Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.


292
DEUTCHMAN AND PARTYKA
form-graphite. With advanced processes like chemical vapor deposition (CVD) and ion
beam enhanced deposition, it is possible to influence, to
a
certain degree, the energy and
charge states of the particles in the vapor phase, thus allowing some control over the
energy state (stable or metastable) and crystallographic and stoichiometric form of the
deposited films. Thus it is feasible to synthesize a variety of diamond and diamondlike
films with
a
range of mechanical, chemical, optical, electronic, and themla1 properties.’-”
Practical applications for the various films on actual engineered components will be deter-
mined by the film properties desired (i.e.. hardness, resistivity, optical transmission, etc.)
and the nature of the deposition process used to produce the films.
3.0
FILM DEPOSITION TECHNIQUES
The development
of
techniques and technologies capable
of
the deposition
of
continuous
thin diamond and diamondlike carbon films has been sparked by advances in the semicon-
ductor and thin film deposition indu~try.”~ These new techniques enable diamond film
deposition on the surfaces
of
both semiconductor and nonsemiconductor materials for
potential use in
a
wide variety of applications, both electronic and nonelectronic. Two
distinct coating methodologies have been developed, one relying
on
deposition from an
excited plasma discharge sustained in
a
low pressure atmosphere of hydrocarbon gases
(plasma-assisted CVDD), and one relying on the direct deposition of carbon films, either
without or with simultaneous bombardment by an intense flux
of
high energy ions (ion
beam enhanced deposition).
3.1
Plasma-Assisted Chemical Vapor Deposition (PACVD)
Techniques
Deposition of diamondlike carbon films with the plasma-assisted CVD technique proceeds
by exciting hydrogen-hydrocarbon-argon gas mixtures either in
a
glow discharge””’ or
with microwave radiation’””* (Fig.
1).
In both cases a plasma is produced, and free carbon
atoms are generated by the thermal decomposition of the hydrocarbon gas component.
The carbon atoms liberated in the plasma have enough energy to allow tetragonal car-
bon-carbon (diamond) bonding, making possible the condensation of diamond and dia-
mondlike carbon films. The films produced are actually mixtures
of
trigonally bonded
carbon (graphite), tetragonally bonded carbon (diamond), and other allotropic crystalline
To dissociate the hydrocarbon starting gas and provide enough thermal energy to
allow formation of trigonal and/or tetragonal carbon bonding, temperatures in the plasma
discharge must exceed 2000°F. Deposition rates on the order of
1
pmhr are achievable.
The presence of free hydrogen in the processing gas helps to promote film growth with
higher concentrations of tetragonally bonded diamond versus trigonally bonded graphite.
This occurs because graphitic bonds are much more chemically reactive than are diamond
bonds, resulting in selective etching
of
the graphite component
of
the films by free hydro-
gen gas in the plasma. However the presence
of
hydrogen trapped in the deposited diamond
films can produce high levels of tensile stresses, leading to embrittlement and buckling.
Applications for diamond films formed by the PACVD techniques are limited to
those in which the substrate can be raised
to
temperatures
in
excess
of
2000°F. Also. the
diamond films deposited by this technique grow epitaxially and therefore condense on
and adhere best to crystalline substrates like silicon and germanium. Therefore diamond
films deposited by PACVD techniques are best suited for applications in semiconductor
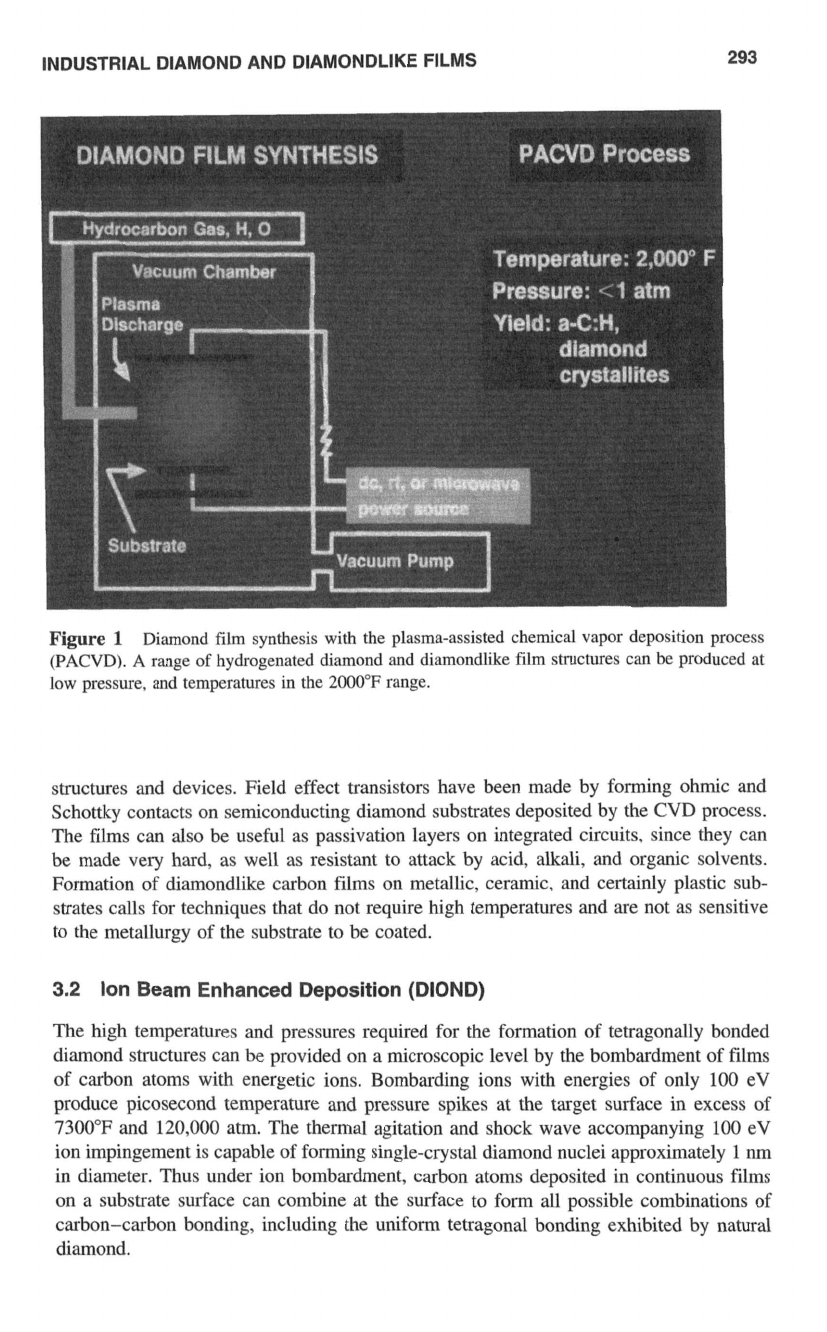
INDUSTRIAL DIAMOND AND DIAMONDLIKE FILMS
293
Figure
1
Diamond film synthesis with the plasma-assisted chemical vapor deposition process
(PACVD).
A
range
of
hydrogenated diamond and diamondlike film structures can be produced at
low pressure, and temperatures in the
2000°F
range.
structures and devices. Field effect transistors have been made by forming ohmic and
Schottky contacts on semiconducting diamond substrates deposited by the
CVD
process.
The films can
also
be useful as passivation layers on integrated circuits, since they can
be made very hard, as well as resistant to attack by acid, alkali, and organic solvents.
Formation of diamondlike carbon films on metallic, ceramic, and certainly plastic sub-
strates calls
for
techniques that do not require high temperatures and are not as sensitive
to the metallurgy of the substrate to be coated.
3.2
Ion Beam Enhanced Deposition (DIOND)
The high temperatures and pressures required for the formation of tetragonally bonded
diamond structures can be provided on a microscopic level by the bombardment of films
of carbon atoms with energetic ions. Bombarding ions with energies of only
100
eV
produce picosecond temperature and pressure spikes at the target surface in excess of
7300°F and
120,000
atm. The thermal agitation and shock wave accompanying
100
eV
ion impingement is capable of forming single-crystal diamond nuclei approximately
1
nm
in diameter. Thus under ion bombardment, carbon atoms deposited in continuous films
on a substrate surface can combine at the surface to form all possible combinations of
carbon-carbon bonding, including the uniform tetragonal bonding exhibited by natural
diamond.
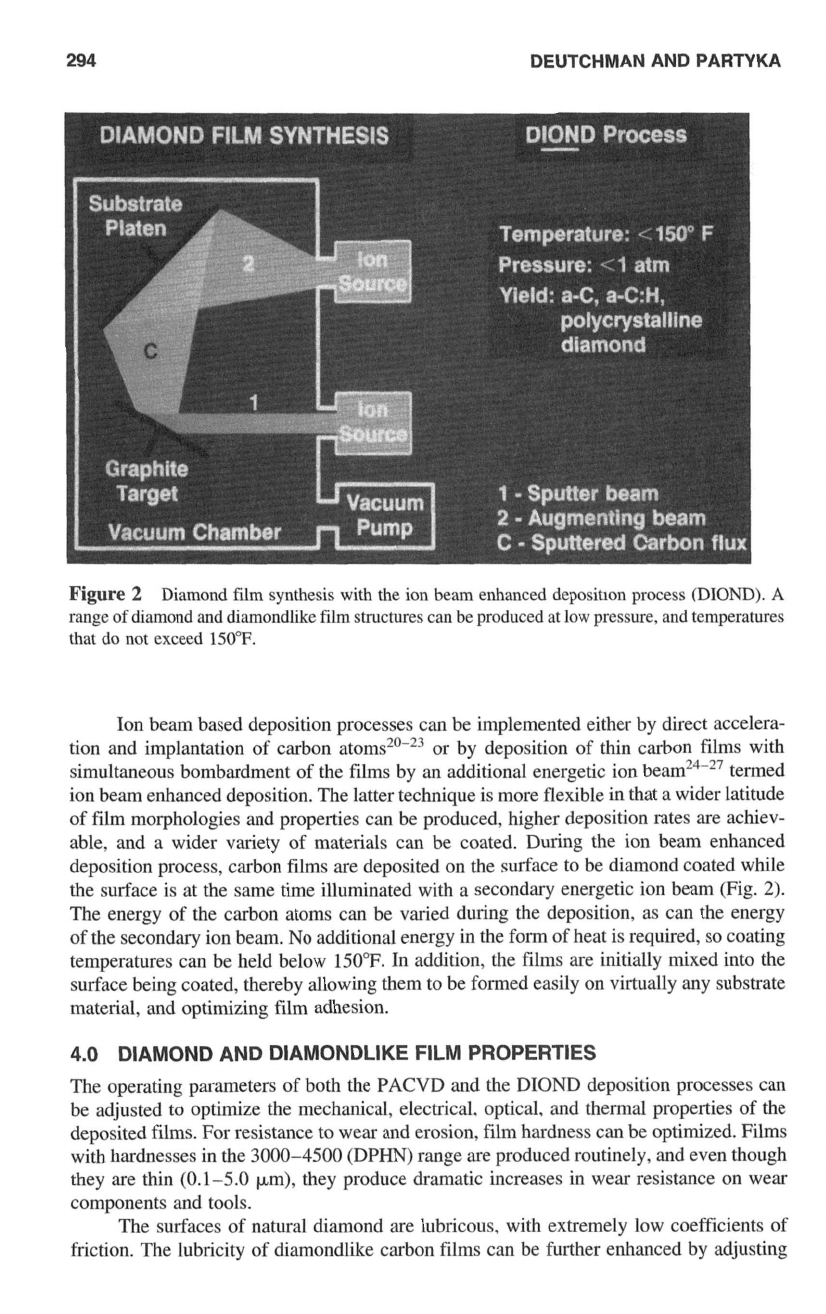
294
DEUTCHMAN AND PARTYKA
Figure
2
Diamond film synthesis with the ion beam enhanced depositlon process
(DIOND).
A
range
of
diamond and diamondlike film structures can be produced at
IOW
pressure, and temperatures
that
do
not exceed
150°F.
Ion beam based deposition processes can be implemented either by direct accelera-
tion and implantation of carbon or by deposition of thin carbon films with
simultaneous bombardment of the films by an additional energetic ion beam’4-27 termed
ion beam enhanced deposition. The latter technique is more flexible in that a wider latitude
of
film morphologies and properties can be produced, higher deposition rates are achiev-
able, and a wider variety of materials can be coated. During the ion beam enhanced
deposition process, carbon films are deposited on the surface to be diamond coated while
the surface is at the same time illuminated with a secondary energetic ion beam (Fig.
2).
The energy of the carbon atoms can be varied during the deposition, as can the energy
of the secondary ion beam.
No
additional energy in the
form
of heat is required,
so
coating
temperatures can be held below 150°F. In addition, the films are initially mixed into the
surface being coated, thereby allowing them to be formed easily on virtually any substrate
material, and optimizing film adhesion.
4.0
DIAMOND AND DIAMONDLIKE FILM
PROPERTIES
The operating parameters
of
both the
PACVD
and the DIOND deposition processes can
be adjusted to optimize the mechanical, electrical. optical, and thermal properties
of
the
deposited films.
For
resistance to wear and erosion, film hardness can be optimized. Films
with hardnesses in the
3000-4500
(DPHN) range are produced routinely, and even though
they are thin (0.1-5.0 Fm), they produce dramatic increases in wear resistance on wear
components and tools.
The surfaces of natural diamond are lubricous, with extremely low coefficients
of
friction. The lubricity of diamondlike carbon films can be further enhanced by adjusting
INDUSTRIAL DIAMOND AND DIAMONDLIKE FILMS
295
process parameters to increase concentrations of trigonally bonded graphitic structures.
Coefficients of friction as low as
0.001
have been produced in diamondlike films rubbing
against steel.
Optical properties can also be optimized by adjusting process parameters. Dia-
mondlike films that are optically transparent in the visible and infrared regions of the
electromagnetic spectrum, as well as films that
are
colored, can also be produced.
Films with an extremely wide range of electrical resistivities can be deposited by
varying process parameters. By adjusting the relative concentration of trigonally and tetrag-
onally bonded carbon atoms. the resistivities of the films can be varied from as low as
1
flan to as high as
1
X
10"
cm.
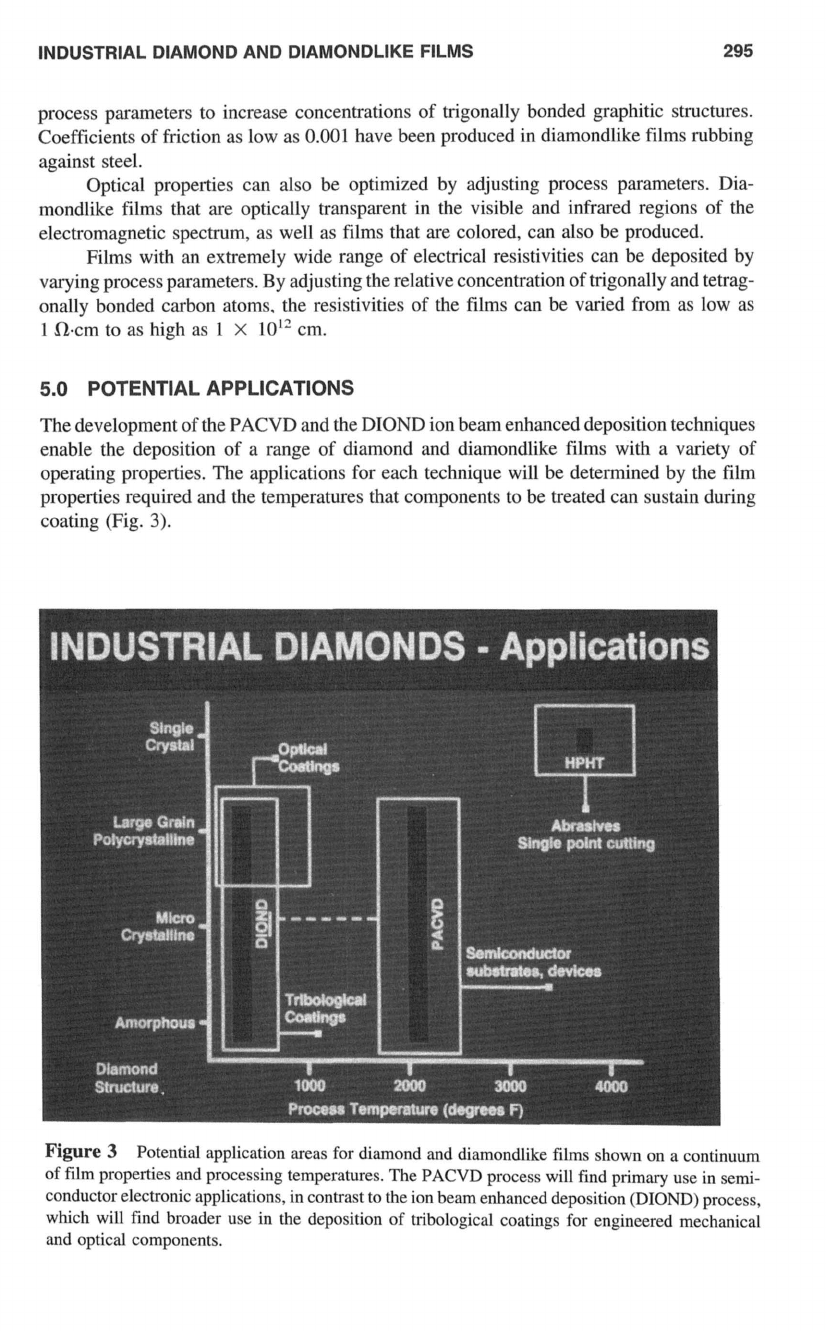
5.0
POTENTIAL APPLICATIONS
The development of the PACVD and the DIOND ion beam enhanced deposition techniques
enable the deposition of a range of diamond and diamondlike films with a variety of
operating properties. The applications for each technique will be determined by the film
properties required and the temperatures that components to be treated can sustain during
coating (Fig.
3).
Figure
3
Potential application areas
for
diamond and diamondlike films shown on a continuum
of
film properties and processing temperatures. The
PACVD
process will find primary use in semi-
conductor electronic applications, in contrast to the ion beam enhanced deposition
(DIOND)
process,
which will find broader use in the deposition
of
tribological coatings
for
engineered mechanical
and optical components.

296
DEUTCHMAN AND PARTYKA
The high temperature
PACVD
process is finding primary applications in the semi-
conductor device area, since semiconducting and ceramic substrate materials can sustain
high processing temperatures. Applications
in
the semiconductor electronics area include
production of active semiconducting elements (amplifiers, oscillators, and electro-optic
elements) that operate at higher switching speeds and higher temperatures (and thus power
levels), high efficiency heat sink layers. and insulating layers for protection against oxida-
tion and chemical contamination.
The availability of the
DlOND
ion beam enhanced deposition process, which is able
to produce a range of diamond and diamondlike carbon films at low temperatures, and
depositable on virtually any substrate material, makes a number of applications for dia-
mond coatings feasible. Wear-resistant surfaces for a wide variety of precision tools and
wear components (e.g., gears and bearings) are now being produced. The dielectric proper-
ties
of
the films allow them to be used as protective coatings for the magnetic recording
media on fixed disks for computers, as well as the magnetic recording heads themselves.
Since the films can be made optically transparent, optical components like lenses and
mirrors can be surfaced for resistance
to
erosion and scratches. The films can also be used
to reduce surface friction
on
components as well as to produce graphitic self-lubricating
layers for applications
in
which conventional lubricants cannot be used.
The availability
of
processes capable
of
depositing diamond and diamondlike films
on
the surfaces of engineered components offers opportunities to improve the operating
performance of many existing products and to develop a wide variety
of
new ones
in
the
semi-conductor, electronic. optical. computer. and mechanical components industries. Key
to
the successful use and application of these new industrial diamond and diamondlike
films are a solid understanding
of
the range of film properties that can be produced and
proper selection
of
the techniques used to deposit them.
REFERENCES
1,
G.
Graff.
High
Tec.hrlol..
7, 44
(
19x7).
2.
J.
C.
Angus et
al.,
Thirr
Solid
Filrrrs,
I
1
X.
3
1
1
(
1984).
3.
C.
Weissmantel, in
Tlrirl
Filr~~,s,fiort~
Frerr
A/or~rs
mtl
Prrrfides.
K.
J.
Klabunde. Ed. Orlando,
4.
J.
C.
Angus,
Thin
Solid
Filrrrs.
142,
145
(
1986).
S.
D.
B.
Kerwin
et
al.,
Tlrirr
Solid
Filrrrs,
I4X.
31
1
(1987).
6.
R.
Messier et
al.,
Thirr
Solid
Filrrls,
153.
I
(
1987).
7.
R.
C.
DeVI-ies.
Arlrur.
Rev.
Muter.
Sci.,
17,
161
(
1987).
8.
J.
C.
Angus
ct
al.,
Sciertcr,
241.
913
(1988).
9.
D.
S.
Whitmcll
et
al.,
7hr
Solid
Filrrrs.
35,
255
(
1976).
FL:
Academic
Press,
198.5.
p.
153.
IO.
B.
V.
Spitsyn
et
al.,
J.
CrT.st.
Grou~th.
52. 219
(1981).
1
I
,
K.
Enke.
Tlrirr
Solid
Filrrrs.
80. 227
(
198
1
).
12.
S.
Matsumoto et
al.,
J.
Appl.
Phys.,
21,
1
I
X3
(
1982).
13.
E.
T.
Prince,
J.
Vrrc.
Sci.
Techrrol.
A3.
3,
694
(
1985).
14.
J.
D.
Warner
et
al.,
J.
VW.
Sci.
Techrrol.
A-?,
3,
900
(
1985).
15.
A.
R.
Nyaiesh
et
al.,
J.
Vcrc.
Sci.
7ethrnl.
A.?,
3.
6
IO
(
1985).
16.
0.
Matsumoto
ct
al..
Thirr
Solid
Filrtl.s,
128,
341
(1985).
17.
0.
Matsumoto
et
al.,
Tl~irr
Solid
Filrm,
146, 283
(
1986).
1
X.
N. Fujimori
et
al.,
VWNIIIII.
36,
99
(
19x6).
19.
S.
Aiscnherg
et
al.,
J.
Appl.
Phys..
42.
2953
(1976).
20.
E.
G.
Spenser
ct
al.,
Appl.
Plrys.
Le//.,
29.
228
(
1976).
21.
J.
H.
Freeman
ct
al.,
Nucletrr
hsfrrurt.
12fetlrotl.s.
135,
1
(1976).

INDUSTRIAL DIAMOND AND DIAMONDLIKE FILMS
22.
T.
Miyazawa et al.,
J.
Appl.
Plys..
SS,
188
(1984).
23.
J.
W. Rabalais et
al.,
Science.
239, 623
(1988).
24.
C.
Weissmantel,
Thin
Solid
Film.
92,
SS
(1982).
25.
M.
J.
Mirtich
et
al.,
Thin
Solid
Filrns,
131, 245 (1985).
26.
C.
Weissmantel et al.,
J.
Vac.
Sci.
Techr~ol.
A4,
6, 2892 (1985).
27.
A.
H.
Deutchman et al.,
Irzd.
Hedng,
LV(7),
12
(1988).
297
This Page Intentionally Left Blank

31
Tribological Synergistic Coatings
Walter
Alina
Generd
Mrrgnnplate
Corporation, Linden, New Jersey
1
.O
INTRODUCTION
The solution of wear and many related problems for any application is very much experi-
ence dependent.
A
scientific basis for resolving these problems unfortunately has not yet
been found. Using experience and history, it is possible to recommend
a
number of poten-
tial solutions; however, the ultimate proof is
in
the actual trial of the application. This is
because there are
so
many variables within each application that the slightest change could
make
a
difference in the selection of the appropriate coating. Even though applications
appear to be identical, there are always slight differences such that the same coating
selection will not always perform
in
the same manner.
The production of synergistic coatings on steel (Nedox) or aluminum (Tufram) is
based on the principle of infusion of
a
dry lubricant or polymer into the coatings. General
Magnaplate has developed
a
family of such coatings (Nedox), each one representing spe-
cific properties, such
as
hardness, lubricity, corrosion protection, and dielectric strength.
The standard hardfacing for steel is an electroless nickel coating. There are
a
number of
electroless nickels that vary the phosphorus content and consequently have differences in
hardness and corrosion resistance. Choice of such
a
coating varies and is based on the
application requirements.
Synergistic coatings for aluminum (Tufram) have been used successfully for many
years. The system can accommodate almost all aluminum alloys, provided a copper content
of
5%
and
a
silicon content of
7%
are not exceeded. Higher percentages of these constitu-
ents (set up too great a change in substrate resistivity, hence) prevent the buildup of
required film thickness.
The prime purpose of the Tufram system is to produce films having properties such
as improved wear resistance, better surface release (lower coefficient
of
friction), good
corrosion resistance, and high dielectric strength.
299

300
ALINA
The principle of these coatings is based on a hardcoat after which a polymer or dry
lubricant is infused into the coating substrate.
All
coatings are used
in
a
wide variety
of
industries. Some are
in
compliance with
the regulations of the
U.S.
Food and Drug Administration and can be used in food and
medical applications.
The improvement in wear resistance to alunlinum ranges from
5
to
25
times that
without the coating. It is difficult to put an exact number on the improvement. since it
varies from one application
to
another.
2.0
WHAT ARE SYNERGISTIC COATINGS?
Synergistic coatings are not really “coatings”
in
the conventional sense of the word. They
are created during multistep processes that combine the advantages
of
anodizing or hard-
coat plating with the controlled infusion of low friction polymer and/or dry lubricants.
These “coatings” become an integral part of the top layers
of
the base metal rather than
merely a surface cover. Since the resulting surfaces are superior
in
performance both to
the base metal and to the individual components of the coatings. the proprietary processes
that produce them are identified as “synergistic.” Why do they work?
The Tufram process, a family of coatings for aluminum alloys, converts the hydrated
alunlinum oxide
A1,03.H,0
and replaces the
H,O
of the newly formed ceramic surface
with inert polymeric material that provides a self-luhricating surface.
In
the process. the
aluminum crystals expand and form porous anchor crystals that remain hygroscopic for
a short period of time.
The particles of the specific polymer selected are then introduced under controlled
conditions of properly balanced solutions, time, and temperature, to permanently interlock
with the newly formed crystals.
This results
in
a harder-than-steel continuous lubricating plastic-ceramic surface of
which the polymeric particles become an integral part (Fig.
1
).
Synergistic coatings are a great asset
in
solving many wear problems, but how were
these problems resolved years ago? Their solution was expensive, time-consuming, and
very frustrating.
Normally a surface was built up with
0.005-0.020
in.
of hard chrome. then precision
machined to a specification. After this surface had been polished to
4
RMS
or better,
it
basically qualified as
a
wear surface because it was hard and the
4
RMS microfinish
reduced the coefficient
of
friction. If the coefficient of friction of such
a
surface were
compared to the coefficients for synergistic coatings (see Table
1
L
the latter would be
seen to have much lower numbers, consequently lower frictional values.
It is important
to
have some familiarity with the coefficient of friction between
materials. Table
1
gives both static and dynamic coefficients of friction
of
many basic
coatings. Each fractional number implies the frictional forces applied against two surfaces.
The coefficients
in
Table
1
are to be considered
only
as a guide. Many factors can
alter figures derived from these laboratory constants. including:
Applied loads
Point loading
Loading stresses
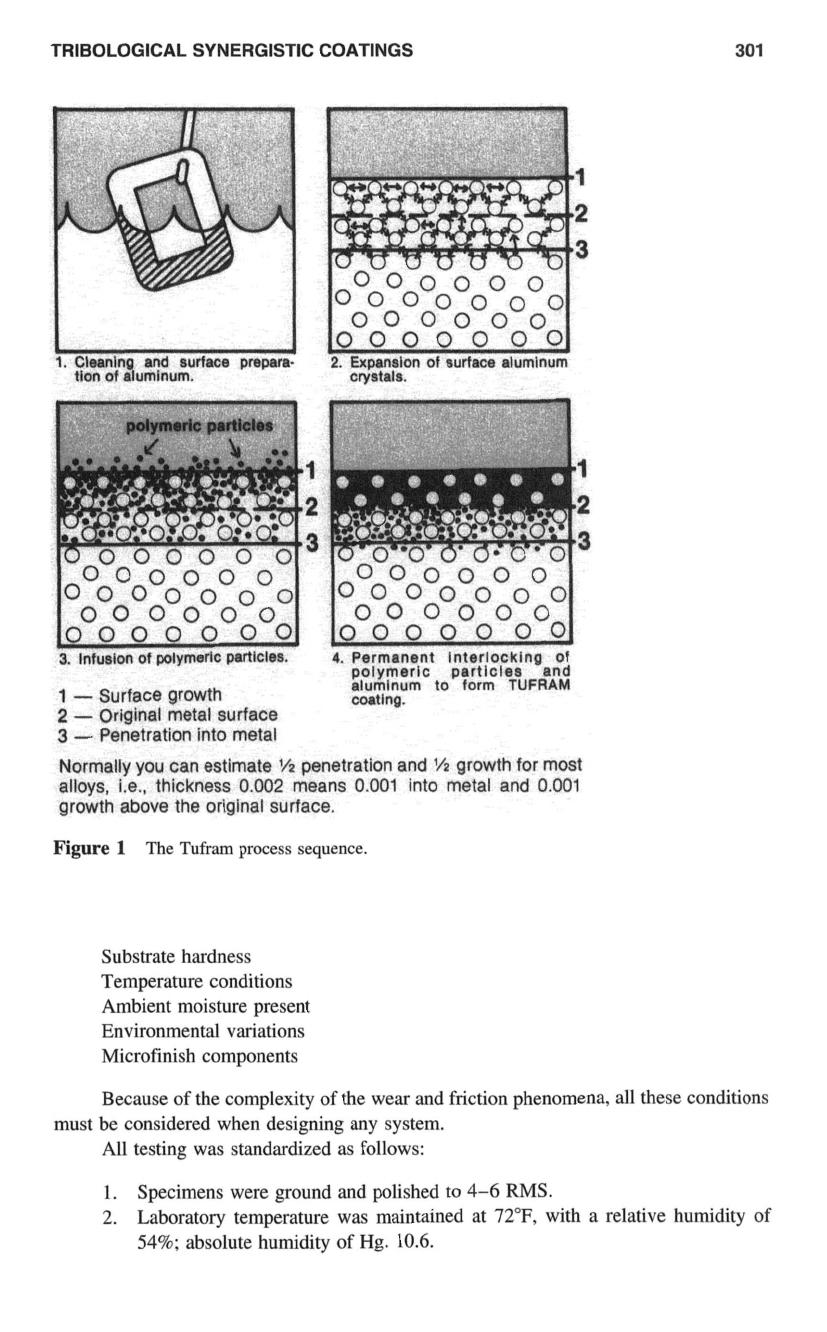
TRIBOLOGICAL SYNERGISTIC COATINGS
301
J
1.
Cleaning and surface prepara-
tion of aluminum.
polymeric particles
L
000000
0000000
000000
0000000
1
2
3
3.
Infusion of polymeric particles.
4.
Permanent Interlocking of
polymeric particles and
alumlnum
to
form
TUFRAM
coatlng.
l
-
Surface growth
2
-
Original metal surface
3
-
Penetration into metal
Normally you can estimate
V2
penetration and
'/2
growth for most
alloys, i.e., thickness
0.002
means
0.001
into metal and
0.001
growth above the original surface.
Figure
1
The
Tufram
process sequence.
Substrate hardness
Temperature conditions
Ambient moisture present
Environmental variations
Microfinish components
Because
of
the complexity of the wear and friction phenomena, all these conditions
All
testing was standardized
as
follows:
must be considered when designing any system.
1.
Specimens were ground and polished to
4-6
RMS.
2.
Laboratory temperature was maintained at
72T,
with a relative humidity
of
54%;
absolute humidity
of
Hg.
10.6.
