Shani G. Radiation Dosimetry: Instrumentation and Methods
Подождите немного. Документ загружается.

Ch-05.fm Page 330 Friday, November 10, 2000 12:01 PM

331
6
Calorimetry
CONTENTS
I. Introduction ............................................................................................................................................................331
II. Water Calorimeter ..................................................................................................................................................331
III. Graphite Calorimeter..............................................................................................................................................340
IV. Other Material Calorimeters ..................................................................................................................................344
References .......................................................................................................................................................................348
I. INTRODUCTION
Calorimetry has been used for a long time as a technique
for establishing the absorbed dose. Graphite calorimetry
has been used to establish absorbed dose standards for
use in radiation therapy. A conversion process is necessary
to convert from dose to graphite to dose to water. In other
radiation measurement areas, too, calorimetry is recog-
nized as a good approach for establishing absorbed dose
standards. The basic assumption is that all (or a known
fraction) of the absorbed radiation energy appears as heat,
so that the measurement of absorbed dose reduces to a
measurement of a temperature change. Most calorimeters
developed for the purposes of radiation dosimetry have
been constructed from graphite because of the perceived
difficulties of working with a liquid system. Graphite has
been chosen because its radiation absorption characteris-
tics are similar to those of water, and thermally isolated
segments can be machined and configured so as to permit
the measurement of absorbed dose to graphite. Given the
dose to graphite, the dose to water is obtained using a
conversion process. The conversion from dose to graphite
to dose to water introduces additional uncertainty.
The main technical difficulty is the problem of con-
structing a thermally isolated segment in which to measure
the temperature change. Any wall which might be used to
hold a small mass of water (of the order of 1 g) would
significantly perturb the measurement. A calorimetric
determination of the dose at a point requires that a ther-
mally isolated element has been arranged so that no sig-
nificant heat transfer occurs during the irradiation. If
T
is the measured temperature rise in this element, then the
absorbed dose to the material,
D
m
, is given by
(6.1)
where
c
m
is the specific heat of the calorimetric material
and
k
HD
is the heat defect. The heat defect, which can be
positive or negative, is given by
k
HD
(
E
a
E
h
)/
E
a
(6.2)
where
E
a
is the energy absorbed by the irradiated material
and
E
h
is the energy which appears as heat.
The approach to equilibrium temperature in time and
space is governed by the heat equation. The effect of con-
ductive heat transfer on the measured temperature change
can be estimated using the equation
(6.3)
where
T
is the temperature and
t
is the time. The thermal
diffusivity,
, is equal to
/
c
where
is the thermal
conductivity,
is the density, and
c
is the specific heat.
Beyond the maximum of the depth-dose curve, the varia-
tion of the dose with depth,
D
(
z
), can be represented
approximately by
(6.4)
where
D
0
is the dose at depth Z
0
and
for
60
Co
-rays is
approximately 0.05 cm
1
.
II. WATER CALORIMETER
When a large water calorimeter is irradiated by a beam
directed vertically downward, apart from the build-up
region, the temperature will in fact decrease with increasing
depth. Thus, at depths well beyond the peak of the depth-
dose distribution, the liquid will be stable with respect to
convection. Domen [1] constructed a water calorimeter in
which no effort would be made to thermally isolate the
volume element in which the dose was to be measured.
The essential features of Domen’s calorimeter are shown
D
m
c
m
T 1 k
HD
()
T t
2
T
Dz() D
0
e
z z
o
()
CH-06.fm Page 331 Friday, November 10, 2000 12:02 PM
332
Radiation Dosimetry: Instrumentation and Methods
in Figure 6.1. The calorimeter consisted of a 30-cm cube
of once-distilled water. During irradiation, the water was
assumed to be motionless. Although the water was ther-
mally isolated from the environment, it was not sealed
against the exchange of gases with the atmosphere. The
water temperature at a depth of 5 cm was measured using
two thermistors sandwiched between thin polyethylene
films. The calorimeter was irradiated with a
60
Co
-ray
beam directed vertically downward.
For low-linear-energy-transfer (LET) radiation, about
70% of the energy is deposited in spurs. In water, the
primary spur products are highly reactive, but some may
escape from the region of the spur before reacting. Others
may undergo reactions within the spur to give rise to stable
products which diffuse throughout the liquid. For a given
LET, each species which escapes from the spur can be
assigned a yield,
G
, which is independent of the compo-
sition of the dilute aqueous solution.
For water, the species which escape from the spurs have
been identified and their
G
-values have been measured for
a wide range of LET. Figure 6.2 shows how the
G
-values
of the eight spur products change with LET. As the LET
increases, adjacent spurs begin to overlap, so that reactions
within the spurs become more important. The result is that
fewer of the highly reactive species, such as and OH,
escape from the region of the spur before reacting.
Fleming and Glass [3] constructed an absorbing ele-
ment which consisted of discs of aluminum and plastic,
each thick enough to fully stop the proton beam. The discs
were in good thermal contact with each other and ther-
mally isolated from the environment, so that the temper-
ature of the assembly could be measured. If the same total
energy was delivered by the proton beam to either the
aluminum or the plastic, any difference in the temperature
rise must be due to a difference in the heat defect of the
two materials.
Selbach et al. [4] measured the heat defect of water
but used low-energy (17–30 kV) x-rays. Figure 6.3 shows
the experimental arrangement used by Selbach et al. [4] The
water-filled absorber was 30 mm in diameter and 15 mm
long. A mu-metal disc 1 mm thick was immersed in the
water and its position along the axis of the radiation field
could be varied by using magnets. The disc was thick enough
to be almost totally absorbing, and measurements were made
with the disc either completely forward or completely back.
The reference material used by Selbach et al. was mu-metal,
an alloy consisting of 75% Ni, 18% Fe, 5% Cu, and 2% Cr
by weight, and they assumed that its heat defect was zero.
Assuming no heat-loss corrections and total absorption of
the x-ray beam, Equation (6.2) can be used to show that the
heat defect of water is [2]
(6.5)
where
T
w
and
T
m
are the measured temperature
changes of the composite absorber.
Ross et al. [5] have described a technique for measur-
ing the heat defect of water which is based on the total
absorption of low-energy (1–5 MeV) electrons. They com-
pared the temperature rise induced in water by the electron
beam with the temperature rise caused by a known amount
of electrical energy. Any difference (after making any nec-
essary corrections) must be due to the heat defect of the
water used.
The essential features of their apparatus are as fol-
lows. The electron-beam energy was determined with an
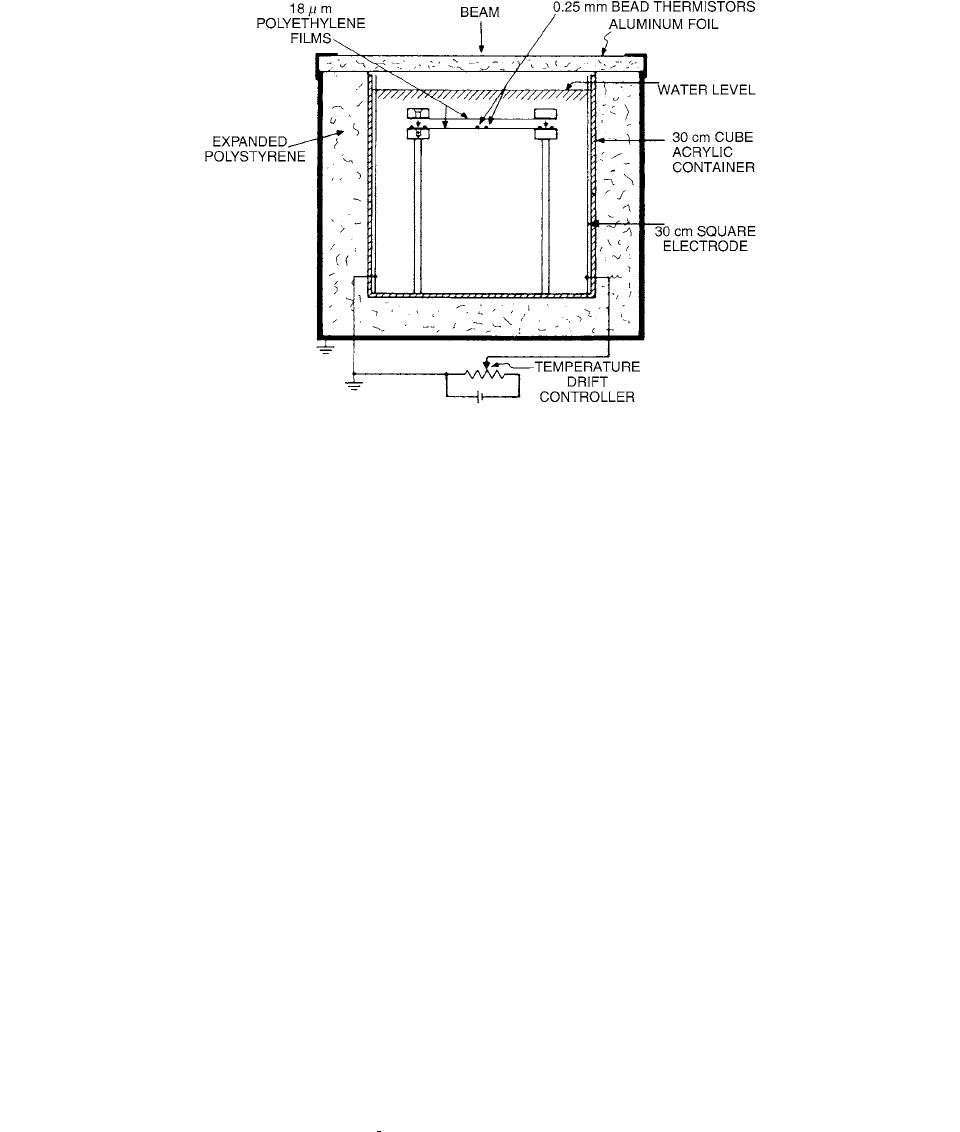
FIGURE 6.1
Schematics of Domen’s absorbed dose to water calorimeter. The large mass of motionless water was thermally isolated
from the environment. The thermistors were fixed in position and protected from the water by polyethylene film. The large electrodes
at opposite sides of the container were used to control temperature drifts. (From Reference [2]. With permission.)
e
aq
k
HD
w
1 T
w
/T
m
()
CH-06.fm Page 332 Friday, November 10, 2000 12:02 PM
Calorimetry
333
uncertainty of 0.2% using a magnetic spectrometer. The
charge delivered to the absorber was measured with an
uncertainty of 0.3% using a toroidal monitor. The beam
entered the water through a Mylar window 23
m thick.
The water was stirred during both irradiation and electrical
heating in order to minimize the effects of temperature gra-
dients. The temperature rise was measured for both modes
of heating and the temperature rise per unit input energy
calculated. The heat defect was then obtained from
(6.6)
where
T
r
and
T
e
are the temperature changes per unit
energy for radiation and electrical heating, respectively.
A calorimeter in which water quality was carefully
controlled was developed by Ross et al. [5]; it is shown
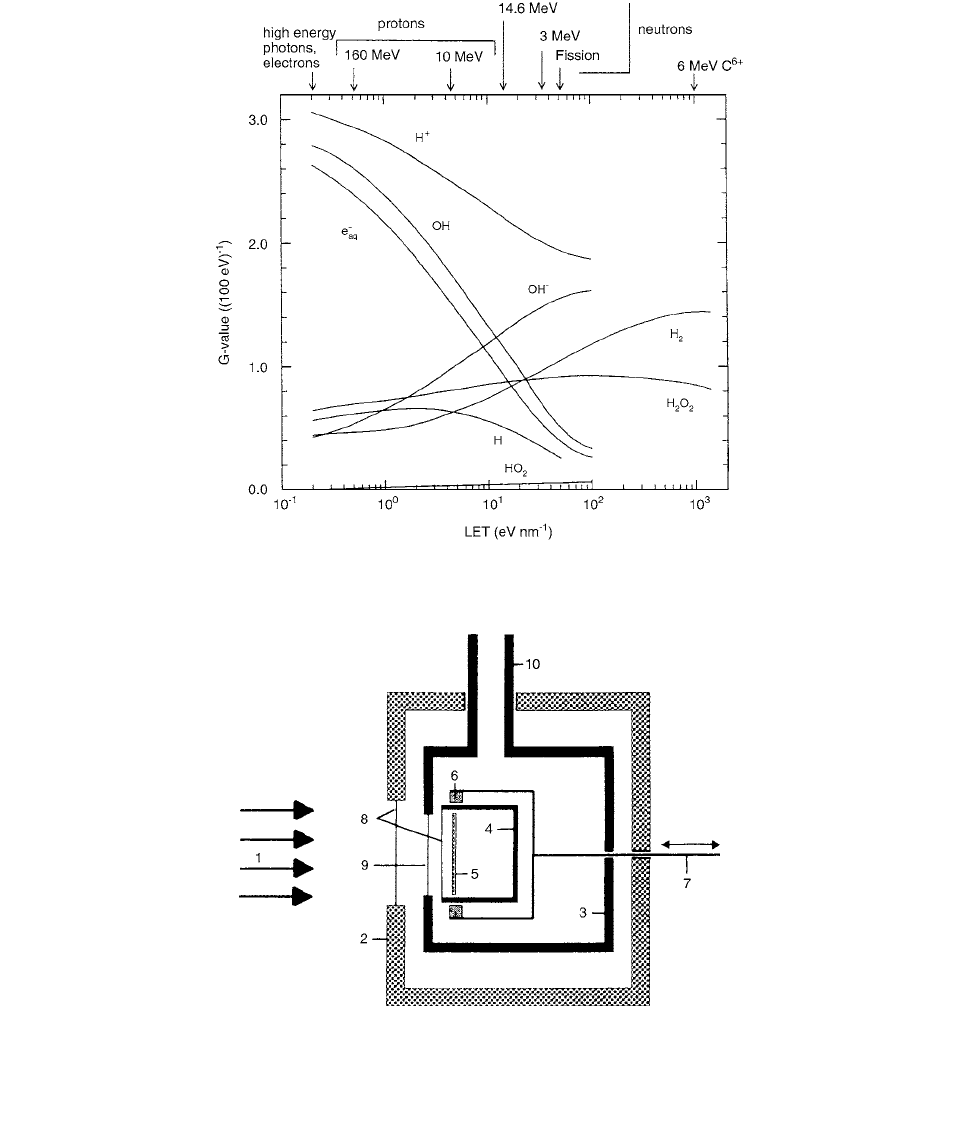
FIGURE 6.2
G
-values of various spur products as a function of the track-averaged LET. The smooth curves summarize the trends
in the measured data, except for H
and OH
. (From Reference [2]. With permission.)
FIGURE 6.3
Section through the measuring system of Selbach et al. [4] The principal components are identified by numbers as
follows: 1, x-ray beam; 2, calorimeter casing; 3, vacuum container; 4, water-filled absorber; 30 mm in diameter and 15 mm long;
5, mu-metal disc which can be moved through the water using magnets; 6, permanent magnets; 7; assembly for changing the location
of the mu-metal disc; 8, plastic foils; 9, beryllium window; 10, vacuum container. (From Reference [2]. With permission.)
k
HD
w
1 T
r
T
e
()
CH-06.fm Page 333 Friday, November 10, 2000 12:02 PM
334
Radiation Dosimetry: Instrumentation and Methods
in Figure 6.4. The water was purified by passing it through
a particulate filter, an ion-exchange column, a 5 nm filter, and
an ozonizer. Finally, the water was doubly distilled and stored
in quartz containers. Approximately 100 ml of water was
held in a thin-walled glass vessel which was thermally iso-
lated from the environment. The water was stirred, and the
temperature rise was measured using thermistors. Facilities
were provided for bubbling the water with various gases and
for sealing the vessel from the atmosphere. If the measured
temperature change was
T
, then
(6.7)
The factor
k
FM
accounts for the effect of the glass in
contact with the water.
Domen [7] has modified his original calorimeter design
so that water quality is carefully controlled. Figure 6.5
shows the revised calorimeter in which the water in the
vicinity of the measuring thermistors is enclosed in a clean
glass vessel. The water is prepared using a filter, deionizer,
and organic absorber. The rest of the water in the calo-
rimeter does not need to be of high quality, since any heat
defect in it will not affect the temperature measurement
in the vicinity of the thermistors. The diameter of the glass
vessel is about 33 mm, and its length is such that it holds
90 cm
3
of water. The thickness of the glass wall is about
0.3 mm. The diameter of the vessel is a compromise based
on two considerations. If the diameter were too small,
excess heat generated in the glass by the radiation field
would affect the measured temperature rise. On the other
D
w
c
m
T
1 k
HD
w
()[]k
FM
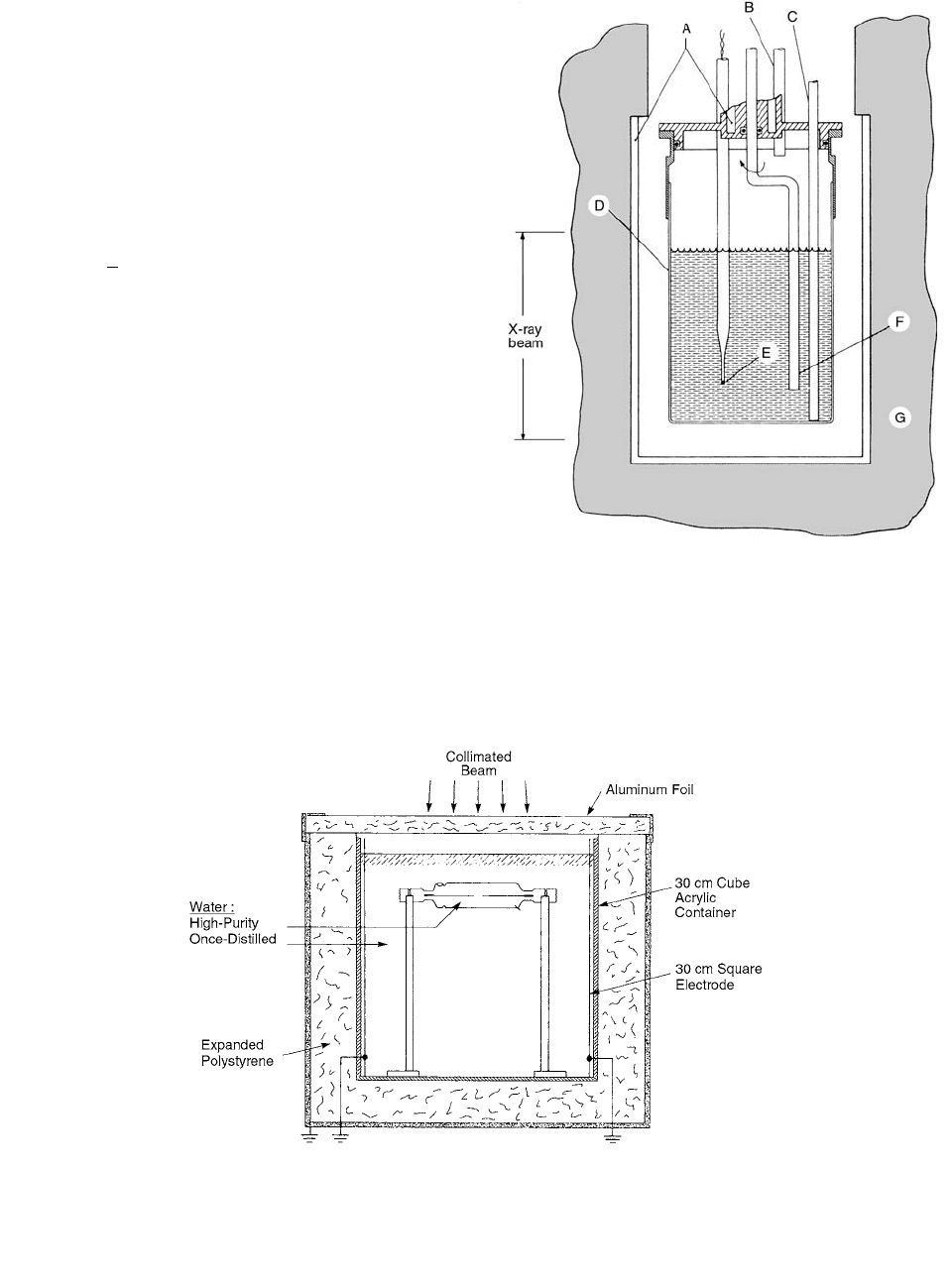
FIGURE 6.4
Cross-section view of Ross’s calorimeter. The
water volume is 5 cm in diameter and 5 cm high. A: channel for
temperature-regulated water; B: outlet tube for gas flow; C: glass
filling and bubbling tube; D: thin-walled (0.040 g cm
2
) Pyrex
calorimeter vessel; E: one of two thermistors; F: glass stirring
paddle; G: styrofoam insulator. (From Reference [2]. With per-
mission.)
FIGURE 6.5
Main features of Domen’s sealed water calorimeter. The main body of the calorimeter consists of a large mass of
motionless water thermally isolated from the environment. The measuring thermistors are located in a sealed glass vessel in which
the water quality is carefully controlled. With this design, the heat defect of the water at the point of measurement is known, and
the vessel also acts as a convective barrier, potentially permitting measurements in horizontally directed radiation fields. (From
Reference [2]. With permission.)
CH-06.fm Page 334 Friday, November 10, 2000 12:02 PM
Calorimetry
335
hand, if the diameter were too large, the vessel would not
serve as a convective barrier. For the diameter chosen,
Domen showed that the effect of excess heat should be less
than 0.1% 60 s after a 60-s irradiation. Furthermore, he
estimated that the glass should act as an effective convective
barrier, permitting measurements to be made in a horizon-
tally directed electron beam.
The determination of the dose to water using a water
calorimeter is absolute in the sense that it does not require
the application of radiation-dependent parameters such as
stopping power ratios, replacement corrections, ion collec-
tion efficiency,
60
Co exposure calibration factors, etc. Indeed,
the determination requires only the specific heat of water,
and the calibration of the calorimeter depends upon an accu-
rate thermometer. If the thermal defect of water is zero, i.e.,
all absorbed energy is converted to heat, then the temperature
change of the water multiplied by the specific heat is equal
to the absorbed dose. (The thermal defect is (
T
e
T
0
)/
T
e
,
where
T
e
is the expected temperature rise and
T
0
is the
observed temperature rise of an irradiated material.) [8]
Figure 6.6 shows a cross section of the calorimeter
with x-rays incident upon it from below.
The core was filled with high-purity water that has
dissolved oxygen removed by bubbling ultra-high-purity
nitrogen through it for several hours. To eliminate the
possibility of convection currents, the core is maintained
at 4.0°C by circulating refrigerated water through the
jacket that surrounds it.
Calibrations are done with the thermistors in the glass
capillary tubes of the core, and the core is filled and
submerged in the reservoir of a refrigerated circulator.
To minimize the effects of ambient temperature varia-
tions, this apparatus is placed in a 4°C cold room for the
period of one week that it takes to complete a calibration.
The resistance of the thermistors is determined using the
same Wheatstone bridge. Water temperatures in the 2–10°C
range and thermistor power levels in the range 5–200
microwatts are routinely used in the calibration procedure.
In order to investigate experimentally the overall cor-
rection factor for a cylindrical ionization chamber, water
calorimetry was used by Seuntjens et al. [9] An important
limitation of water calorimeters open to impurities is the
heat defect arising from chemical reactions induced by the
radiolysis of water. Using different types of closed-vessel
calorimeters containing water with well-defined additives,
agreement between experiments and model calculations of
the relative heat defect caused by the radiolysis of water
was obtained. [9] Using high-purity deoxygenated water,
Schultz et al. obtained agreement between water calorimetry
and ionization chamber dosimetry.
A schematic drawing of the calorimeter is shown in
Figure 6.7. The calorimeter essentially consisted of a
cylindrical PMMA tube with a length of 15 cm, a diameter
of 4 cm, and a wall thickness of 0.5 mm, containing high-
purity water suspended in a large water phantom. For its
construction, the different parts of the cylinder were glued
together using acrylate glue flushed with air for several
hours, rinsed numerous times with high-purity water, and
pre-irradiated up to several kGy in order to reduce influ-
ences of the heat defect.
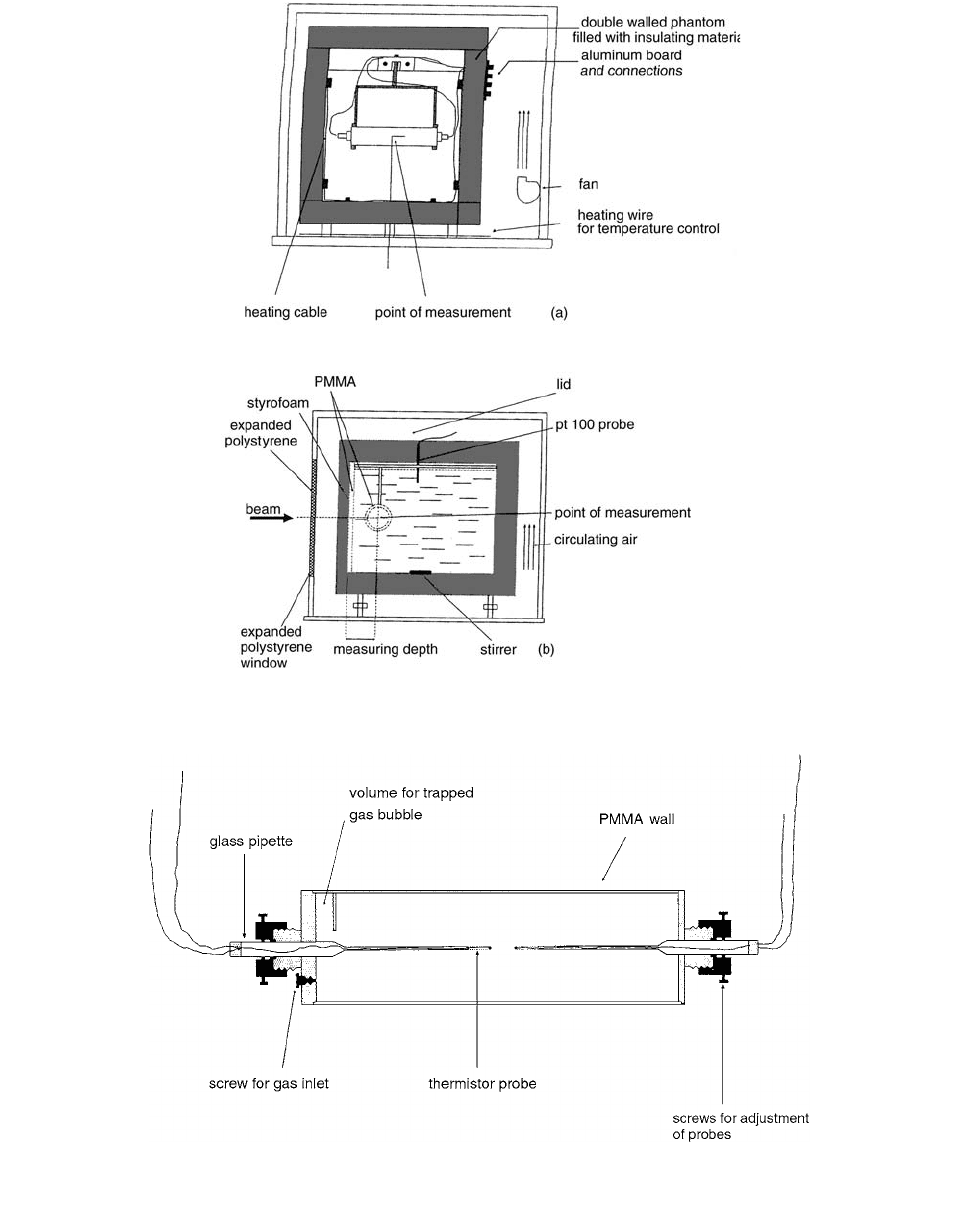
The calorimeter tank was a 30-cm
30-cm
30-cm
(inner dimensions) double-walled phantom with 4-cm wall
thickness and a PMMA front window (8.25-mm thickness).
In the bottom of the calorimeter, a small magnetic stirrer is
built in for agitation of the water. The water temperature is
measured with two small PT100 resistance probes. [9]
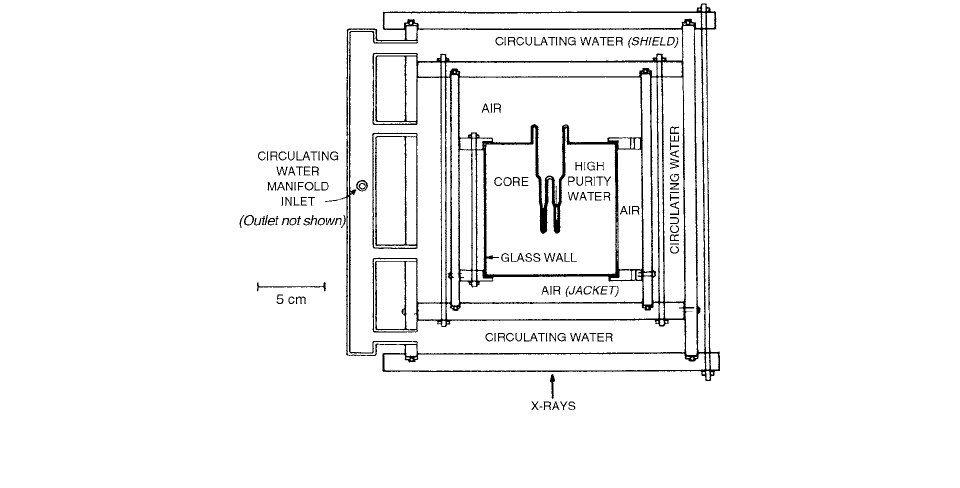
FIGURE 6.6
Cross section of the water calorimeter. Omitted from this drawing are two Teflon-seated glass valves and a 3-cm
3
,
nitrogen-filled expansion chamber, located at the distal end of the core. (From Reference [8]. With permission.)
CH-06.fm Page 335 Friday, November 10, 2000 12:02 PM
336
Radiation Dosimetry: Instrumentation and Methods
The water temperature can be adjusted using insulated
heating cable mounted in the phantom. The calorimeter core
(calorimeter detector vessel) was suspended in the water
phantom and adjusted using a tiny spacer between the inner
side of the phantom front window and the detector vessel
so as to set the point of measurement (the center of the
thermistors) at a geometrical depth of 5 cm from the outside
of the phantom PMMA window. The calorimeter core is
FIGURE 6.7
Schematic drawing of the water calorimeter and enclosing box: (
a
) front view; (
b
) side view. (From Reference [9].
With permission.)
FIGURE 6.8
Schematic drawing of the calorimeter detector vessel (PMMA) with thermistor probes. The vessel can be bubbled with
various gases to establish various chemical systems. (From Reference [9]. With permission.)
CH-06.fm Page 336 Friday, November 10, 2000 12:02 PM
Calorimetry
337
shown schematically in Figure 6.8. The temperature increase
is measured using thermistor probes. The calorimeter is
irradiated by a horizontal beam with a field diameter of
at least the total length of the detector vessel (15 cm).
The thermistor probes essentially consisted of small
commercial glass thermistor probes (Thermometrics, Inc.,
type P20, outer glass diameter 0.5 mm, length 5 mm),
glued into the end of small glass capillaries using epoxy.
The thermistors were serially connected to the one-arm
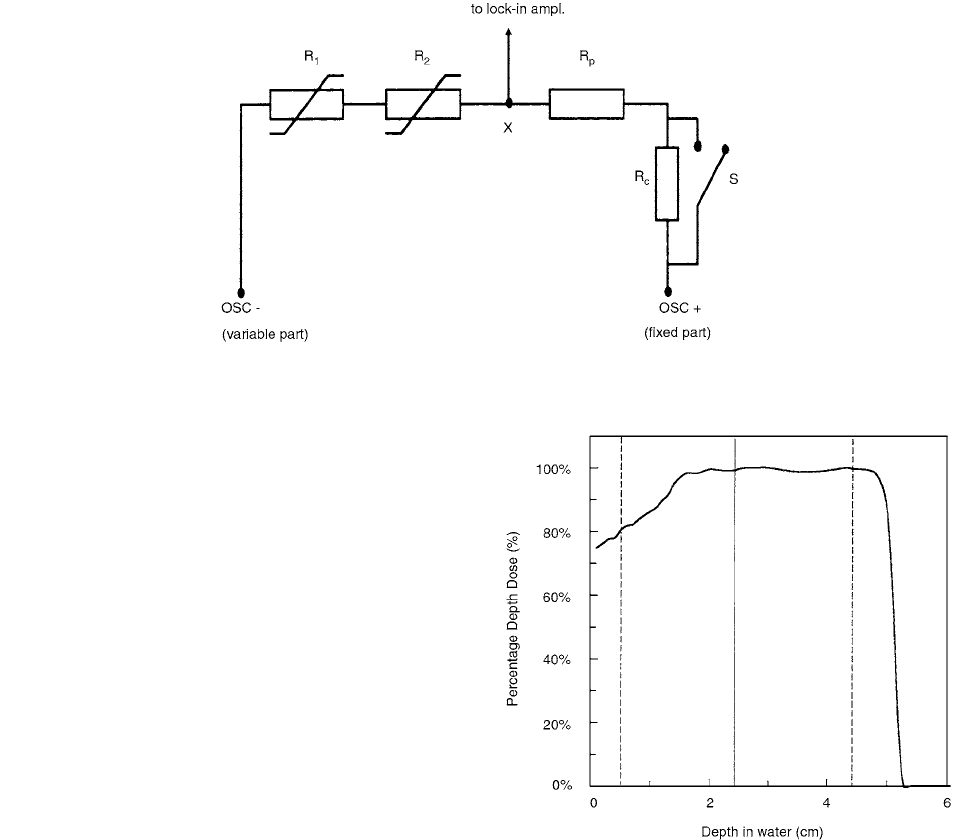
bridge circuit, shown in Figure 6.9.
The thermistor power level was varied in the range
1–200
W for ten temperature points in the range 15–30°C.
Because the temperature was varied over more than 10 K
and the relation between ln
R
and 1/
T
is slightly nonlinear
the fit
(6.8)
was used.
Due to the low dose rates, long irradiation times (typ-
ically 300 s) are required to obtain a sufficiently large
signal. For this purpose, corrections have to be applied for
heat loss (or gain) at the point of measurement by the non-
uniformity of the dose distribution. These corrections are
also needed due to excess heat from non-water materials
caused by their lower thermal capacity and their different
radiation absorption characteristics.
Palmans et al. [10] reported water calorimeter oper-
ation in an 85-MeV proton beam and a comparison of
the absorbed dose to water measured by ionometry with
the dose resulting from water calorimetric measurements.
The results showed that pure hypoxic water and hydrogen-
saturated water yielded the same response with practically
zero heat defect, in agreement with the model calculations.
The absorbed dose inferred from these measurements was
then compared with the dose derived from ionometry by
applying the European Charged Heavy Particle Dosimetry
(ECHED) protocol. Restricting the comparison to cham-
bers recommended in the protocol, the calorimeter dose
was found to be 2.6%
0.9% lower than the average
ionometry dose.
For 85-MeV protons, the range in water amounts to
50.5 mm, thereby irradiating the entire cylindrical core of
the calorimeter. The percentage depth-dose curve is shown
in Figure 6.10.
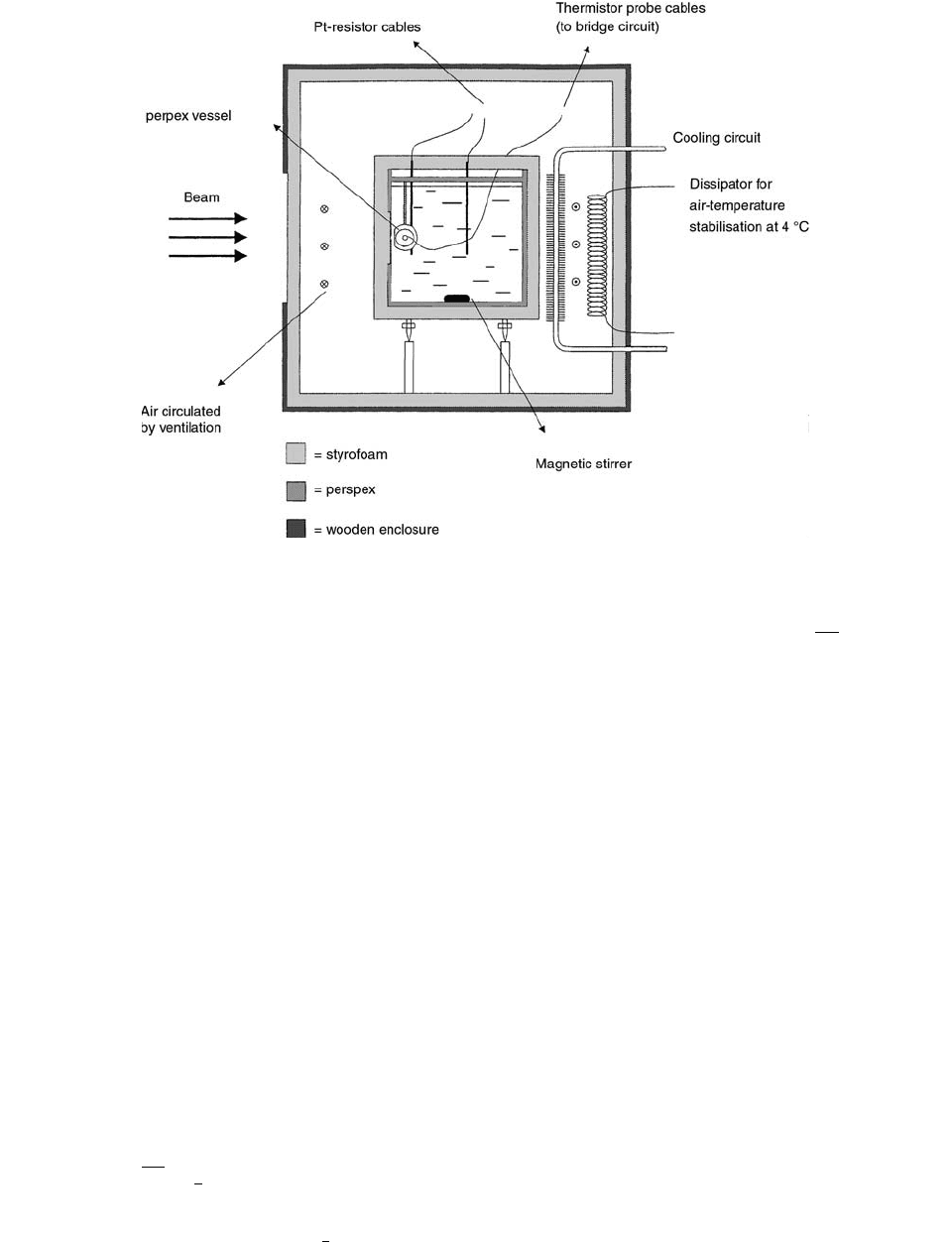
Figure 6.11 shows a schematic side view of the water
calorimeter. The calorimeter consists of a 30-cm cubic
water phantom, stabilized at 4°C in order to remove any
concern related to convection. The water temperature is
measured using calibrated platinum resistor probes at dif-
ferent positions in the phantom. The temperature increase
FIGURE 6.9
Schematic diagram of bridge circuit. (From Reference [9]. With permission
lnR a bT cT
2
FIGURE 6.10
Percentage depth-dose curve in water for the
modulated 85-MeV proton beam. The full vertical line shows
the position of the measuring point; the dashed lines show the
upstream and downstream limits of the calorimeter detection
vessel. (From Reference [10]. With permission.)
CH-06.fm Page 337 Friday, November 10, 2000 12:02 PM
338
Radiation Dosimetry: Instrumentation and Methods
due to radiation is measured in the center of a 4-cm-diameter
acrylic vessel (14-cm length, 0.5-mm wall thickness) using
glass thermistor probes (0.5 mm in diameter at the probe
end), each of 24 k
at 4°C.
In water calorimetry, dose to water is immediately
derived from the temperature change by multiplication with
the specific heat capacity (
c
w
) of water at the measuring
temperature, which is known with high accuracy. Specifi-
cally, we considered correction factors for conductive heat
losses (
k
c
) and the chemical heat defect (
h
). The effect of
the thermistor probe ends (0.5-mm diameter) and the vessel
wall (0.5-mm PMMA) on the dose at the measuring point
has been studied in detail for medium-energy x-rays and
high-energy photons, where it has been found to be smaller
than 0.2%
0.1%. Therefore, considering the scatter prop-
erties of protons compared to photons, the effect is probably
very small for protons. The correction factor (
k
sc
) for this
effect for protons has, therefore, been ignored and an uncer-
tainty of 0.2% has been assigned. By measuring the overall
fractional thermistor resistance change of the two ther-
mistors and applying the following equation [10],
(6.9)
absorbed dose to water was derived. represents the
average thermistor sensitivity which results from the
calibration against standard thermometry. The average
fractional thermistor resistance change of the two
thermistors was derived from linear extrapolations of
pre-irradiation drift and post-irradiation drift to mid-run
and measurement of the bridge output voltage change
(“irradiation-run”). This voltage change is converted into
an overall fractional bead resistance change by increas-
ing the opposite bridge arm with a known resistor and
measuring the output voltage change (“calibration-run”).
Corrections of approximately 0.05% for change in ther-
mistor power dissipation, and therefore excess heat, are
made for both irradiation and calibration runs.
The heat defect
h
is defined as
(6.10)
where
E
a
represents the energy absorbed and
E
h
is the
energy appearing as a temperature rise. For low LET radi-
ation and for water containing well-defined impurities, it
has been shown that the heat defect can be calculated
based on the primary yields (
G
-values
number of mol-
ecules formed due to an energy deposition of 100 eV) of
the species produced 10
7
s after the passage of the sec-
ondary electron through water, and assuming the species
are homogeneously distributed throughout the solution.
The method is based on the calculation of product yields
in the irradiated solution long (i.e., seconds to minutes)
after the passage of the radiation using reaction kinetics
FIGURE 6.11
Schematic drawing of the water calorimeter used by Palmans et al. (side view). (From Reference [10]. With
permission.)
D
w
c
w
R
R
--------
S
1
k
c
k
sc
1
1 h
----------------
S
RR
h
E
a
E
h
E
a
-------------------
CH-06.fm Page 338 Friday, November 10, 2000 12:02 PM
Calorimetry 339
and long after evaluation of the overall energy balance
using published enthalpies of formation. [10]
Absorbed dose to water at the point of measurement
in the calorimeter was derived from the ion chamber read-
ing M using the ECHED code of practice
(6.11)
where
N
k
is the air-kerma calibration factor for a
60
Co
photon beam and C
p
is an overall dose conversion factor
given by
(6.12)
where the stopping power ratio of water to air is taken
from ICRU Report 49 [17] for the effective proton energy
(E
P
)
eff
at the point of measurement. For (W
air
/e)
p
the value
of 35.2 J/C is recommended in the protocol. For consis-
tency, the other chamber-dependent factors in the calibra-
tion beam (
A
wall
and k
m
) are taken from the IAEA code of
practice for all chambers. Here A
wall
is a correction factor
for scattering and attenuation in the chamber wall, and k
m
is a correction factor for the non-air equivalence of the
chamber construction materials. For the comparison with
water calorimetry, values for A
wall
(equal to IAEA values
for the chambers used) and k, recommended in the
ECHED protocol, were used, yielding doses deviating by
no more than 0.4% for the same chamber reading.
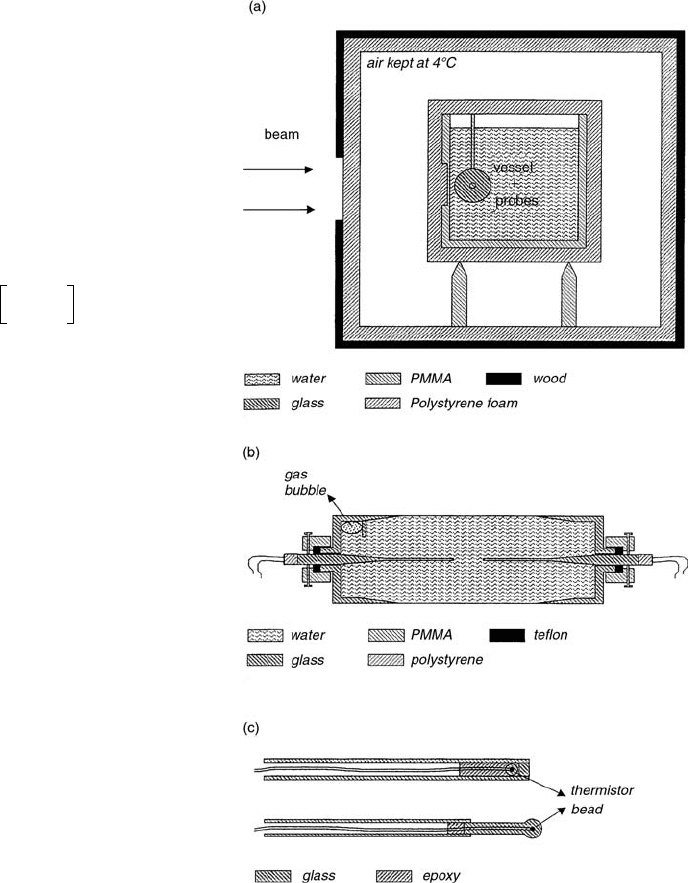
Figure 6.12a shows a schematic side view of the water
calorimeter positioned in its enclosure. The calorimeter
tank consists of a 30-cm
30-cm 30-cm water phan-
tom, thermally isolated by polystyrene foam. Isolation of
the enclosure allows temperature stabilization of the air
surrounding the calorimeter phantom. The operating tem-
perature is 4°C, in order to remove any concern related to
convection. The water temperature is continuously mea-
sured with calibrated Pt-resistor probes inserted at differ-
ent positions in the water phantom. The temperature
increase due to irradiation is measured at the center of a
cylindrical vessel using small thermistors that are embed-
ded in the tip of small glass probes (Figure 6.12c). Two
probe types are shown, one in which the thermistor bead
is epoxied in the end of the probe tip and one where the
bead is sealed in a small glass rod. Both types have been
used for the
60
Co measurements, but only the second type
has been used in the 5-MV and 10-MV photon beams. No
significant difference in calorimeter response has been
observed due to the use of the two different types. This is
also confirmed by excess heat calculations. Figure 6.12b
shows the cylindrical glass vessel and the positioning of
the glass probes. The vessel contains deionized and three-
times-distilled water and has a diameter of 4 cm, a length
of 14 cm, and a wall thickness varying from 0.3 mm in
the central part corresponding to the central axis of the
beam to 1.6 mm at the lateral edges. The chemical water
systems inserted into the vessel for the present study are
Ar-saturated pure water and Hz-saturated pure water. [11]
Absorbed dose to water is derived from the tempera-
ture increase due to irradiation at the location of the ther-
mistors,
T, as
(6.13)
where c
w
is the specific heat capacity of water at the measur-
ing temperature. The temperature change T is measured
D
w
M N
k
C
p
C
p
1 g()A
wall
k
m
S
---
air
water
W
air
e()
P
W
air
e()
C
-------------------------
FIGURE 6.12 Schematic design of the Gent sealed water cal-
orimeter: (
a) phantom and enclosure, (b) glass vessel with posi-
tion mechanism for thermistor probes, and (
c) tip of the
thermistor probes. (From Reference [11]. With permission.)
D
w
c
w
Tk
c
k
sc
k
dd
1
1 h
--------------
CH-06.fm Page 339 Friday, November 10, 2000 12:02 PM
