Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


5.9 On the Efficiency of Consilient Protein-based Machines
205
tration step used
(0.1
N -
1.0N),
these numbers
are slightly different from the values in Table
5.4 for the concentration step of (ON -
1.0N).
The concentration step of a factor of 10
(0.1
N
- 1.0 N) is chosen so that the chemical energy
input at physiological temperature, 37°C
(310°K), would be 2.3RT log(lO) = 1.42 kcal/
mole, for utilization in the denominator of
Equation (5.25). Therefore,
ecM[poly(GVGVP),NACl] =
0.50kcal/mole/l.42kcal/mole = 0.35 (5.26)
For CaCl2 there is as yet no experimental
value for AHt(0.1N - l.ON) determined from
calorimetry.Thus, to have an estimate, the same
slope will be assumed as for NaCl, that is,
AGH[poly(GVGVP), CaCl2(0.1N - l.ON)] =
(0.50/12.5)(6.6
X
0.9) = 0.24. Under this approx-
imation,
ecM = [poly(GVGVP), CaCl2] =
0.24 cal/mole/1.42 cal/mole = 0.17 (5.27)
In this approximate way, the efficiency limit
when using the increment of
(0.1
N - l.ON)
with a chemical energy per mole of 1.42 kcal at
37°C is 0.35 for NaCl and an even smaller value
of 0.17 for CaCl2. The effectiveness of salt in
driving hydrophobic association increases dra-
matically when there are charged side chains
with which to ion pair.
5,9.3.2 CaCh and NaCl as Chemical
Energy Inputs to a Charged Model
Protein: Impact of Ion Pairing on Driving
Hydrophobic Association
In the absence of titration data for accurate
A|LI
and An values, in the absence of corrections
to activities (see above), and without direct
calorimetry data to estimate AHt for the specific
salt and model protein systems of interest, gross
comparison derives from the AGHA(salt) values
Usted in Table 5.4A, as determined in section
5.3.4.
5.9.3.2.1 For Model Protein i Using CaCl2 as
the Chemical Energy Input
For Model Protein i in Table 5.5 and from data
in Figure 5.10 and Table 5.3, the protonation of
-COQ-
(E70F) to give -COOH (E70F) gives a
slope of (9kcal/mole)/(380°C). As ion pairing
constitutes partial neutralization, we will
assume this slope. For CaCl2, in the 0.0125 N to
0.05 N concentration interval and as shown in
Figure 5.12A and listed in Table 3e of Luan and
Urry,^^ at low ion concentrations the steepest
slope is estimated to have a AT/N value of
-618°C/N, which gives
AGHA
= -14.6kcal/mole-
pentamer/N-calcium ion.
It should be emphasized that this value is rel-
evant in only a very narrow concentration range,
causing only partial folding and unfolding of
the model protein. Nonetheless, a sense of the
increased effectiveness of ion pairing in driving
hydrophobic association derives on division of
14.6 by 0.27 from Table 5.4. By this estimate,
calcium ion pairing with polymer carboxylate as
compared with the calcium chloride salt effect on
an uncharged polymer approximates to be some
50 times more efficient in the use of chemical
energy to produce a change in the Gibbs free
energy for hydrophobic association,
AGHA-
5.9.3.2.2 For Model Protein i Using NaCl as
the Chemical Energy Input
For NaCl in the 0.15 N to 0.25 N concentration
interval and also as shown in Figure 5.12A and
Usted in Table 3e of Luan and Urry,^^ at low ion
concentrations the steepest slope is estimated to
have a AT/N value of-395° C/N.This gives AGHA
= -9.35 kcal/mole-pentamer/N-sodium ion.
Division by 0.57 for NaCl with poly(GVGVP)
of Table 5.4 provides an estimate of better than
a 15'fold increase in the effectiveness ofNa^ ion
pairing with -COO~, that
is,
Glu-COO~ ... Na^,
in driving hydrophobic association than when
there is no site for ion pairing.
5.9.3.2.3 For Model Protein x' and
Poly[0.76(GVGVP),0.24(GKGVP)] Using the
NaCl Energy Input
First an estimate of the lowering of the Gibbs
free energy of hydrophobic association,
AGHA,
obtained for poly[0.76(GVGVP),0.24
(GK^GVP)],
derives from the data in Table 3d
of Luan and Urry.^^ The steepest slope, AT(/N,
(0.05
N to 0.20 N) arising from ion pairing of
-NHs^
with Cr, gives a value for NaCl of

206
5.
Consilient Mechanisms for Diverse Protein-based Machines
-177° C/N. Next, the value for AT/AGHA of
12.5kcal/mole/230°C derives from Figure 5.10
using Model Protein x' of Table 5.5. On multi-
plication, AGHA(Lys-NH3^ ... CI"; steepest ion-
pairing slope) ~ - 9.6kcal/mole-pentamer/N-
chloride ion. Division of this number by 0.57 of
Table 5.4 for NaCl with poly(GVGVP) gives a
relative effectiveness of approximately 17.
Again, we see the dramatic effect of ion pairing
on driving hydrophobic association.
5.9.3.3 Acid as Energy Input for Model
Protein with Titrable Functional Groups
The acid-base titration curve is sigmoid in
shape as is commonly the case for equilibrium
processes wherein fraction of completion of the
reaction is plotted versus log[C]. In the case of
the acid-base titration, pH = -log[H^]. Because
of this, some basis must be decided upon for the
measurement of the relative proton chemical
energy required to drive from 0% to 100%
completion. Here we utilize the steepest part of
the sigmoid and talk of the efficiency for oper-
ating in this linear range, which would give the
maximal efficiency for the process. The curve
for a dilute weak acid, described by the
Henderson-Hasselbalch equation, exhibits a
steepest slope of 0.9 pH units for going from
degree of ionization, a, of 0 to 1, that is, from
the protonated to the charged state.
To make comparisons with the above salt
effects, however, accurate values of An from
acid-base titrations and correction of concen-
trations to activities should be considered. At
this time, however, several different graphical
representations of relative efficiencies are pos-
sible.
These include comparison of the relative
effectiveness of changes in chemical potential,
A|i,
to drive Tt from just above to below the oper-
ating temperature, comparison of the relative
A|iAn areas determined from acid-base titration
curves,
and comparison of the significance of
dif-
ferent degrees of positive cooperativity, that is,
the impact of changes in the Hill coefficient.
5.9.4 Graphical Insights into
Relative Efficiencies for Chemical
Energy Input
Three different graphical representations
provide insight into relative efficiency in
response to a chemical energy input. The first
shows the relative amount of chemical energy
required to lower the onset temperature for
hydrophobic association from just above the
operating temperature to a temperature just
sufficient to cross the width of the transition
zone of Figure 5.5. For (GVGVP)n that
would be to lower Tt from the physiological
temperature of 37°C where the polymer is
hydrophobically dissociated to 25°C where
the model protein would be almost completely
hydrophobically associated, as depicted in
Figure 5.33.
A second representation comes from the per-
spective of acid-base titration curves. The rela-
tive efficiency, for example, of two different
proton-driven molecular machines becomes
apparent by the relative areas of AjiixAn, where
A|Li
(= -2.3RT ApH) is measured by a step along
the pH axis and An by a step along the y-axis,
in terms of the change in number of moles
required to convert from one state to the other.
This is demonstrated in Figure 5.34.
A third measure of relative efficiency derives
from comparison of the Hill coefficients where
the larger Hill coefficient indicates a more
efficient conversion of chemical energy into
the hydrophobically associated state. This is
demonstrated in the inset in Figure 5.34.
5.9.4.1 Relative Efficiency Limits for
Chemical Energy Inputs Using the
Consilient Mechanism
The efficiency limit of the consilient mechanism
for a chemical energy input is written as
ecM =AGHA/A^An (5.28)
AjiAn is the chemical energy where A|i is the
change in chemical potential and is the Gibbs
free energy per mole of the chemical used
to drive hydrophobic association, given by
2.3RT log{[C2]} - log{[Ci]} or equivalently
2.3RT log{[C2]/[Ci]} where the approximation
of concentration is used for activity and the
change in chemical potential is positive when
[C2] >
[Ci].
An is the change in number of moles
of the chemical used to achieve the hydropho-
bic association, for example, the number of
moles of functional groups titrated.

5.9 On the Efficiency of Consilient Protein-based Machines
207
The relative efficiency of two systems,
r|(system-l)/r|(system-2) = T|I/T|2, can be
expressed as the ratio of efficiency limits for the
two systems when being compared using the
same chemical energy.
^i/^i
=T|
(system-1)/T|(system-2) =
ecM
(system-l)/ecM (system-2)
ili/tl2 =(AGHA/AnAn)^/(AGHA/AnAn)2
(5.29)
5.9.4.2 Relative Efficiencies by Chemical
Energy Required to Move the Cusp
of Insolubility Across Biology's
Transition Zone
When concerned with the physiological operat-
ing temperature of 37°C, a model protein with
its Tt(b)-value at 37°C will not have appreciably
hydrophobically associated. Any variable that
lowers the Tt(b)-value to
25°C,
which is the width
of the transition zone for (GVGVP)25i, will
have essentially completed the transition to the
hydrophobically associated state, as depicted in
Figure 5.33. The variable will have moved the
cusp of insolubility across the transition zone
for biology. In particular, the interest is in
the variable of the chemical energy per mole
required for a ATt just sufficient to traverse the
transition zone from hydrophobically dissoci-
ated at 37°C to hydrophobically associated at
25°C.
Two examples are represented in Figure 5.15
for Model Proteins I and ii of Table 5.5, where
the TfValue is plotted as a function of an
increase in chemical energy, that is, in this case
an increase in the concentration of calcium ion.
Section
5.4.3.2
considered the relative efficien-
cies on the basis of graphical comparisons. With
respect to Equation (5.29), that comparison
assumed the values of (AGHA)I and (AGHA)2 and
of Aui and An2 to be equivalent. The approxi-
mation becomes T|I/T|2 ~ A|X2/A|ii, which gives
the ratio of 0.116/1.12 -
0.1.
With adequate
dif-
ferential calorimetry and titration data, the rel-
ative efficiencies in terms of relative efficiency
limits can be determined accurately. Actual effi-
ciency ratios for converting chemical energy
into mechanical work using cross-linked
matrices are calculated below in section 5.9.5.3.
5.9.4.3 Relative Efficiencies by AjiAn
Areas of Acid-base Titration Curves
Another ready visual means of approximating
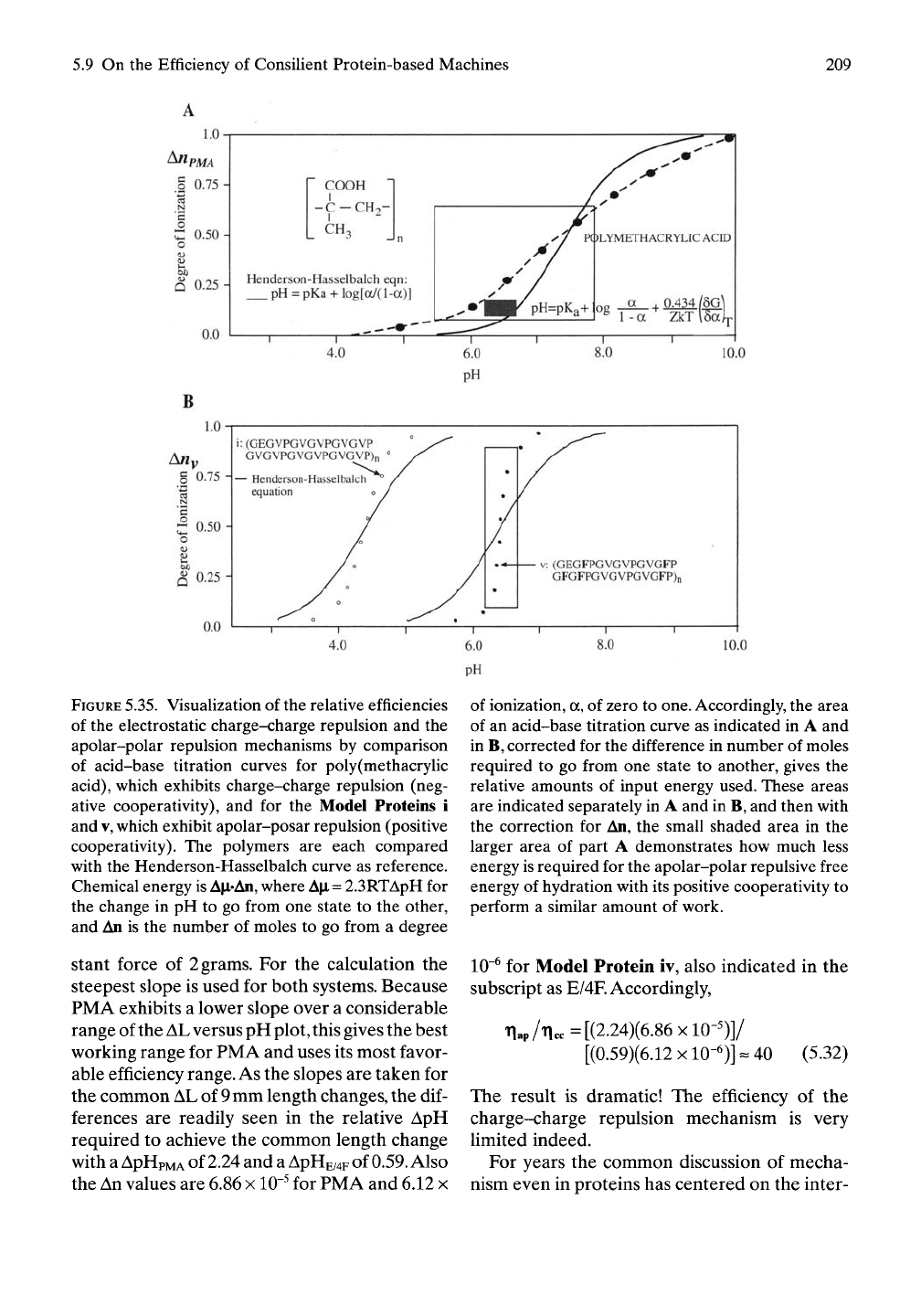
relative efficiencies is given in Figure 5.34 for
the series of Model Proteins i through v. In par-
ticular, Model Proteins i and v are compared by
the boxes indicated. When the titrations are
carried out with the same concentration of car-
boxyls for each model protein and the same
concentration of titrant, the vertical displace-
ment is proportional to An and the horizontal
displacement is proportional to A|i. Accord-
ingly, comparison of the area, AjiiAUi, as indi-
cated for Model Protein i with the area, A|XvAnv,
approximated for Model Protein v gives the
relative efficiencies more accurately than was
done above for the data in Figure 5.15.
Improved accuracy here would require choos-
ing accurately the same interval for the degree
of ionization, a, for example, the interval of 0.1
to 0.9.
5.9.4.4 Relative Efficiencies by
Comparison of Hill Coefficients
The most efficient operational design would be
for the machine to operate over the range of
the acid-base titration curve with the steepest
An/A|i slope. Because the Hill coefficient, n,
as defined in Equation (5.20) is a measure of
the slope, it provides for ready comparison of
efficiencies. The Hill coefficients for Model
Proteins i through v are listed in Figure 5.34,
and the slopes are plotted in the inset. Accord-
ingly, the comparison of the efficiencies of
Model Proteins i and v simply becomes T|i/T|v =
1.5/8.0
=
0.19.
Thus,
by increasing the hydropho-
bicity by the replacement of five Val (V)
residues by five Phe (F) residues, as indicated
in Table 5.5, increases the efficiency of the
protein-based machine by just over fivefold.
5.9.5 Relative Chemomechanical
Transductional Efficiencies of the
Electrostatic Charge-Charge
Repulsion and Consilient Mechanisms
by Experimental Determination of
A|LI,
An, and fAL
The comparison of relative efficiencies above
considered only those polymers functioning by

208
5.
Consilient Mechanisms for Diverse Protein-based Machines
the consiUent mechanism. This involved the
inverse temperature transition for hydrophobic
association. Using the same functional group,
the carboxyl, and its interconversion between
-COO"
to -COOH, it is now possible to
compare directly the chemomechanical trans-
ductional efficiencies of poly(methacrylic acid),
[_CH2-(CH3)C(COOH)-]n, abbreviated as
PMA, to that of Model Protein iv in Table 5.5.
PMA utilizes the electrostatic charge-charge
repulsion mechanism, and Model Protein iv uti-
lizes the apolar-polar repulsive free energy of
hydration of the inverse temperature transition
developed in this chapter.
The same graphical insights utilized above
can be used to obtain insight into relative effi-
ciency. These can then be compared with the
direct experimental determination of the effi-
ciency of each polymeric system and of the rel-
ative efficiencies. The relative A|iAn areas can
be observed for the polymers as well as their
Hill coefficients. Finally, the acid-base titration
curves are given for the cross-linked elastic
matrices held at fixed force and the change in
length determined.
5.9.5.1 Relative Efficiencies by A^An
Areas and Hill Coefficients of Polymer
Acid-Base Titration Curves
5.9.5.1.1 Comparison of the AjiiAn Areas
The acid-base titration curve for PMA is given
in Figure 5.35A, where the change in degree of
ionization and change in pH are given as the
box or AjLiAn area over which the polymer func-
tions effectively in contraction/relaxation. In
Figure 5.35B occurs the acid-base titration for
Model Protein v and the A|LiAn box for its con-
traction/relaxation cycle. The AjiiAn box for
Model Protein v in Figure 5.35B is transposed
as a small shaded box onto the box for PMA in
Figure 5.35A, with a gross correction for the
difference in An. Even so, the difference in effi-
ciencies can be visually appreciated. This visual
comparison would more accurately carry over
to relative efficiencies if the change in length at
the same fixed force resulted in similar fAL
terms for the mechanical work performed.
5.9.5.1.2 Comparison of the Hill Coefficients
As shown in the inset of Figure 5.34, the Hill
coefficient for PMA is 0.5, whereas that of
Model Protein v is 2.7. Thus, without consider-
ation of differences in An, the relative efficiency
would be 0.5/8.0 = 0.06 or one-sixteenth as effi-
cient as the model protein. After the statement
of efficiency for chemomechanical transduction
immediately below, the relative efficiency of
the two mechanisms will be given using Model
Protein iv as the representative of the con-
silient mechanism. The ratio of Hill coefficients
for the latter model protein is 0.5/2.7 or differ-
ing only by a factor of 5 rather than 16. The
experimentally determined relative efficiency is
still very large.
5.9.5.2 Statement of Efficiency for
Chemomechanical Transduction
The proper expression for the efficiency of a
chemomechanical engine is
\\
=
fAL/A|LiAn (5.30)
where f is the fixed force or weight being lifted
and AL is the distance that the weight is raised.
The quantities A|x and An, as considered above,
are the change in chemical potential and the
change in number of moles of proton required
during the energy conversion. In what follows
a stress-strain/acid-base titration apparatus is
used to obtain the information required for
Equation (5.30).
5.9.5.3 Relative Chemomechanical
Efficiencies of Electrostatic Repulsion and
Consilient (Inverse Temperature
Transition) Mechanisms
The quantity that we wish to calculate is given
by Equation (5.31) where the subscript ap
stands for the apolar-polar repulsive free
energy of hydration mechanism and cc stands
for the charge-charge repulsion mechanism:
r\^Jr\cc=[fAL{ap)/A\iAniap)]/
[fAL{cc)/A\iAn{cc)] (5.31)
The experimental data for AL and AJLI are
obtained from the graph in Figure 5.36 for a con-
5.9 On the Efficiency
of
Consilient Protein-based Machines
A
209
1.0
^PMA
Z 0.50
o
Q 0.25
^
0.0
COOH
"
-C-CH2-
I
^
Henderson-Hasselbalch
eqn:
pH = pKa +
log[cx/(l-a)]
yy P0LYMETHACRYLIC ACID
I
4.0
pH=pKa+
r^
1-a
ZkTl^lp
n—
8.0
10.0
pH
B
1.0
Ani;
0.75
I
0.50
o
^
0.25
0.0
i:
(GEGVPGVGVPGVGVP
GVGVPGVGVPGVGVP)n
— Henderson-Hasselbalch
equation
•
v:
(GEGFPGVGVPGVGFP
GFGFPGVGVPGVGFP)n
FIGURE
5.35.
VisuaUzation of
the
relative efficiencies
of the electrostatic charge-charge repulsion and
the
apolar-polar repulsion mechanisms
by
comparison
of acid-base titration curves
for
poly(methacrylic
acid),
which exhibits charge-charge repulsion (neg-
ative cooperativity),
and for the
Model Proteins
i
and
V,
which exhibit apolar-posar repulsion (positive
cooperativity).
The
polymers
are
each compared
with the Henderson-Hasselbalch curve as reference.
Chemical energy
is
A[i*An,
where
Ap,
=
2.3RTApH for
the change
in
pH
to
go from one state
to
the other,
and An is the number
of
moles
to
go from
a
degree
stant force
of
2
grams.
For the
calculation
the
steepest slope is used
for
both systems. Because
PMA exhibits
a
lower slope over
a
considerable
range of the
AL
versus pH plot, this gives the best
working range for PMA and uses its most favor-
able efficiency
range.
As the slopes are taken for
the common AL
of
9 mm length changes, the
dif-
ferences
are
readily seen
in the
relative
ApH
required
to
achieve
the
common length change
with a
ApHpMA
of 2.24 and a
APHEMF
of 0.59. Also
the An values are 6.86
x
10"^
for
PMA and 6.12
x
10.0
of ionization,
a, of
zero
to
one.
Accordingly, the area
of an acid-base titration curve as indicated in
A
and
in
B,
corrected for the difference in number of moles
required
to go
from one state
to
another, gives
the
relative amounts
of
input energy used. These areas
are indicated separately in
A
and in B, and then with
the correction
for
An,
the
small shaded area
in the
larger area
of
part
A
demonstrates how much less
energy
is
required for the apolar-polar repulsive free
energy
of
hydration with its positive cooperativity
to
perform
a
similar amount
of
work.
10
^
for
Model Protein
iv,
also indicated
in the
subscript as
E/4F.
Accordingly,
Tlap/Tlcc=[(2.24)(6.86xl0-^)]/
[(0.59)(6.12xl0-^)]-40
(5.32)
The result
is
dramatic!
The
efficiency
of the
charge-charge repulsion mechanism
is
very
limited indeed.
For years
the
common discussion
of
mecha-
nism even
in
proteins has centered
on
the inter-

210
5.
Consilient Mechanisms for Diverse Protein-based Machines
6
5
4H
3
2H
H
2g constant force-
PMA
E4F
compact
conformation
unfolds
cp qoO\
3 4
T I I
9 10 11 12
FIGURE
5.36. Experimental plots of cross-hnked
matrices, each loaded with a 2 gram weight, that con-
tract on lowering pH to lift the weight through the
distances, AL, as indicated. These data provide a
direct experimental comparison with the relative
efficiencies for chemomechanical transduction of
Tjcc (charge-charge repulsion mechanism) using
poly(methacrylic acid) and of Tjap (apolar-polar
repulsion mechanism) using elastic Model Protein iv
of Table 5.5. The An values are 6.86 x 10"^ mole for
PMA and 6.12 x 10"^ mole for E/4F, Model Protein
iv. The calculation given in the lower part demon-
strates the apolar-polar repulsion mechanism of the
model protein to be about 40 times more efficient
than the charge-charge repulsion mechanism of
PMA, poly(methacrylic acid). See text for further
discussion. (Unpublished data of D.W. Urry, L.
Hayes, and J. Lee.)
action of charges, the attraction of opposite
charges and the repulsion of like charges. In
dominantly aqueous systems, however, salts dis-
solve to become ions because the free energy
of separated hydrated ions is more favorable
than the ion-pair associations in the crystal. The
phase separation process of inverse tempera-
ture transitions occurs in the absence of any
charge at all, but charge under the influence
of a hydrophobic domain regains its affinity
for association even in a dominantly aqueous
system and becomes a most potent means of
controlling phase separation.
It seems quite evident that the function of
biological macromolecules, and proteins in par-
ticular with their ease to evolve the most effi-
cient mechanism for accessing and utilizing its
energy sources biological systems as discussed
in Chapter 6, would utilize the inverse tem-
perature transition mechanism developed and
demonstrated in this chapter.
Clearly, it would seem not unreasonable to
propose the consilient mechanism as the domi-
nant mechanism in protein structure formation
and function. The comprehensive hydrophobic
effect should be the foundation from which to
engineer protein materials for medical and non-
medical uses.
References
1.
E.O.Wilson, Consilience, The Unity of Knowl-
edge. Alfred E.
Knopf,
New York, 1998, page 8,
gives a definition of the word consilience as pro-
viding a "common groundwork of explanation."
2.
Following E.O. Wilson, we speak of a consilient
mechanism as a "common groundwork of ex-
planation" for each of two interlinked physical
processes in the function of protein-based
machines of
biology.
The first physical process is
that of hydrophobic association, herein devel-
oped as the comprehensive hydrophobic effect
(CHE).
CHE derives its consiHence from being
the dominant mechanism capable of achieving
all of the diverse energy conversions for which
biology is renown and more general yet from
being relevant to all amphiphilic polymeric
systems, that
is,
for all polymers that contain both
oil-like (hydrophobic) and polar (e.g., charged)
constituents. The second physical process is that
of entropic elastic force development, which is
essential to efficient energy conversion, espe-
cially when motion is involved; this force devel-
opment is most evident as an increase in elastic
force under isometric conditions. The elastic
consilient mechanism derives its consilience
from being relevant to all polymeric systems, of
whatever composition, whenever deformation
results in a decrease in chain backbone mobility,
for example, the damping of internal chain
dynamics on extension. This second physical
process, which constitutes a consiHent mecha-
nism for ideal or entropic elastic, will be delin-
eated as the elastic consilient mechanism.
3.
E.O. Wilson, Consilience: The Unity of Knowl-
edge. Alfred E.
Knopf,
New York, 1998, pp. 4-5.
4.
D.W. Urry, "Physical Chemistry of Biological
Free Energy Transduction as Demonstrated by
Elastic Protein-based Polymers." / Phys. Chem.
B,
101,11007-11028,1997.
5. ''Concepts Introduced During Development
of Elastic Protein-based Polymers for Free

References 211
Energy Transduction: The conclusions of this
article can also be given in terms of the follow-
ing chronological listing of the concepts
introduced during the development of elastic
protein-based polymers for free energy trans-
duction: (1) the concept of the damping of inter-
nal chain dynamics on extension as the source of
entropic elastomeric force, called the librational
entropy mechanism of elasticity, (2) the concept
of Tt, the temperature of the hydrophobic
folding and assembly transition, being used as
the fundamental measure of hydrophobicity and
providing a practical on-off switching capacity;
(3) the generally obvious concept that raising the
temperature from below to above Tt is a means
of performing mechanical work by cross-linked
elastic protein-based polymers; (4) the concept
of the ATt-mechanism wherein the value of Tt is
changed, rather than the temperature, as a
means of achieving free energy transduction; (5)
the concept of energy conversion by means of
the coupling of different functional moieties by
being part of the same hydrophobic folding and
assembly domain arising out of, for example, (a)
hydrophobic-induced pKa shifts, (b) hydropho-
bic-induced shift in redox potential, and (c)
demonstrated coupling of carboxyl and redox
functions to result in electrochemical trans-
duction; (6) the concept of the competition for
hydration between apolar (hydrophobic) and
polar (e.g., charged) moieties to give rise to pKa
shifts and positive cooperativity; (7) the concept
of 'poising' for achieving higher efficiencies;
(8) essential equivalence of the inverse tem-
perature transition of a phase separation and
the intramolecular phase separation of the
hydrophobic folding of a globular protein or
assembly of the protomer subunits to form a
multisubunit globular protein such as phospho-
fructose kinase, that
is,
the extension to globular
proteins; and (9) extension to all polymers
where, however, the degree of expression of the
above effects is limited due to the lack of the
many advantages of protein-based polymers of
Table I." (From Urry.4)
6. D.W. Urry, "Molecular Machines: How Motion
and Other Functions of Living Organisms Can
Result from Reversible Chemical Changes."
Angew. Chem. [German], 105, 859-883, 1993;
Angew. Chem. Int. Ed. Engl, 32,819-841,1993.
7.
D.W. Urry, "Elastic Biomolecular Machines:
Synthetic Chains of Amino Acids, Patterned
After Those in Connective Tissue, can Trans-
form Heat and Chemical Energy into Motion."
Sci. Am. January 1995, 64-69.
8. D.W. Urry, "The Change in Gibbs Free Energy
for Hydrophobic Association: Derivation and
Evaluation by means of Inverse Temperature
Transitions." Chem. Phys. Letters, 399,177-183,
2004.
9. D.W. Urry and T.M. Parker, "Mechanics of
Elastin: Molecular Mechanism of Biological
Elasticity and its Relevance to Contraction." /.
Muscle Res. Cell Motil., 23, issue 5-6 (2002);
Special Issue: Mechanics of Elastic Biomole-
cules, H. Granzier, M. Kellermayer, W Linke,
Eds.
10.
Recall that oil-like groups contain hydrocar-
bon; the classic example is the CH2 unit, the
most common chemical grouping of oil. The
principal ingredient of vinegar is acetic acid,
which when negatively charged is written as
CH3-COO", but our use of the term vinegar-
like also includes positively charged species like
amine functions, for example, -NHs^, and it can
even include very polar groups like oxygen and
the peptide group
itself.
Of particular interest
in protein function are the vinegar-like groups
that can exist in either of two or more states,
such as charged or uncharged.
11.
The vinegar-Uke side chains can exist in either
of two different states, for example, the car-
boxyl/carboxylate, COOH/COO", chemical
couple and the amino/ammonium, -NH2/NH3^,
chemical couple. As demonstrated below, the
uncharged state of the couple favors associa-
tion of oil-like domains, whereas the charged
state of the couple disrupts association of oil-
like domains by having destroyed the special
hydration of oil-like groups in the process of
achieving its own hydration.
12.
The word consilient is used as the adjective
form of the noun consilience. As listed in
Webster's Third New International Dictionary,
consilience contains the sense that there exist
pervasive, fundamental laws of nature underly-
ing related disciplines that provide a common
groundwork of understanding. Here we refer to
the consiUent mechanism related to hydropho-
bic association as a water-dependent, pervasive,
and fundamental process by which the macro-
molecules of living organisms function in pro-
viding the essential diverse energy conversions
of Life. In general, if we speak of the consilient
mechanism without further delineation, it will
be the consilient mechanism of hydrophobic
association. When referring to elastic, reference
will be to the consilient mechanism for entropic
(ideal) elasticity or simply to the elastic con-
silient mechanism.

212
5.
Consilient Mechanisms for Diverse Protein-based Machines
13.
(GVGVP)n uses the single letter amino acid
residue abbreviation for the repeating pen-
tapeptide sequence (glycyl-valyl-glycyl-valyl-
prolyl)n. Also, the three letter abbreviation
would be (Gly-Val-Gly-Val-Pro)n. The peptide
residue is given in general as -NH-CHR-CO-
and the R-group side chain is -H for G,
-CH-(CH3)2 for V, and -CH2-CH2-CH2- for P,
with the three CH2 moieties bridging from the
N (replacing the H) to the central atom of the
backbone of the peptide residue, C. Thus the V
and P residues contain the oil-like groupings
of atoms, which are in fact the hydrocarbon
moieties of oil. The interconnecting peptide
group
itself,
-CO-NH-, formed between the
defined residues represents a vinegar-hke
grouping, even though it is without a net charge.
It is considered polar, as there are substantial
partial charges on the atoms, but the sum of the
partial charges of the four atoms in the peptide
moiety is essentially zero. These structural
considerations were introduced in Chapter 2
and are treated in more detail below in section
5.2.
14.
For a comparison per gram with the transitions
of melting and vaporization of water, these
numbers per pentamer would need to be
divided by 23, which is (409 grams/mole-
pentamer divided by
18
grams/mole-water).
15.
F.
Franks, "Protein Destabilization at Low Tem-
peratures."
^^v.
Protein Chem.,46,107-139,1995.
16.
PL. Privalov, "Cold Inactivation of Enzymes."
Crit.
Rev. Biochem. Mol BioL,25,281-305,1990.
17.
The term inverse transition was first used in con-
nection with the increase in order of the antibi-
otic stendomycin on raising the temperature
(D.W. Urry and A. Ruiter, "Conformation of
Polypeptide Antibiotics. VL Circular Dichroism
of Stendomycin." Biochem. Biophys. Res.
Commun., 38,800-806,1970). The term became
specifically inverse temperature transition in
relation to coacervation of elastin fragments
that exhibited a phase separation with
increased order on raising the temperature
(B.C.
Starcher, G. Saccomani, and D.W. Urry,
"Coacervation and Ion-Binding Studies on
Aortic Elastin." Biochim. Biophys. Acta, 310,
481^86,1973,
and D.W. Urry, B. Starcher, and
S.M. Partridge, "Coacervation of Solubilized
Elastin Effects a Notable Conformational
Change." Nature, 222, 795-796,1969).
18.
P.J. Flory, Principles of Polymer Chemistry.
Cornell University Press, Ithaca, New York,
1953,
Figure 121.
19.
M. Manno, A. Emanuele,
V.
Martorana, PL. San
Biagio, D. Bulone, M.B. Palma-Vitorelh, D.T.
McPherson, J. Xu, T.M. Parker, and D.W. Urry,
"Interaction of Processes on Different/time
scales in a bioelastomer capable of performing
energy conversion." Biopolymers,
59,51-64,2001.
20.
F. Sciortino, K.U. Prasad, D.W. Urry, and
M.U. Palma, "Self-Assembly of Bioelastomeric
Structures From Solutions: Mean Field Critical
Behavior and Flory-Huggins Free-Energy of
Interaction." Biopolymers, 33,743-52,1993.
21.
B.A. Cox,B.C. Starcher, and
D.W.
Urry,"Coacer-
vation of a-Elastin Results in Fiber Formation."
Biochim. Biophys. Acta., 317,209-213,1973.
22.
B.A. Cox, B.C. Starcher, and D.W. Urry, "Coac-
ervation of Tropoelastin Results in Fiber For-
mation." /. Biol. Chem., 249, 997-998,1974.
23.
D.W. Urry, M.M. Long, and H. Sugano, "Cychc
Analog of Elastin Polyhexapeptide Exhibits an
Inverse Temperature Transition Leading to
Crystallization."/. Biol. Chem.,253, 6301-6302,
1978.
24.
W.J. Cook, H.M. Einspahr, T.L. Trapane, D.W.
Urry, and C.E. Bugg, "Crystal Structure and
Conformation of the CycHc Trimer of a Repeat
Pentapeptide of Elastin, Cyclo-(L-Valyl-L-
prolylglycyl-L-valylglycyl)3."/.Am. Chem. Soc,
102,
5502-5505,1980.
25.
J.A.V. Butler, "The energy and entropy of
hydration of organic compounds." Transaction
Faraday Society, 33, 229-238,1937.
26.
H.S. Frank and M.E. Evans, "Free Volume and
Entropy in Condensed Systems: III. Entropy in
Binary Liquid Mixtures; Partial Molal Entropy
in Dilute Solutions; Structure and Thermody-
namics in Aqueous Electrolytes." /. Chem.
Phys., 13, 507-532,1945.
27.
A convenient estimate of the value of Tt is
shown in Figure 5.IB, where an increase in tur-
bidity of the solution is plotted with an increase
in temperature and the 50% point for maximal
turbidity measures
Tj.
As shown in Figure 5.1C,
this measure of Tt occurs near the onset of the
transition as measured by differential scanning
calorimetry (DSC), where it is referred to as
Tb and the shaded area of the curve gives
the exothermic heat of the transition, AHt, for
(GVGVP)25i. It should be further noted that a
more accurate, but not yet precise measure of
ASt uses a mean temperature for the transition,
which we may denote as T^. The accurate
measure of ASt sums over the DSC curve in
small increments of heat released divided by
the mean temperature for the increment. For

References 213
convenience, we refer to Tt, but the differences
should be kept in mind when carrying out cal-
culations as in 5.1.3.4.
28.
D.W. Urry, T.L. Trapane, and K.U. Prasad,
"Phase-Structure Transitions of the Elastin
Polypentapeptide-Water System Within the
Framework of Composition-Temperature
Studies." Biopolymers, 24, 2345-2356,1985.
29.
D.W. Urry, D.T McPherson, J. Xu, H. Daniell,
C. Guda, D.C. Gowda, N. Jing, and T.M. Parker,
"Protein-Based Polymeric Materials: Syntheses
and Properties." In The Polymeric Materials
Encyclopedia: Synthesis, Properties and Appli-
cations, CRC Press, Boca Raton, pp. 7263-7279,
1996.
See Figure 6.
30.
As dialysis membranes are calibrated against
globular proteins and as below Tj these protein-
based polymers are unfolded and disassembled,
it was expected that the polymer that could pass
through the membrane by a process of reptition
(reptilian motion) would be much larger than
50,000 Da. After preparing the polymers by
recombinant DNA technology, where the chain
length was precisely known, for example, just
over 100,000Da for (GVGVP)25i, it was appar-
ent that the chemically synthesized protein-
based polymers retained by the 50,000 cut-off
membranes were over 100,000 Da.
31.
F Sciortino, M.U. Palma, D.W. Urry, and K.U.
Prasad, "Nucleation and Accretion of Bioelas-
tomeric Fibers at Biological Temperatures and
Low Concentrations," Biochem. Biophys. Res.
Commun. 157,1061-1066,1988.
32.
Several points require consideration on identi-
fication of AHt(CH2) - Tt(GVGIP)ASt(CH2) as
-AGHA(CH2).
The points include the separabil-
ity assumption of Equations (5.3) and (5.4), the
relevance of the model protein to such identifi-
cation, and the choice of reference state in
order that the nonlinearity of hydrophobic-
induced pKa shifts be included. From the data
of Butler,^^ the separability is reasonable for a
simple CH2 group, but examination of the cal-
culated result is required to be satisfied
whether or not extension to more complex sub-
stituents is warranted. As the inverse tempera-
ture transition of (GVGVP)n has been
experimentally shown to involve no Raman
detectable changes in secondary structure,^^ the
elastic-contractile model proteins of focus here
reasonably represent the best known model
available for such an effort. It should be noted,
however, that NMR studies on the temperature
and solvent dependence of peptide NH and
^^CO chemical shifts suggest minor changes in
the exposure of peptide NH and CO groups to
solvent during the transition'^^'^^ To include the
nonlinear effects, such as the hydrophobic-
induced pKa shifts, where pKa = AG/2.3RT, the
choice of reference state becomes important.
For situations where these nonlinear effects are
significant, ASt(GXGVP) is the preferred refer-
ence state. This requires extrapolation of
ASt(GXGVP) given for fx = 0.2 in Table 5.1 to
fx = 1, as was done for Tj. Also this requires
DSC data for the modified composition that is
not always easy to obtain. When DSC data is
not available for the perturbed state. Equation
(5.8) can yet be used with ASt(GVGVP) =
ASt(ref), for certain comparison such as ratios
of interest. The complete derivation of Equa-
tion (5.10b) and its evaluation by means of
dif-
ferential scanning calorimetry (DSC) data is
given in reference 8, which should be sought for
a more in depth treatment.
33.
Bioluminescence presents an interesting case
where a chemiluminescence occurs in which
the build up of the chemical required many
stepwise energy inputs. Also there could possibly
be a stretch-induced component, for example, as
seen at night in the wake of ships at sea.
34.
If this were not the case we would be faced with
the puzzhng question. Since the value of T, is
higher when there are fewer CH2 units in the
polymer, and therefore less hydrophobic
hydration, why should a higher temperature be
required to melt the lesser amount of more
poorly structured hydrophobic hydration? The
thermally-induced inverse temperature transi-
tion does not result from a thermal destructuring
of hydrophobic hydration, as we, with others,
have indicated in the past (See for example the
upper right illustration in Figure 2.8).^
35.
D.W.
Urry, S-Q. Peng,
J.
Xu, and
D.T.
McPherson,
"Characterization of Waters of Hydrophobic
Hydration by Microwave Dielectric Relax-
ation."/.y4m6r. Chem. 5'oc.,119,1161-1162,1997.
36.
20 Mrad means a radiation-absorbed dose of 20
million Roentgens.
37.
To be an ideal elastic band means that the
energy expended during stretching of the band
is entirely recovered on relaxation of the
deforming force.
38.
This aspect of the consilient mechanism has
been called the ATt-mechanism, because the
energy conversion occurs not by a change in
temperature but rather by a change in the tran-
sition temperature.

214
5.
Consilient Mechanisms for Diverse Protein-based Machines
39.
Here we note more general terms: polar for
vinegar-like or for charged or ionized and
apolar for oil-like or hydrophobic, but recog-
nize that there exist a continuum of groups
from polar to apolar.
40.
D.W. Urry, D.C. Gowda, S.-Q. Peng, and T.M.
Parker, "Non-linear Hydrophobic-induced pKa
Shifts: Implications for Efficiency of Conver-
sion to Chemical Energy." Chem. Phys. Lett,
239,
67-74,1995.
41.
D.W. Urry, S.Q. Peng, L.C. Hayes, D.T.
McPherson, Jie Xu, T.C. Woods, D.C. Gowda,
and A. Pattanaik, "Engineering Protein-based
Machines to Emulate Key Steps of Metabolism
(Biological Energy Conversion)." Biotechnol
5/o^«g., 58,175-190,1998.
42.
D.W. Urry, L. Hayes, C.X. Luan, D.C. Gowda,
D.
McPherson, J. Xu, and T. Parker, "ATt-
Mechanism in the Design of Self-Assembling
Structures," In Self-assembling Peptide Systems
in Biology, Medicine and Engineering. A.
Aggeli, N. Boden, S. Zhang, Eds., Kluwer Aca-
demic Publishers, Dordrecht, The Netherlands,
2001,
pp. 323-340.
43.
Energy is the product of two quantities, the
change in the intensive variable times the change
in the corresponding extensive variable. For
mechanical energy, the intensive variable is the
applied force, f, and the extensive variable is
the distance over which the force is applied, AL.
The amount of mechanical energy involved in a
process becomes the product of the force to
produce the change in motion times the change
in length or position, that is, fAL. Chemical
energy is the product of the extensive variable,
the number of a molecular species,
n,
such as that
of a particular ion utilized in the process and the
intensive variable, called chemical potential (|x =
RT Ina where a is the activity, which becomes
concentration at low concentrations that limit
effects of ion-ion interactions).
44.
D.W. Urry and M.M. Long, "Conformations of
the Repeat Peptides of Elastin in Solution: An
AppHcation of Proton and Carbon-13 Magnetic
Resonance to the Determination of Polypep-
tide Secondary Structure." CRC Crit. Rev. Bio-
chemistry, 4,1-45,1976.
45.
D.W. Urry, "Characterization of Soluble
Peptides of Elastin by Physical Techniques." In
Methods in Enzymology, 82, 673-716, 1982,
(L.W Cunningham and D.W. Frederiksen, Eds.)
Academic Press, Inc., New York, New York.
46.
D.W. Urry, CM. Venkatachalam, M.M. Long,
and K.U. Prasad, "Dynamic p-Spirals and A
Librational Entropy Mechanism of Elasticity."
In Conformation in Biol. (R. Srinivasan and R.H.
Sarma, Eds.) G.N. Ramachandran Festschrift
Volume, Adenine Press, USA, 11-27,1982.
47.
D.W. Urry, "Thermally Driven Self-assembly,
Molecular Structuring and Entropic Mecha-
nisms in Elastomeric Polypeptides." In Mol.
Conformation and Biol. Interactions (P.
Balaram and
S.
Ramaseshan, Eds.) Indian Acad,
of Sci., Bangalore, India, pp. 555-583,1991.
48.
D.W Urry, T. Hugel, M. Seitz, H. Gaub, L.
Sheiba, J. Dea, J. Xu, and T Parker, "Elastin: A
Representative Ideal Protein Elastomer." Phil.
Trans. R. Soc.
Lond.,
B 357,169-184, 2002.
49.
D.W. Urry, T. Hugel, M. Seitz, H. Gaub, L.
Sheiba,
J.
Dea,
J.
Xu, L. Hayes,
F.
Prochazka, and
T Parker, In Ideal Protein Elasticity: The Elastin
Model, P. Shewry and A. Bailey, Eds., Cam-
bridge University Press, (in press)
2003.
50.
L.B. Sandberg, J.G Leslie, C.T. Leach, V.L.
Torres, A.R. Smith, and D.W. Smith, "Elastin
Covalent Structure as Determined by Solid
State Amino Acid Sequencing." Pathol. Biol.,
33,
266-274,1985.
51.
H. Yeh, N. Ornstein-Goldstein, Z. Indik, P
Sheppard, N. Anderson, J.C. Rosenbloom, G.
Cicila, K. Yoon, and J. Rosenbloom, "Sequence
Variation of Bovine Elastin mRNA due to
Alternative Splicing." /. Collagen Rel. Res., 1,
235-247,1987.
52.
When the number of repeats is large,
poly(GVGVP) is equivalent to poly(VPGVG),
because the polymers differ only by the partic-
ular 2 or 3 residues that begin or terminate the
polymer. For the remainder of the 1,000 or so
residues, the polymers are identical.
53.
G.J. Thomas, Jr., B. Prescott, and D.W Urry,
"Raman Amide Bands of Type-II P-Turns
in Cyclo-(VPGVG)3 and Poly(VPGVG), and
Implications for Protein Secondary Structure
Analysis." Biopolymers, 26, 921-934,1987.
54.
D. Volpin, D.W. Urry, I. Pasquali-Ronchetti,
and L. Gotte, "Studies by Electron Microscopy
on the Structure of Coacervates of Synthetic
Polypeptides of Tropoelastin." Micron, 1, 193-
198,1976.
55.
D.W Urry, CM. Venkatachalam, M.M. Long,
and K.U. Prasad, "Dynamic P-Spirals and a
Librational Entropy Mechanism of Elasticity."
In Conformation in Biology, R. Srinivasan and
R.H. Sarma,Eds., G.N. Ramachandran Festschrift
Volume, Adenine Press, USA, 11-27,1982.
56.
D.K. Chang and D.W. Urry, "Polypentapeptide
of Elastin: Damping of Internal Chain Dynam-
ics on Extension." /. Computational Chem., 10,
850-855,1989.
