Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


5.8 Integration of Cooperativities Due to Apolar-Polar and Charge-Charge Repulsion
195
too much hydrophobic hydration, at a given
temperature above Tj, causes the domains
immediately to reassociate. Should an ionizable
group, say, a carboxyl (-COOH), within the dis-
sociating hydrophobic domain ionize to form
the carboxylate (-COO"), it must obtain its
required hydration by destructuring nascent
hydrophobic hydration. The result is an in-
crease in free energy of the charged hydropho-
bic domain. Now there are two possibilities.
Either there is sufficient proton in solution
that the carboxylate recovers its proton, or a
second carboxylate forms and cooperates
with the first in destroying hydrophobic hydra-
tion such that the free energy of each of the
two carboxylates is less than that of the lone
carboxylate. Now formation of each new
carboxylate does so with a lower free energy.
Accordingly, as shown in Figure 5.30, the first
carboxylate forms with a higher pKa, and each
subsequent carboxyl ionizes with a lower pKa.
The last carboxyl to form carboxylate com-
pletes the positive cooperativity for the model
protein.
5.7.9.5 Basis for the pKa Shift Remaining
After Complete Hydrophobic Dissociation
When considering the residual pKa shifts for
the model protein series above, we need to be
clear that even Model Protein i with no pheny-
lalanine (Phe, F) residues exhibits a mean pKa
shift to 4.5 so that the residual shift due solely
to the three Phe (F) residues of Model Protein
iii is only 0.3 units or 0.43 kcal/mole-Glu. On the
other hand, the total AGap for Model Protein v
with a ApKa of 2.87 would be
4.1
kcal/mole-Glu
when at a temperature sufficiently above the Tf
value. Another relevant point is that in Figure
5.25B a residual hydrophobic hydration
remains for Model Proteins i and ii with less
than two carboxylates per 100 residues, which
residual hydrophobic hydration remains as
ionization continues to 3.4 carboxylates per
100 residues. The understanding is that the
hydrophobic residues tend to arrange on the
backside of the model protein from the car-
boxylate groups and are thereby shielded by
the polymer chain from the otherwise over-
powering carboxylates. Thus derives the image
of an apolar-polar repulsion. It should be
emphasized that the carboxylates, even though
immersed in water, do not achieve full hydra-
tion when their pKa is greater than 4.
Accordingly, there results a residual pKa shift,
after complete hydrophobic dissociation, as the
result of the sequence-imposed proximity of the
charged and hydrophobic side chains.
5.8 Integration of
Cooperativities Due to
Apolar-Polar and
Charge-Charge Repulsion into
Acid-Base Titration Theory
5.8.1 Hydrophobically Associated and
Hydrophobically Dissociated: Two
States for Diverse Allostery
Over the last century, one of the more enig-
matic, yet fundamental properties exhibited by
protein-based machines of biology has been
positive cooperativity. So impressed was Monod
by this phenomena that he is reported to have
considered positive cooperativity "the second
secret of life," with the first secret being the
structure of DNA.^^"^ The initial example goes
back to the first decade of the twentieth century,
the binding of oxygen by hemoglobin.^^'^^^
Many remarkable researchers have made
exceptional contributions over many years—
Wyman,^^ Adair,^^^ of course Perutz,^^"^ and then
in the 1960s Monod and coworkers,^^^'^^^ as well
as Koshland, Nemethy, and Filmer.^^^
By means of mathematical formalism
steeped in symmetry of multisubunit globular
proteins, Monod and coworkers described the
cooperative binding of oxygen by hemoglobin,
which effort warranted Nobel recognition.^^^ In
the general perspective, each of the identical or
near identical globular subunits could exist in
two different states of order, hence Monod's
use of the term allostery. The physical basis for
the process was not specifically addressed,
however, other than to credit a "conformational
change." In fact, the place wherein we have
found a mechanism had been discounted, with

196
5.
Consilient Mechanisms for Diverse Protein-based Machines
the perspective that "One may set aside the
simple problem of fibrous proteins. Being used
as scaffolding, shrouds and halyards, they fulfill
these requirements by adopting relatively
simple types of translational symmetries."^^^
Thus,
what we describe in this volume, using
model proteins of inherent translational sym-
metry, had not been anticipated.
Another element, taught by the consilient
mechanism that stands out as not having been
anticipated prior to the current work but con-
stitutes the scientific core of this volume, is
contained in the words of Gregorio Weber in
one of the currently outstanding treatments of
protein interactions, "A complete description of
the energetics of hemoglobin, or any other
oligomeric protein, is well-nigh impossible.
It would involve not only the determination
of the energetic couplings of any number of
Ugands with each other and with the subunit
interactions but also the variations of these
quantities with pH, temperature and pres-
sure."^^^
It is here that the consiHent mecha-
nism, a name for chronicling the comprehensive
hydrophobic effect, has something to con-
tribute. Not only the variables of chemical
potential (e.g., ligand binding and pH), temper-
ature, and pressure, but also the variables of
applied potential, mechanical force, and elec-
tromagnetic frequencies all sum (+ and -) to
give the resultant change in Gibbs free energy
for hydrophobic association/dissociation,
2|AG'HA, as depicted in Figure 5.33. This
constitutes a statement for a
AGHA
additivity
principle.
Here, by introducing AGHA, which by com-
paring ionized and neutral states, can be equiv-
alent to AGap(=2.3 RT ApKa) in the absence of
charge-charge repulsion, we bring positive
cooperativity into the formahsm for acid-base
titrations.
As appUed to our model proteins, this
represents a problem in allostery quite equiva-
lent to that of hemoglobin. In each repeating
unit of hemoglobin there are two states—
oxygen bound and oxygen free. For our model
proteins with an ionizable functional group in
each repeat, there are ionized and nonibnized
states.
In the case of hemoglobin, the principle
variable is the fraction of ligand bound Y, and
it enters in the form Y/(l - Y). This is equiva-
lent in the acid-base titration theory to the
degree of ionization, a, and it enters as a/(l -
a).
For another example, for model proteins
containing redox couples, there is the degree of
reduction, a', and it enters into the formalism
as a7(l - a'). Thus, the following formalism is
relevant, independent of whether one treats
ligand binding to allosteric proteins, the ioniza-
tion process in an acid-base titration curve, or
the reduction process in a potentiometric titra-
tion. In our view of positive cooperativity,
whether in the model protein-based machines
of our design or in the protein machines of
biology, in all cases two states—the hydropho-
bically associated state and the hydrophobically
dissociated state—provide the common
allosteric denominator with the fundamental
fraction being the degree of dissociated and
associated states.
5.8.2 The Henderson-Hasselbalch
Equation for Dilute Weak Acids
For an isolated polypeptide containing one ion-
izable residue such as an aspartic or glutamic
acid residue without significant hydrophobicity
in the remaining residues, the Henderson-
Hasselbalch equation applies. The derivation
begins with the statement of an equilibrium con-
stant,
Ko,
that describes the relationship between
two states, in our case -COOH and -COO".
Ko =[COO-][H^]/[COOH]:
g-AGo/RT _ j^Q-AGo/2.3RT
(5.14)
where AGo is the change in Gibbs free energy
on going from reactants and products. By
taking the logarithm to the base 10 and intro-
ducing the definitions that pH = -log[H^] and
pKo = -logKo, Equation (5.14) becomes
pH = pKo + log{[COO-]/[COOH]} (5.15)
With the definitions a = [COO-]/{[COOH] -H
[COO"]} for the fraction of ionized species and
(1 - a) = [COOH]/{[COOH] + [COO"]} for the
fraction of nonionized species, substitution
into Equation (5.15) results in the well-known
Henderson-Hasselbalch equation, that is,
pH = pKo + log[a/(l - a)] (5.16)
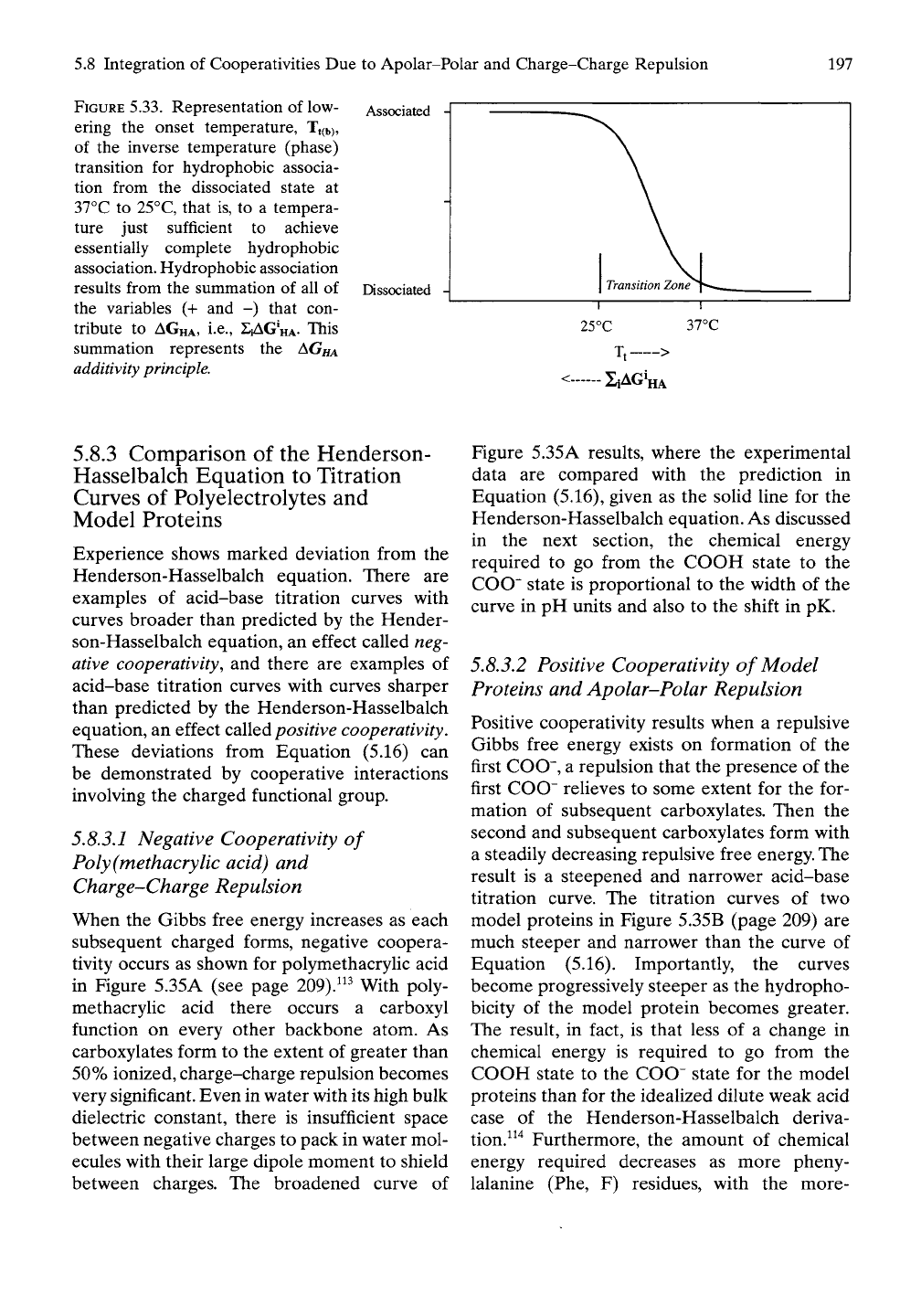
5.8 Integration of Cooperativities Due to Apolar-Polar and Charge-Charge Repulsion
197
FIGURE
5.33.
Representation of low- Associated
ering the onset temperature, Tt(b),
of the inverse temperature (phase)
transition for hydrophobic associa-
tion from the dissociated state at
37°C to 25°C, that is, to a tempera-
ture just sufficient to achieve
essentially complete hydrophobic
association. Hydrophobic association
results from the summation of all of Dissociated -|
the variables (+ and -) that con-
tribute to AGHA, i.e., SJAG'HA. This
summation represents the AG/^
additivity
principle.
1
Transition Zone
25°C 37°C
1, >
SiAG^HA
5.8.3 Comparison of the Henderson-
Hasselbalch Equation to Titration
Curves of Polyelectrolytes and
Model Proteins
Experience shows marked deviation from the
Henderson-Hasselbalch equation. There are
examples of acid-base titration curves with
curves broader than predicted by the Hender-
son-Hasselbalch equation, an effect called neg-
ative cooperativity, and there are examples of
acid-base titration curves with curves sharper
than predicted by the Henderson-Hasselbalch
equation, an effect called positive cooperativity.
These deviations from Equation (5.16) can
be demonstrated by cooperative interactions
involving the charged functional group.
5.8J.1 Negative Cooperativity of
Poly (methacrylie acid) and
Charge-Charge Repulsion
When the Gibbs free energy increases as each
subsequent charged forms, negative coopera-
tivity occurs as shown for polymethacrylic acid
in Figure 5.35A (see page 209).^^^ With poly-
methacrylic acid there occurs a carboxyl
function on every other backbone atom. As
carboxylates form to the extent of greater than
50%
ionized, charge-charge repulsion becomes
very significant. Even in water with its high bulk
dielectric constant, there is insufficient space
between negative charges to pack in water mol-
ecules with their large dipole moment to shield
between charges. The broadened curve of
Figure 5.35A results, where the experimental
data are compared with the prediction in
Equation (5.16), given as the solid line for the
Henderson-Hasselbalch equation. As discussed
in the next section, the chemical energy
required to go from the COOH state to the
COO"
state is proportional to the width of the
curve in pH units and also to the shift in pK.
5,8.3.2 Positive Cooperativity of Model
Proteins and Apolar-Polar Repulsion
Positive cooperativity results when a repulsive
Gibbs free energy exists on formation of the
first
COO",
a repulsion that the presence of the
first
COO"
relieves to some extent for the for-
mation of subsequent carboxylates. Then the
second and subsequent carboxylates form with
a steadily decreasing repulsive free energy. The
result is a steepened and narrower acid-base
titration curve. The titration curves of two
model proteins in Figure 5.35B (page 209) are
much steeper and narrower than the curve of
Equation (5.16). Importantly, the curves
become progressively steeper as the hydropho-
bicity of the model protein becomes greater.
The result, in fact, is that less of a change in
chemical energy is required to go from the
COOH state to the COO" state for the model
proteins than for the idealized dilute weak acid
case of the Henderson-Hasselbalch deriva-
tion.^^"^ Furthermore, the amount of chemical
energy required decreases as more pheny-
lalanine (Phe, F) residues, with the more-
198
5.
Consilient Mechanisms for Diverse Protein-based Machines
hydrophobic R-group -CH2-C5H6, replace less-
hydrophobic valine (Val, V) residues, v^ith the
R-group -CH2(CH3)2.
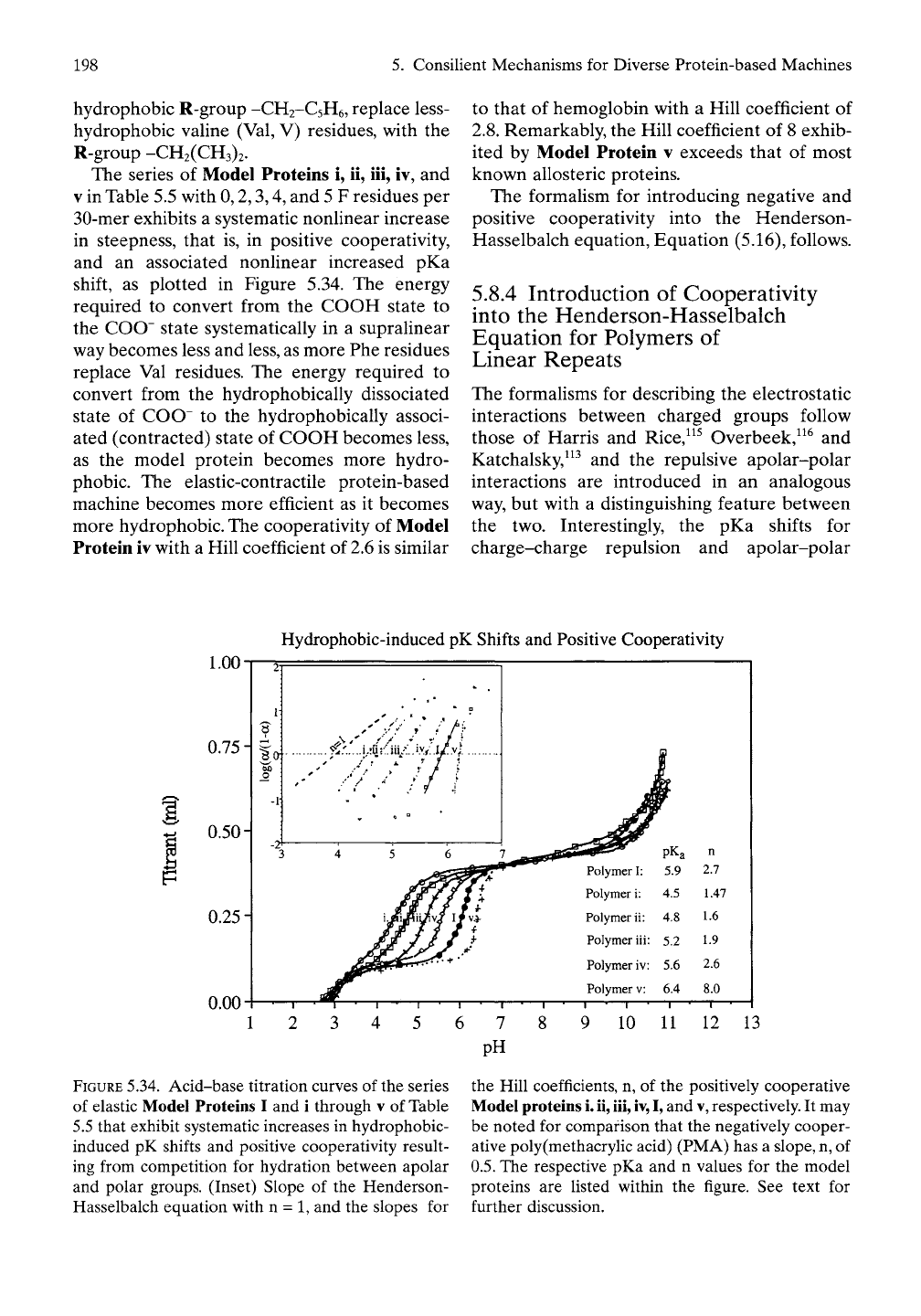
The series of Model Proteins i, ii, iii, iv, and
V
in Table 5.5 with 0,2,3,4, and 5 F residues per
30-mer exhibits a systematic nonlinear increase
in steepness, that is, in positive cooperativity,
and an associated nonlinear increased pKa
shift, as plotted in Figure 5.34. The energy
required to convert from the COOH state to
the COO" state systematically in a supralinear
way becomes less and less, as more Phe residues
replace Val residues. The energy required to
convert from the hydrophobically dissociated
state of COO" to the hydrophobically associ-
ated (contracted) state of COOH becomes less,
as the model protein becomes more hydro-
phobic. The elastic-contractile protein-based
machine becomes more efficient as it becomes
more hydrophobic. The cooperativity of Model
Protein iv with a Hill coefficient of 2.6 is similar
to that of hemoglobin with a Hill coefficient of
2.8.
Remarkably, the Hill coefficient of 8 exhib-
ited by Model Protein v exceeds that of most
known allosteric proteins.
The formalism for introducing negative and
positive cooperativity into the Henderson-
Hasselbalch equation. Equation (5.16), follows.
5.8.4 Introduction of Cooperativity
into the Henderson-Hasselbalch
Equation for Polymers of
Linear Repeats
The formalisms for describing the electrostatic
interactions between charged groups follow
those of Harris and Rice,^^^ Overbeek,^^^ and
Katchalsky,^^^ and the repulsive apolar-polar
interactions are introduced in an analogous
way, but with a distinguishing feature between
the two. Interestingly, the pKa shifts for
charge-charge repulsion and apolar-polar
Hydrophobic-induced pK Shifts and Positive Cooperativity
1.00
0.75
0.50
0.25
0.00
pKa
Polymer I: 5.9
Polymer i: 4.5
Polymer ii: 4.8
Polymer iii: 5.2
Polymer iv: 5.6
Polymer v: 6.4
—i—•"—I—'—I—'—T"
12 3 4 5 6 7
pH
—I—
10
n
2.7
1.47
1.6
1.9
2.6
8.0
—1—•—I—'•
11 12 13
FIGURE
5.34. Acid-base titration curves of the series
of elastic Model Proteins I and i through v of Table
5.5 that exhibit systematic increases in hydrophobic-
induced pK shifts and positive cooperativity result-
ing from competition for hydration between apolar
and polar groups. (Inset) Slope of the Henderson-
Hasselbalch equation with n = 1, and the slopes for
the Hill coefficients, n, of the positively cooperative
Model proteins
i.
ii,
iii,
iv,
I,
and
v,
respectively. It may
be noted for comparison that the negatively cooper-
ative poly(methacrylic acid) (PMA) has a
slope,
n,
of
0.5.
The respective pKa and n values for the model
proteins are listed within the figure. See text for
further discussion.

5.8 Integration of Cooperativities Due to Apolar-Polar and Charge-Charge Repulsion
199
repulsion are additive. The cooperativities, on
the other hand, are of opposite sign and can
cancel.
Thus,
when the free energy of interaction
represented by the pK shift and the free energy
of interaction represented by the change in
cooperativity are the same, as is the case in
Figure 5.29, the apolar-polar repulsive free
energy for hydration dominates, that is, there is
no significant charge-charge repulsion.
5.8.4.1 Multiple Acid-base Equilibria in
Protein-based Polymers with Widely
Sequence Displaced Functional Groups
For the set Model Proteins i through v in Table
5.5,
one glutamic acid residue (Glu, E) repeats
every 30 residues. This means that there are 95
backbone and side chain atoms between the
ionizable functions, and for Model Protein i
there are 36 such repeats. There then exist 36
equiUbrium constants, K; through Kj^, one for
the ionization of each carboxyl function. An
overall equiUbrium constant can be written,
K^^, which is the product of all 36. This can be
written in general as
[(COO-)J[HlV[(COOH)J (5.17)
Taking the logarithm to the base 10,
nlogK-nlog[H^] +
log{[(COO-)J/[(COOH)J} (5.18)
and again defining pH = -log[H^], pK = -logK,
dividing by n, and rearranging gives
pH = pK + (l/n)log{[(COO-)J/[(COOH)J}
(5.19)
5.8.4.2 Introduction of Effect of
Substantial Hydrophobic Hydration
As shown in Figure 5.34, in the interactions
among carboxylates while under the influence
of increasing numbers of hydrophobic residues,
pK ^ pKo, but rather pK = pKo + ApK, that is,
the pKa shifts of section 5.7.8. Furthermore, it
is assumed that when one chain starts to ionize,
due to the positive cooperativity, it completely
ionizes before the next chain starts such that, to
an adequate approximation, the experimentally
measurable degree of ionization, a ~
[(COO-)J/[(COOH)„] and n is replaced by a
phenomenological n to correspond with this
assumption. Equation (5.19) can now be
restated as,
pH = pKo + ApK + (l/n) log[a/(l - a)] (5.20)
Equation (5.20) retains the form of the
Henderson-Hasselbalch equation, but it has
been generalized to introduce pK shifts and
cooperativity into the expression. This pheno-
menological introduction of cooperativity is
analogous to the treatments describing the oxy-
genation of hemoglobin. The quantity n is
equivalent to a Hill coefficient.^^ Furthermore,
in point 4 of section 5.7.8.2, we noted for pK
shifts of about 1 that the Wyman equation,^^
AAGo = RT(1 - l/n)/a(l - a), when evaluated
at the pK at a = 0.5 and the relevant Hill
coef-
ficients
(n),
gave the same value for the energy
of interaction as that calculated for the pK
shift. In particular, by Equation (5.13), AGap =
2.3RTApK, and when evaluating at a = 0.5,
AAGo = 4RT(1 - l/n). Thus, as occurred in the
data in Figure 5.29 where AGap ~
AAGo,
the rela-
tionship between ApK and Hill coefficient, n,
becomes ApK = 1.74 (1 - l/n).This suggests that
positive cooperativity and hydrophobic-
induced pK shifts can be different representa-
tions of the same interaction energy.
Larger pK shifts, however, have been
observed than are calculable by the Wyman
equation. For example, a pK shift of 6 has been
observed for aspartic acid in a very hydropho-
bic polymer (see Figure 5.32), whereas from the
Wyman equation, ApK = 1.74 (1 - l/n), the ApK
could not exceed 1.74. Also with a reference pK
value of 4.0 for the Glu carboxyl, a pK shift of
2.4 is seen in Figure 5.34 with a corresponding
Hill coefficient of 8. For larger Hill coefficients
and pK shifts, the limitation of the correlation
is likely due to limiting approximations of the
Wyman equation that have been overcome, for
example, by the Monod treatment.
Additionally, as shown in Figure 5.31A, pK
shifts separate into an apolar-polar repulsive
component that can be relaxed by a change in
hydrophobic association and an apolar-polar
repulsive component that is dictated by
sequence and thereby retained even in the most
unfolded and disassembled state. Expectedly,
the pKa shift, relaxed by a disruption of all

200
5.
Consilient Mechanisms for Diverse Protein-based Machines
hydrophobic association, is the component that
results in positive cooperativity and in Hill
coefficients of greater than 1.
To continue with the derivation, because K =
^-AG/RT ^ ^Q-AG/2.3RT^
j^gj^^
^ -AGo/2.3RT = -pKo,
and Equation (5.20) can be rev^ritten in terms
of changes in Gibbs free energy, AG, to give
pH =
AGo
/2.3RT +
AAGo
/2.3RT
+ (l/n)log[a/(l-a)]
(5.21)
Derivation of Equation (5.21) considers
hydrophobic-induced pK shifts and hydropho-
bic-induced increases in positive cooperativity.
In Figure 5.30, however, there is experimental
deUneation between the hydrophobic domain
where the above considerations dominate and
the electrostatic domain where charge-charge
repulsion dominates with a negative coopera-
tivity.
Thus we require an expression that would
properly include both effects.
5.8.5 Toward a Generalized
Acid-Base Titration Expression
5,8,5.1 Introduction of the Harris and
Rice Treatment of Cooperativity
Following Harris and Rice,^^^ the last term of
Equation (5.8) can be effectively restated as
log[a/(l - a)] + (aAG/aa)T/2.3RT, where
(3AG/9a)T = 0 at a = 0.5, to give
pH = pKo + ApK + log[a/(l - a)]
+ (^AG/aa)T/2.3RT (5.22)
that
is,
the last term of Equation (5.22) replaces
the Hill coefficient with a term that provides
for the change in Gibbs free energy as the
degree of ionization changes, (3AG/3a)T, which
changes the steepness of the curve from that
given by the simple Henderson-Hasselbalch
equation.
5,8.5.2 Introduction of the Effects of Both
the Charge-Charge Repulsion, AG^o and
Apolar-Polar Repulsive Free Energy of
Hydration, AG^^
As discussed above, there are two primary
mechanisms in aqueous dominated media for
pK shifts and changes in the steepness of the
experimental titration
curve.
These are referred
to as electrostatic-induced, principally charge-
charge repulsion (cc) and as hydrophobic-
induced, that is, the apolar-polar repulsive free
energy of hydration (ap). The general expres-
sion can now be written specifically to include
these two primary interactions as
pH = pKo + ApKcc + ApKap +
log[a/(l-a)] + {[((9AG/^a)J^.
+ [OAG/(9a)J^J/2.3RT
(5.23)
Although the ApKcc and ApKap values are of the
same sign and add, the partial derivatives are of
the opposite sign and counter each other. In par-
ticular, [(3AG/3a)T]c-c is the result of a negative
cooperativity with a curve that is broader than
given by the Henderson-Hasselbalch equation,
and [(3AG/3a)T]a-p is the result of a positive
cooperativity with curve that is steeper than
given by the Henderson-Hasselbalch equation.
5.8.5.3 Experimental Delineation ofAGcc
and AGap in Series of Model Proteins
The experimental titration data for the series of
Model Proteins I, i, ii, iii, iv, and \ are listed
in Table 5.5 and shown in Figure 5.34, where
the differential effects of charge-charge and
apolar-polar repulsion become apparent. For
Model Proteins i, ii, iii, iv, and v, for each sys-
tematic shift in pKa there occurs a correspond-
ing increase in Hill coefficient. Model Protein
I, however, does not fall within the series,
because it exhibits a pKa shift without an
increase in magnitude of the Hill coefficient.
Model Protein I and iv exhibit essentially the
same Hill coefficient of 2.7, whereas the pKa
values differ by 0.3 pH units.
On inspection of the sequence of Model
Protein
I,
it is more hydrophobic with five F plus
two I residues having replaced seven less
hydrophobic V residues in the repeating 30
residue sequence. If this were the only determi-
nant it would be expected to have a greater pKa
and a larger Hill coefficient than Model Protein
v. Model Protein I, however, has two proximal
E residues with carboxylates separated by only
two residues, that
is,
-EPGE-. From the data in

5.8 Integration of Cooperativities Due to Apolar-Polar and Charge-Charge Repulsion
201
Figure 5.30, in poly(GEGIP) the Glu residues
are separated by four residues, -EGIPGE-, and
the pKa shift due to charge-charge repulsion is
>0.35 pH units. The charge-charge repulsion
component of the pKa shift for -EPGE- is
expected to be greater than for -EGIPGE-.
Accordingly, in the titration curve of Model
Protein I, when compared with those of Model
Proteins i, ii, iii, iv, and v, the differential effects
of charge-charge and apolar-polar repulsion
become apparent, and an iterative fitting
process using Equation (5.23) should allow
determination of AGcc and AGap in this mixed
case.
In doing so the recognition of the residual
pKa shift after complete unfolding, shown in
Figure 5.31, should also be delineated.
5.8.5.4 Positive Cooperativity as a
Fundamental Property of the Competition
for Hydration Between Apolar
(Hydrophobic) and Polar (e.g..
Charged) Species
From the analysis of the acid-base titration
data in Figures 5.30 through 5.34, positive coop-
erativity results from the apolar-polar repul-
sive free energy of hydration, that is, from the
competition for hydration between apolar
(hydrophobic) and polar (e.g., charged) species.
The general statement can be that the appear-
ance on the scene of the first polar, for example,
charged, species must do the work of destruc-
turing hydrophobic hydration in order to
achieve adequate hydration for
itself.
To put this into perspective, consider again
the Gibbs free energy for solubility, AG(solu-
bility) = AH - TAS, and recall the discussion of
Butler's findings (see section 5.1.3.3), where
insolubiUty results from the formation of too
much hydrophobic hydration. Too much hydro-
phobic hydration causes the positive (-TAS)
term to become larger than the negative
(exothermic) AH term, that is, when AG(solu-
bility) becomes positive and solubility is lost.
The insolubiUty comes in the form of the asso-
ciation of a pair of hydrophobic domains.
However, the pair of domains undergoes disso-
ciation and association fluctuations. Now, a
polar species can emerge (e.g., COOH -^
COO") proximal to an opening fluctuation, if it
can achieve adequate hydration by destructur-
ing the hydrophobic hydration. In doing so it
lowers the magnitude of the positive (-TAS)
term sufficiently to allow the dissociation to
stand. Even though there may yet be substan-
tial hydrophobic hydration covering the sur-
faces of the previously paired hydrophobic
domains, there is additional water, and the
emergence of the second polar species achieves
its hydration without having to destructure
quite so much hydrophobic hydration, and its
equilibrium constant is more favorable. This is
the description of positive cooperativity at the
molecular level arising from an apolar-polar
repulsive free energy of hydration.
To the best of my knowledge, the hemoglobin
oxygenation curve is historically the first
example of a biologically essential positive
cooperativity. Because of this, it becomes an
important objective to explore the phenome-
nology of hemoglobin's positive cooperativity
and compare it with that of the consilient
mechanism due to an apolar-polar repulsive
free energy of hydration (as is done in
Chapter 7) and, in fact, to do so for a number of
protein-based machines that exhibit positive
cooperativity.
5.8.5.5 Evaluation of [(dAG/da)Tjap Within
the Single Polymer, Model Protein v
From the plot in Figure 5.31A of the titration
of Model Protein v, the pKa of the first car-
boxyls to ionize may be taken as 7.0, whereas
that of the last carboxyls to ionize is about 5.7.
Therefore, in the process of driving hydropho-
bic disassociation of a relaxation or, inversely,
in the process of driving hydrophobic associa-
tion of a contraction, the pKa shifts by some
1.3 pH units. By Equation (5.13), AGap
= 2.3 RT ApKa - 2.3 x 1.987 x 310 x 1.3
= 1.8kcal/mole-carboxyl. Now, because
AGap = J[(9AG/3a)T]apda, we have that
/[(3AG/3a)T]apda « 1.8 kcal/mole-carboxyl. This
is an example wherein only the apolar-polar
repulsive free energy of hydration is relevant,
as the presence of a Glu (E) residue every 30
residues does not give rise to charge-charge
repulsion in the unfolded, state. Also, when
totally unfolded the carboxyl pKa is still shifted

202
5.
Consilient Mechanisms for Diverse Protein-based Machines
from the unperturbed pH value of near 4.0.
Even with the model protein unfolded, there
still exists an apolar-polar repulsive free energy
AGap of 2.3 RT ApKa = 2.3 x 1.987 x 310(5.7 -
4.0) = 2.4kcal/mole-carboxyl. Even w^hen disas-
sembled and unfolded, the sequence of Model
Protein v does not allow the carboxylate
complete access to water unencumbered
by hydrophobic groups. Furthermore, the
maximum pKa shift experienced by the car-
boxyls in this hydrophobic model protein is
7.0 - 4.0 = 3.0, giving a AGap of 4.2kcal/mole-
carboxyl (see Figure 5.31A).
For Model Protein I, there occur 2 glutamic
acid residues (Glu, E) per 30 residues, and
importantly these are separated by only two
residues. In this case there exist significant
values for both AGap and AGcc- Recall that the
free energies add for the pKa shift but are of
the opposite sign for cooperativity. This being
the case, it is possible to write two equations
with the two unknowns, AGap and AGcc, and to
solve the values. In this case, approximate
values for AGap of 2.0kcal/mole-carboxylate
and AGcc of 0.7 kcal/mole-carboxylate pair can
be estimated.
Thus,
when laid out in the form of
Equation (5.23), it becomes possible to begin
separation of different contributions to the
Gibbs free energy of interaction.
5.8.5.6 Relationship of the Consilient
Mechanism to Cold Denaturation and to
Hydrophobic Dissociation on Being Made
More Polar
Hydrophobic association on raising the tem-
perature is the most fundamental aspect of the
consiUent mechanism, arising as it does from
the inverse temperature transition. An equiva-
lent statement would be that hydrophobic
dissociation on lowering the temperature is
fundamental to the consilient mechanism. His-
torically, this has been called cold denaturation
ofenzymes.^^ In our view, those protein systems
that associate on heating to physiological tem-
peratures in order to achieve a functional state
should be considered in terms of the consilient
mechanism.
In fact, any protein function that involves
cycling between hydrophobically associated
and dissociated states by whatever energy
input, for example, whether chemical or elec-
trochemical, should be considered in terms of
the consilient mechanism of the apolar-polar
repulsive free energy of hydration.
5.8.6 Toward a Generalized Theory
for Achieving Function of Protein-
based Machines Founded on
Hydrophobic Association
Equation (5.23) provides a general expression
for fitting the family of curves schematically
shown in Figure 5.19B and experimentally
demonstrated in Figure 5.20A-C. The relevant
energy conversions can be considered as
electro-chemical (A), chemo-mechanical (B),
and electro-mechanical (C). In each case, posi-
tive cooperativity is the result of increasing
hydrophobicity, and it would appear to apply to
the many energy conversions described in
section 5.6. Thus, it would seem necessary only
to cloak the expression in the correct energy
terms,
recognizing as in Equation (5.21) that
pH,
for example, is a free energy divided by 2.3
RT.
It would seem, whatever set of energies
drive function, that a complete description
could be achieved by properly including the
required terms in such an equation.
Hopefully, the above proposed integration of
energy conversion, involving the recognition of
a commonality of protein function that includes
an apolar-polar repulsive free energy of hydra-
tion, might bring us one step closer to the
daunting challenge recognized by Weber^^^ of
achieving a more "complete description of the
energetics" of protein-based machines.
5.9 On the Efficiency
of Consilient Protein-
based Machines
5.9.1 Introductory Remarks
5.9.1.1 The Central Role of the Gibbs Free
Energy for Hydrophobic Association in
Diverse Energy Conversions
The change in Gibbs free energy for hydropho-
bic association,
AGHA,
affects and is affected
by the forms of energy utilized by living organ-

5.9 On the Efficiency of Consilient Protein-based Machines
203
isms.
It originates from inverse temperature
transitions; it establishes the comprehensive
hydrophobic effect, and, because of the perva-
siveness of its role in catalyzing energy conver-
sion, the comprehensive hydrophobic effect
provides the basis for the consilient mechanism.
From our developing perspective and as
demonstrated in part below, it appears that
AGHA provides the foundation for the most effi-
cient mechanism of diverse energy conversion
available in aqueous systems.
5.9,1.2 The Design of Poised Elastic-
Contractile Protein-based Machines
The design of efficient protein-based machines
requires judicious choice of model protein com-
position based on the intended energy source
and operating conditions of temperature,
medium, and so on. Proper design poises the
system immediately to initiate function on
addition of the driving force, the input energy.
Of course, in recognizing this, protein function
by modulating hydrophobic association simply
depends in a complementary way on the many
variables of the Weber concern,^^^ for example,
ligand binding, subunit association, pH, tem-
perature, and pressure, as well as phosphoryla-
tion-dephosphorylation, oxidation-reduction,
absorption of light, and so forth.
From a practical standpoint, for example, Tt
would be set just above the transition zone, as
defined in Figure 5.5, such that the energy input
for which the system is designed can most effi-
ciently lower Tt through the transition zone. For
biological conditions Tt would be poised at
37° C, and the added energy would lower Tt the
width of the transition zone to about
25° C.
The
reaction would be represented by an equilib-
rium constant for hydrophobic association,
KHA, expressible as logKnA = -AGHA/2.3RT,
where AGHA/2.3RT would be plotted versus Y'\
the fraction of hydrophobic association in
analogy to Equation (5.23), and modified to
include cooperativity as shown for a protona-
tion/deprotonation reaction also treated
in Equation (5.23). The chemical couple
COOH/COO", is a specific example, but the
reaction can involve any of the energy conver-
sions represented in the Hexagon in Figure
5.22. Of course. Axiom 5 (see section
5.5.3)
provides a guiding principle for increasing
efficiency of energy conversion, which has the
effect of increasing positive cooperativity.
5.9.1.3
AGHA
Provides an Efficiency
Limit and Becomes the Coupling Process
in Energy Conversion by the
Consilient Mechanism
Interestingly, for each model protein composi-
tion and energy source the consilient mecha-
nism sets an efficiency limit,
CCM-
It is simply the
change in Gibbs free energy for hydrophobic
association, the output energy, divided by the
input energy, that is,
CCM
= AGnA/input energy.
For the coupling of functions, as, for example,
occurs in electro ^-^ chemical transduction,
reduction becomes the energy input that results
in an output energy of
AGHA,
a decrease in the
Gibbs free energy of hydrophobic association
due to an increase in hydrophobicity; the
AGHA
thus produced then becomes the energy input
for the output of a change in chemical energy.
In general, these energy conversions can be
reversible, and
AGHA
emerges as the coupUng
process in energy conversion by the consilient
mechanism.
To elaborate on an electro
<*-•
chemical trans-
duction example, the chemical group to be
reduced can be a biosynthetic modification of
vitamin B3 (niacin, shown in Figure 3.8A) or of
vitamin B2 (riboflavin, shown in Figure 3.8B).
Electrical energy is expended to reduce the
oxidized state of a modified vitamin B3 or B2
attached to the protein, making the protein
more oil-like and lowering the Gibbs free
energy for hydrophobic association,
AGHA,
sufficient to drive hydrophobic association.
This decrease in AGHA changes the free energy
of a carboxylate and causes it to take up a proton
(see Figure 5.20A and associated discussion).
Analyses of these elements are considered
below after a brief general definition of efficiency.
5.9.2 General Statement for Efficiency
of Energy Conversion
The usual symbol for efficiency is r|. The defin-
ition of r\ is the output energy divided by the

204
5.
Consilient Mechanisms for Diverse Protein-based Machines
input energy times 100 so that the efficiency is
given as a percentage:
x\
= (output energy/input energy) 100 (5.24)
5.9.3 Consilient Mechanism's
Efficiency Limit,
CCM,
for Model
Protein Composition and
Energy Source
The Gibbs free energy for hydrophobic associ-
ation,
AGHA,
may be viewed as an output energy
that results from a particular input energy
acting on a specific composition of protein-
based machine. Thus, we define an efficiency
limit for energy conversion by the consilient
mechanism,
CCM,
for a particular energy input
and model protein composition.
ecM =
AGHA
/input energy (5.25)
The same input energy can result in very
different efficiency limits depending on the
protein composition. Furthermore, the same
energy input, acting on the same polymer com-
position, can vary depending on the range of the
intensive variable. For example, for the compo-
sition (GVGVP)n, raising the temperature from
5° to 20° C results in no
AGHA
(see Figure 5.5A).
On the other hand, raising the temperature from
25° to 40° C results in hydrophobic association
that is useful in many ways including the output
of mechanical work. Alternatively, an energy
input acting to lower the value of Tt would have
little effect until Tt begins to enter the transition
zone,
which for most mammals would begin at
about 37° C and be complete by about 25° C (see
Figure 5.5B). In relation to Figure 5.5, Tt on the
X-axis could be plotted versus Y", the fraction of
hydrophobically associated
state.
With Tt above
37° C, Y'' would be zero; as Tt is lowered through
the transition zone, there would be a sigmoidal
increase in Y'' until a value of
1
were reached at
about 25°
C.
Graphically this is simply the
sigmoid of Figure 5.33.
A prevalent example in biology involves a
chemical energy and its intensive variable of
chemical potential, |J., the change in Gibbs free
energy per mole of chemical. For the polymer
composition in Figure 5.12A, at low salt con-
centration the slope ATt(%)/A|x, apparent as
ATt(x)/AN, is steep, whereas at higher concen-
trations the slope is much less. By Equation
(5.9),
AGHA is larger for the low salt concentra-
tion range than the high concentration range.
Thus,
CcM varies significantly depending on the
effectiveness of the energy input in achieving a
AGHA-
The latter quantity is calculable by
employing Equation (5.10b),
AGHA(X)
=
[AHt(ref) - AHt(x)], and using relevant experi-
mental data, much of which are found in Luan
and Urry's review^^ entitled "Elastic, Plastic,
and Hydrogel-Forming Protein-based Poly-
mers"
in the Polymer Data Handbook. Key
results are listed in Table 5.4. Accordingly, in a
plot of Tt versus concentration it is the slope at
any point along the curve, and the calculated
AGHA per mole, that is, |XHA, would be for that
concentration. A good example of the impor-
tance of model protein composition is shown in
Figure 5.15 and the associated discussions of
relative effectiveness in section 5.4.3.2.
5.9.3.1 CaCk and NaCl as Chemical
Energy Inputs to an Uncharged Model
Protein to Drive Hydrophobic Association
We begin with NaCl, for which calorimetry
data, AHt, are available from Table I of
Luan et al.^^^ for this salt interacting with
poly(GVGVP) and used in Equation (5.10b).
Chemical energy is defined as A|iAn, where the
chemical potential,
A|LI,
is 2.3RT log(a7a")- The
a' and a" are the activities of the ions at two
dif-
ferent activities, a' and a'', which equal concen-
tration at sufficiently low ion concentrations,
[C].This assumption will be made here for sim-
plicity, but the activity coefficients required for
accurate calculations with salts are available.^^^
This situation is further complicated by the
determination of An when there is no func-
tional group being titrated. Accordingly the
numbers calculated are taken for a An of 1.
These complications are not significant for
comparison of the effect of different chloride
salts on uncharged polymers, but they do
become relevant when making comparisons
with data from charged model proteins.
For the concentration step from
0.1
N to
l.ON, ATt is 12.5 and
AGHA
=
AHt(0.1
N - l.ON)
= 0.5kcal/mole-pentamer. Note for the concen-
