of 2.4 (= 1.7
X
2.3RT) kcal/mole-Glu.^^^
Those who cannot abandon the concept of
the carboxyls being bound within a local envi-
ronment of low dielectric constant may still
wish to argue that the carboxyl is held within a
low dielectric constant provided by the most
proximal Phe (F) side chains. Thus it becomes
of interest to remove the two most sequence
proximal Phe (F) residues. This is achieved with
Model Protein iii, and the experimental results
are shown in Figure 5.31B. Although the mag-
nitude of the pKa shift is reduced, the conun-
drum remains. The last carboxyl to ionize does
so with a pKa shifted 0.84 pH units from that
of the unperturbed Glu pKa of about 4. Here
there remains a repulsion of 1.2 (= 0.84 x
2.3RT) kcal/mole-Glu even when the more
hydrophobic Phe (F) residues are at a distance
of some 11 residues along the sequence. It
seems inescapable that apolar-polar repulsion,
the effort of the charged and hydrophobic
residues to reach water unperturbed by the
other, reaches out several nanometers. More
detailed scenarios follow.
5.7,9.4 Scenario for pKa Shifts Coupled
with Positive Cooperativity: Starting from
the Hydrophobically Associated State
During an opening fluctuation of a pair of asso-
ciated hydrophobic domains, the formation of
