Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.

This Page Intentionally Left Blank

Chemical Vapor Deposition
1
.O
INTRODUCTION
Chemical vapor deposition (CVD) is a technique
of
modifying properties
of
surfaces
of
engineering components by depositing a layer or layers of another metal or compound
through chemical reactions
in
a
gaseous medium surrounding the component at an elevated
temperature.
In
formal terms, CVD may be defined as
a
technique in which a mixture of
gases interacts with the surface
of
a substrate at
a
relatively high temperature. resulting
in
the decomposition of some
of
the constituents
of
the gas mixture and the formation of
a solid film
of
coating
of
a metal or
;I
compound
on
the substrate.
2.0
PROCESS
A modern CVD system includes a system
of
metering
a
mixture of reactive and carrier
gases.
a
heated reaction chamber. and a system for the treatment and disposal of exhaust
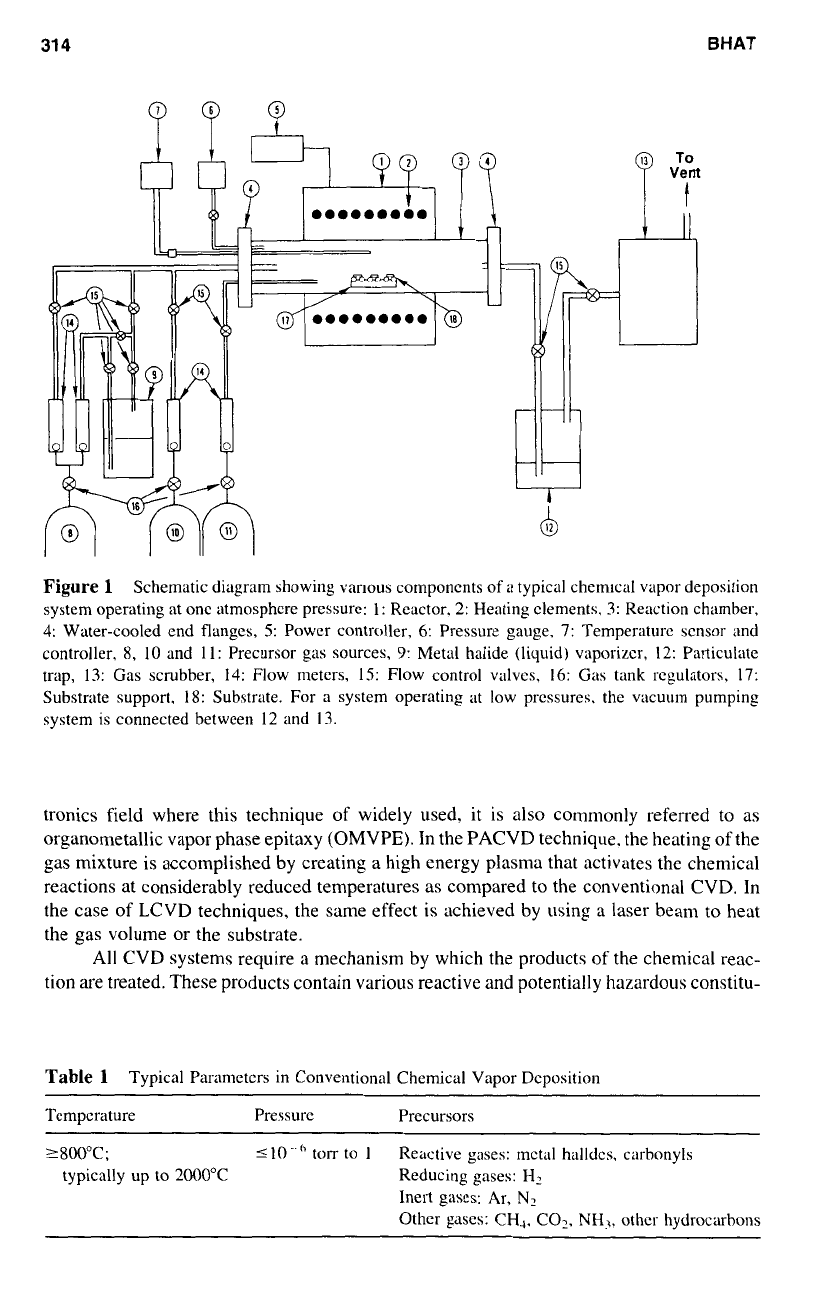
gases. Figure
1
shows the basic arrangement
of
various components of industrial CVD
systems.
The gas mixture (which typically consists of hydrogen, nitrogen or argon, and rex-
tive gases such
as
metal halides and hydrocarbons) is carried into
a
reaction chamber that
is heated to the desired temperature by suitable means. The various techniques include
resistance heating with Kanthal, Globar (Sic) or graphite heating elements. or induction.
In some cases. the substrate is heated directly by passing an electric current through it.
Typical operating parameters for a conventional CVD process are shown
in
Table
I.
Different variations of the conventional method have been developed over the last
few decades. These include moderate-tenlperature CVD (MTCVD), plasma-assisted CVD
(PACVD) and laser CVD (LCVD). In the MTCVD process. the reaction temperature is
reduced
to
below about
850°C
by the use
of
metalorganic compounds
as
precursors. There-
fore. this technique is also referred to
as
metalorganic CVD (MOCVD).
In
the microelec-
31
3
31
4
BHAT
Figure
1
Schematic diagram showing vanous components
of
a
typical chemical vapor deposition
system operating at
one
atmosphere pressure:
1:
Reactor.
2:
Heating elements.
3:
Reaction chamber,
4:
Water-cooled end flanges,
5:
Power controller,
6:
Pressure gauge,
7:
Temperature sensor and
controller,
8,
10
and
11:
Precursor gas sources,
9:
Metal halide (liquid) vaporizer,
12:
Particulate
trap,
13:
Gas scrubber,
14:
Flow meters,
IS:
Flow control valves,
16:
Gas tank regulators,
17:
Substrate support.
18:
Substrate. For a system operating at low pressures. the vacuum pumping
system is connected between
12
and
13.
tronics field where this technique
of
widely used, it is also commonly referred to as
organometallic vapor phase epitaxy (OMVPE).
In
the PACVD technique. the heating of the
gas mixture is accomplished by creating a high energy plasma that activates the chemical
reactions at considerably reduced temperatures
as
compared to the conventional CVD.
In
the case of LCVD techniques, the same effect is achieved by using
a
laser beam to heat
the gas volume or the substrate.
All CVD systems require
a
mechanism by which the products
of
the chemical reac-
tion are treated. These products contain various reactive and potentially hazardous constitu-
Table
1
Typical Parameters in Conventional Chemical Vapor Deposition
Temperature Pressure Precursors
2800°C;
51W"
torr to
1
Reactive gases: metal hnlldcs. carbonyls
typically
up
to
2000°C
Reducing gases: H?
Inert gases: Ar, N2
Other gases: CH4. CO?, NH3, other hydrocarbons

CHEMICAL VAPOR DEPOSITION
31
5
Table 2
Chemical Reactions
in
CVD
Reaction Equatlon
Thermal decomposition,
or
pyrolysis
CHISiCll
-
Sic
+
3HCI
Reduction
WF(,
+
3H’
-
W
+
6HF
Oxidation
SiH,
+
0:
-
Si02
+
2H2
Hydrolysis
2A1CIq
+
3HZO
+
A1201
+
6HCI
Coreduction
TiCIJ
+
+
SH2
-
TiB2
+
IOHCl
ents. as well as particulate matter. which must be trapped and neutralized before the gases
are exhausted to the atmosphere. In addition, since most CVD processes are carried out
at subatmospheric pressures, the pumping equipment must be protected from the relatively
hot, corrosive gases. This is usually done by using nonreactive materials for the pump
components.
The deposition
of
coatings by CVD can be achieved in a number of ways. The
chemical reactions utilized in CVD are shown
in
Table
2.
These reactions between various
constituents occur in the vapor phase over the heated substrate, and a solid film is deposited
on the surface. The coatings are, therefore. termed “overlay” coatings. On the other hand,
a surface film can also be deposited by causing a reaction between the substrate surface
and one or more
of
the constituents of the vapor phase. One example of this technique is
the formation
of
a
nickel aluminide film
on
the surface of nickel by a reaction of aluminum
trichloride and hydrogen from the vapor with the surface of nickel at a high temperature.
Such a coating may, therefore, be called a conversion coating.
3.0
APPLICATIONS
The CVD technique is applicable for the deposition of
a
wide variety of materials, such
as metals, compounds, ceramics, powders, and whiskers. Typical materials deposited by
CVD and applications of the CVD technique are summarized in Tables
3
and
4.
One
of
the earliest applications of CVD was in the manufacture of pigments. Powders of TiO?,
SiO?. carbon black, and other materials such as
A1203,
Si3N4. and BN. have been routinely
made by CVD. In a variation of the conventional CVD technique, powders of nuclear
fuel materials from the fuel rods used
in
nuclear reactors have been coated in
a
fluidized
bed with coatings of Sic. graphite. and ZrC for containment
of
fission products.
Table
3
Typical Materials Deposited by CVD
Material Example
Metals
Al.
As.
Be. Bi, Co. Cr. Cu, Fe.
Ge,
Hf,
Ir.
MO. Nb, Ni,
Os.
Pb, P. Re, Rh, Ru, Sb,
Compounds
11-V1
and
Ill-V
compounds.
borides.
carbldcs.
nltrides, and silicides of
transition
Ceramics
AI2O3.
AIN, B703, BN, Sic, Si3N,,
U02.
Y203,
Zr02,
etc.
Si.
Sn.
Tn. Th, Ti.
U,
V.
W.
Zr,
also
carbon and boron
metals.
as
well
;IS
sulfides, phosphides aluminides. etc.

31
6
BHAT
Table
4
Applications of the CVD Technique
Tribological coatings
Decorative films
Wear-resistant coatings
Superconducting films
High-tempernture coatings for oxidation resistance
Emissive coatings
Dielectric tnsulnting films
Coatings for fiber composltcs
Opticnl/rcflective films
Free-standing structural shapes
Photovoltaic films
Powders
and
whiskers
Because of the nature of the process, CVD is used to deposit high-purity metals
from their halide or carbonyl precursors, and the technique has been especially useful for
synthesizing refractory metals. The most commonly used precursors for CVD are metal
halides. For a successful application
of
CVD,
it
is necessary to be able to decompose the
halides at relatively moderate temperatures (e.g.,
5
1000°C). Thus, many metals whose
halides are stable in this temperature range are difficult to deposit successfully. In many
of these cases. organometallic compounds have been used successfully. Typical examples
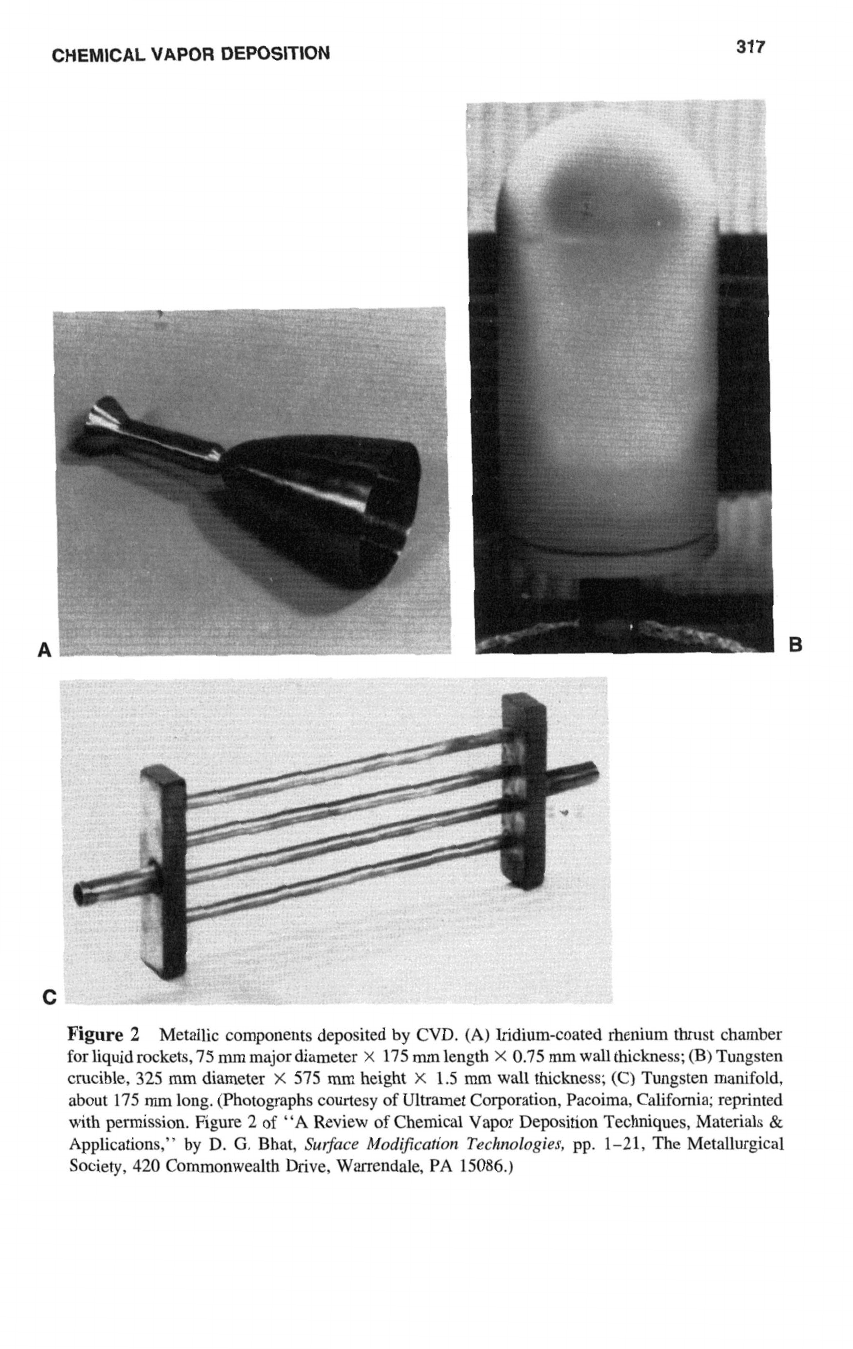
of metallic components deposited by CVD are shown in Figure
2.
In
recent years, another interesting application
of
the CVD technology has achieved
prominence. Deposition of whiskers of metals and refractory compounds is of significant
technological importance because of the potential for development
of
composite materials.
Composites have become a very inlportant new class
of
engineering materials for aerospace
structural applications.
Whiskers are needle-shaped single crystals of materials, typically
1
pm or less in
diameter and several micrometers long. It has been demonstrated that the addition of
whiskers to ceramics, which are inherently brittle. significantly improves their fracture
toughness. Various refractory compounds have been deposited in the form of whiskers
by CVD. These include
A1203.
Cr3C7. Sic. Si2N4. TiB?. TIC, TIN. ZrC. ZrN. and
Zr02.
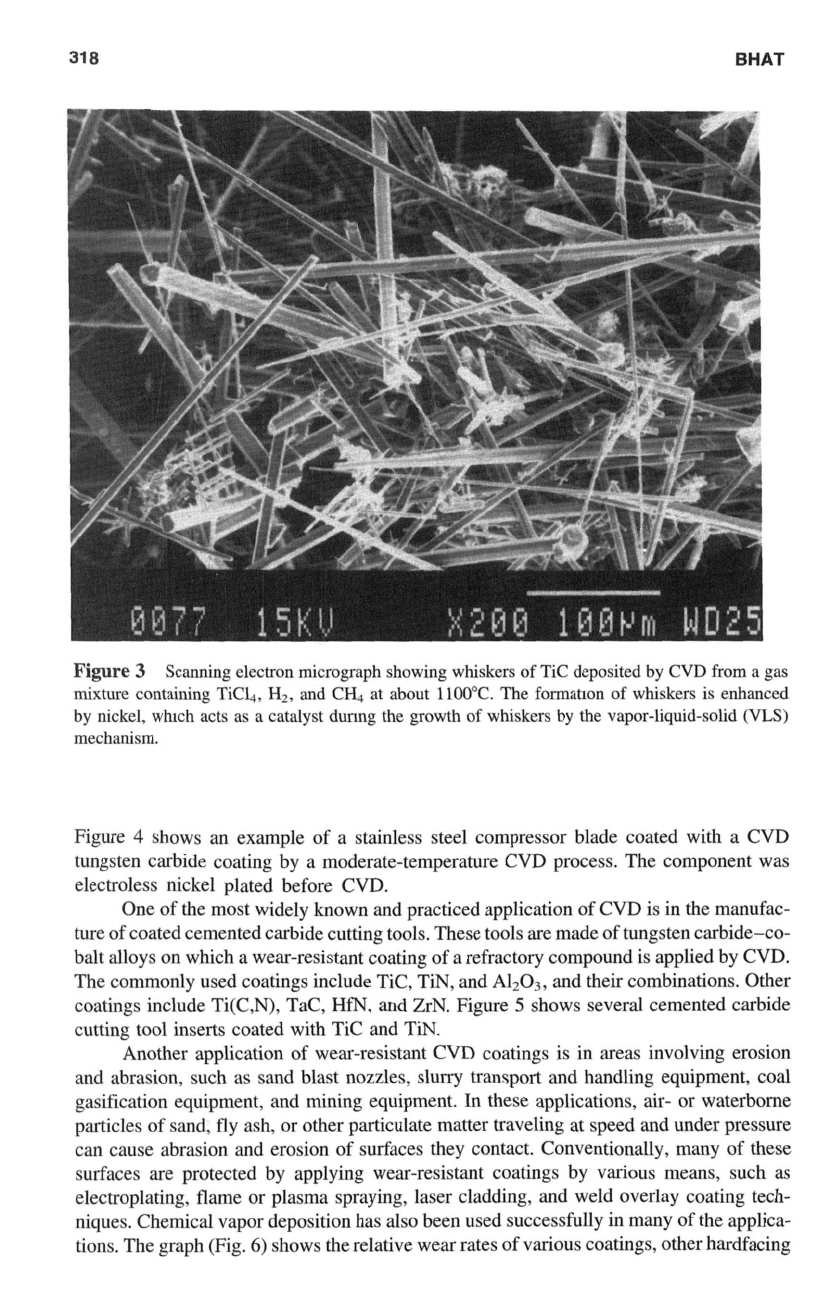
Figure
3
shows an example of TIC whiskers deposited by CVD.
It
is to be expected that
with the increasing prominence of the composite materials in the advanced engineering
components. many more materials will be synthesized in the whisker and fiber forms for
these applications.
As
stated earlier. the conventional CVD calls for relatively high temperatures.
This requirement imposes certain limitations on the type of substrate that can be
successfully used for deposition. Typically. most ceramic materials, graphite. and
refractory metals such
as
tungsten and molybdenum are found to be quite suitable
because of their high thermal and chemical stability
in
typical CVD process environ-
ments. Steels have also been used successfully, but certain precautions must be taken
for best results. For example, most steels other than austenitic or ferritic steels undergo
soli(I stllte phase transformation
in
the 700-800°C temperature range. This transformation
is ;iccon1p;inied by changes
in
microstructure. physical properties. and dimensions
that
COLI^^
be detrimental for the coating or the component
in
the intended application.
h
addition, the chemical stability of steel may be compromised in some CVD coating
operations.
as
in
the case
of
tungsten deposition as a result of the reaction
of
steel
wit11 the tluoride gases. Methods to avoid these possibilities exist; for example. one
can deposit
a
film
of
nickel by electrolytic or electroless means to protect the substrate.
CHEMICAL VAPOR
DEPOSITION
31
7
A
B
C
.
Figure
2
Metallic components deposited by CVD.
(A)
Iridium-coated rhenium thrust chamber
for liquid rockets,
75
mm
major diameter
X
175
mm
length
X
0.75
mm
wall thickness; (B) Tungsten
crucible,
325
mm
diameter
X
575
mm
height
X
1.5
mm
wall thickness; (C) Tungsten manifold,
about 175
mm
long. (Photographs courtesy of Ultramet Corporation, Pacoima, California; reprinted
with permission. Figure
2
of
“A
Review of Chemical Vapor Deposition Techniques, Materials
&
Applications,” by D.
G.
Bhat,
Surface
Mod$cution
Technologies,
pp. 1-21, The Metallurgical
Society,
420
Commonwealth Drive, Warrendale,
PA
15086.)
31
8 BHAT
Figure
3
Scanning electron micrograph showing whiskers
of
Tic
deposited
by
CVD
from
a gas
mixture containing Tick,
Hz,
and CH4 at about
1100°C.
The
formation
of
whiskers is enhanced
by
nickel, which acts as
a
catalyst dunng the growth
of
whiskers
by
the vapor-liquid-solid
(W)
mechanism.
Figure
4
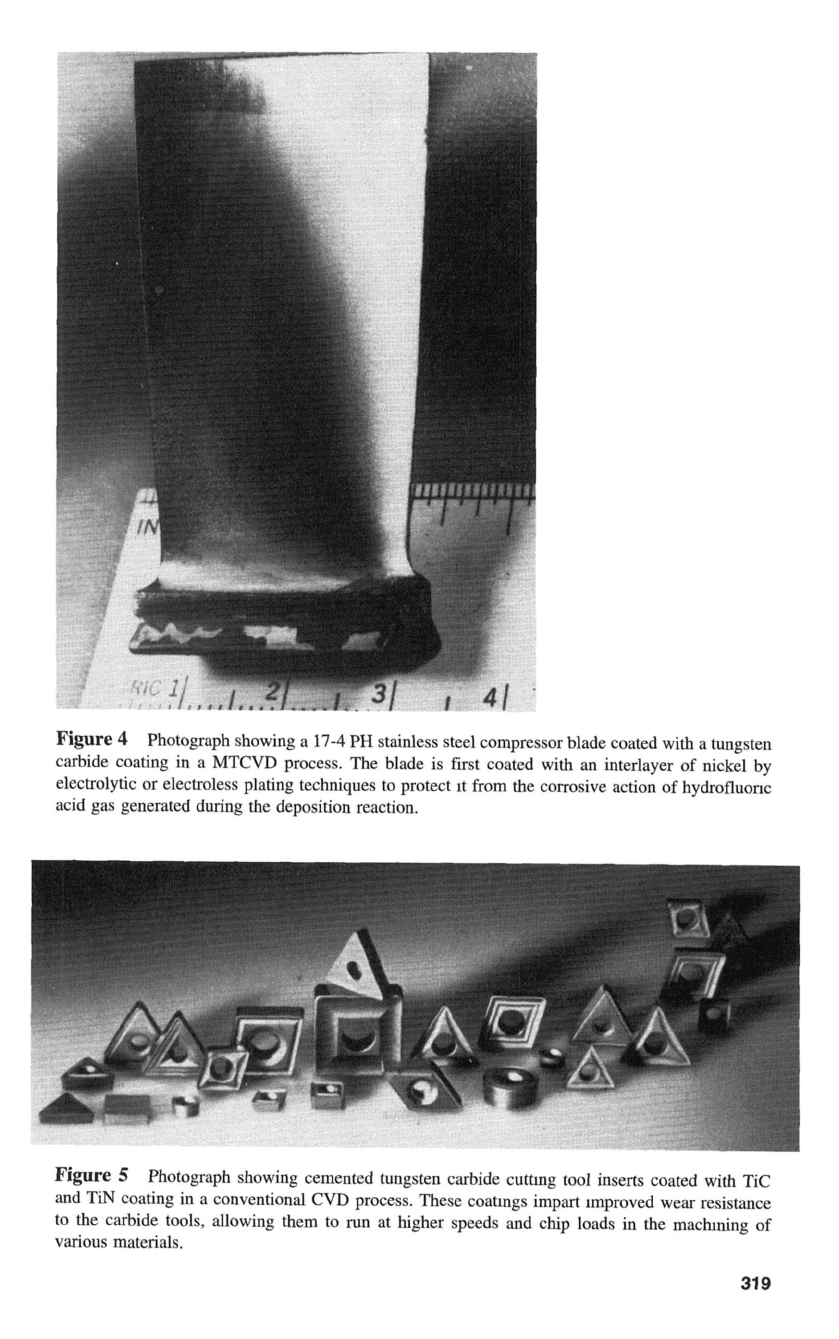
shows an example of a stainless steel compressor blade coated with
a
CVD
tungsten carbide coating by a moderate-temperature CVD process. The component was
electroless nickel plated before CVD.
One of the most widely known and practiced application
of
CVD is in the manufac-
ture of coated cemented carbide cutting tools. These tools are made of tungsten carbide-co-
balt alloys on which a wear-resistant coating
of
a refractory compound is applied by CVD.
The commonly used coatings include Tic, TiN, and A1203, and their combinations. Other
coatings include Ti(C,N), TaC,
HfN,
and ZrN. Figure
5
shows several cemented carbide
cutting tool inserts coated with Tic and TiN.
Another application
of
wear-resistant CVD coatings is in areas involving erosion
and abrasion, such as sand blast nozzles, slurry transport and handling equipment, coal
gasification equipment, and mining equipment. In these applications, air-
or
waterborne
particles of sand, fly ash,
or
other particulate matter traveling at speed and under pressure
can cause abrasion and erosion of surfaces they contact. Conventionally, many of these
surfaces are protected by applying wear-resistant coatings by various means, such as
electroplating, flame
or
plasma spraying, laser cladding, and weld overlay coating tech-
niques. Chemical vapor deposition has also been used successfully in many of the applica-
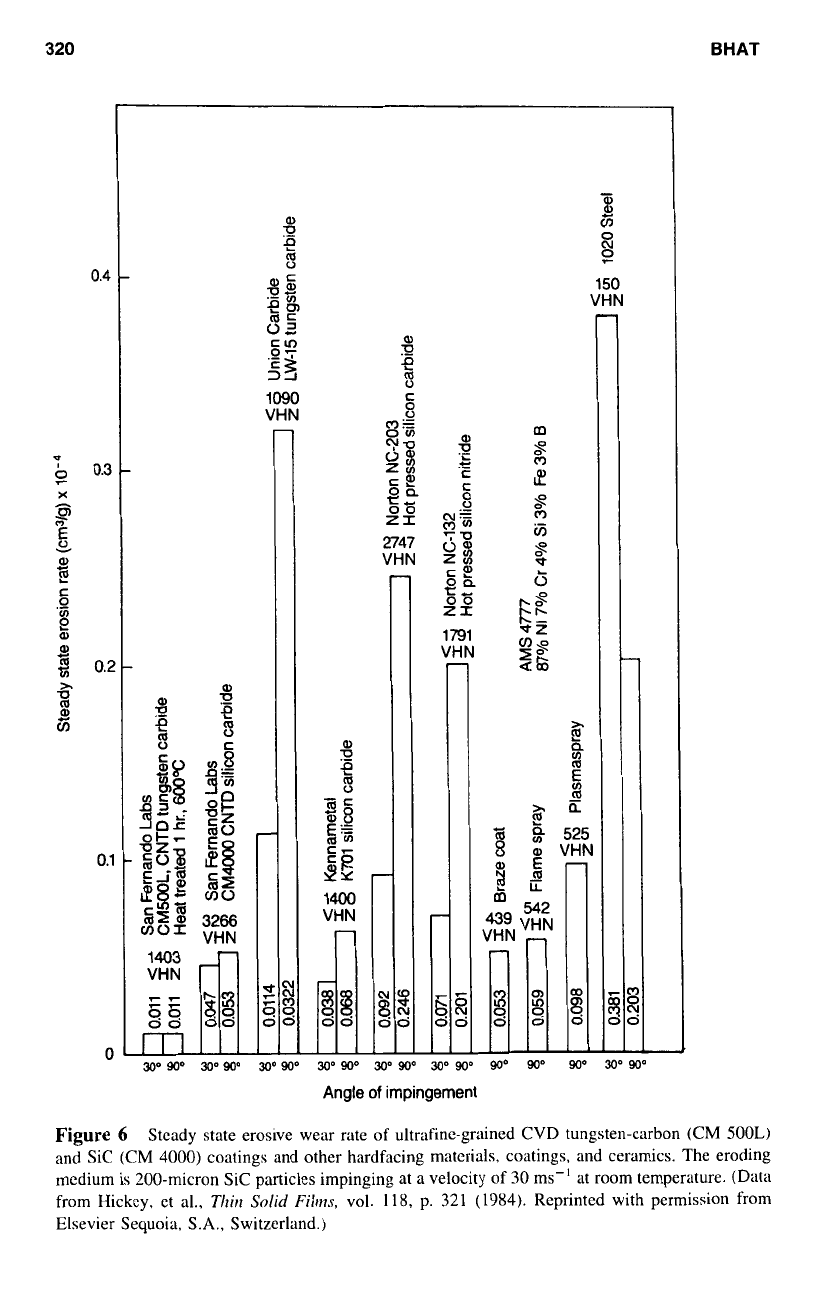
tions. The graph (Fig.
6)
shows the relative wear rates
of
various coatings, other hardfacing
Figure
4
Photograph showing a
17-4
PH
stainless steel compressor blade coated with a tungsten
carbide coating in a MTCVD process. The blade is
first
coated with an interlayer
of
nickel by
electrolytic or electroless plating techniques to protect
It
from the corrosive action
of
hydrofluonc
acid gas generated during the deposition reaction.
Figure
5
Photograph showing cemented tungsten carbide cuttmg tool inserts coated with Tic
and TIN coating in a conventional
CVD
process. These coahngs imp'art Improved wear resistance
to
the carbide tools, allowing them
to
run
at
higher speeds and chip loads in the machlning
of
various materials.
31
9
320
0.4
0
0
'
0.3
X
h
f
v
P
!!
0
c
U)
.-
g
0.2
0
f!
8
a,
VHN
1791
2.
a
U)
m
E
B
5
9
150
VHN
0
(U
00
!
BHAT
Figure
6
Stcady state erosive wear rate
of
ultrafine-grained CVD tungsten-carbon (CM
500L)
and
Sic
(CM
4000)
coatings and other hardfacing materials, coatings, and ceramics. The eroding
medium is '200-micron Sic particles impinging at a velocity of
30
ms" at
room
temperature. (Data
from Hickcy, et al.,
Thrl
Solid
Fibs,
vol.
118,
p.
321
(1984).
Reprinted with permission from
Elsevier Sequoia,
S.A.,
Switzerland.)
CHEMICAL VAPOR DEPOSITION
321
materials and ceramics against sand. indicating that CVD coatings can be successfully
used
in
these applications.
Tribological coatings present another use for CVD coatings: to improve the coeffi-
cient
of
friction between sliding or rolling surfaces
in
contact. thereby reducing wear due
to adhesion. abrasion. or other causes. Typical coatings used
in
these applications include
refractory compounds such
as
carbides. nitrides.
and
borides
of
transition metals. The
important properties of coatings
in
these applications include hardness. elastic modulus.
fracture toughness. adhesion. grain size. and
to
a
certain extent. chemical stability depend-
ing
on
the service environment.
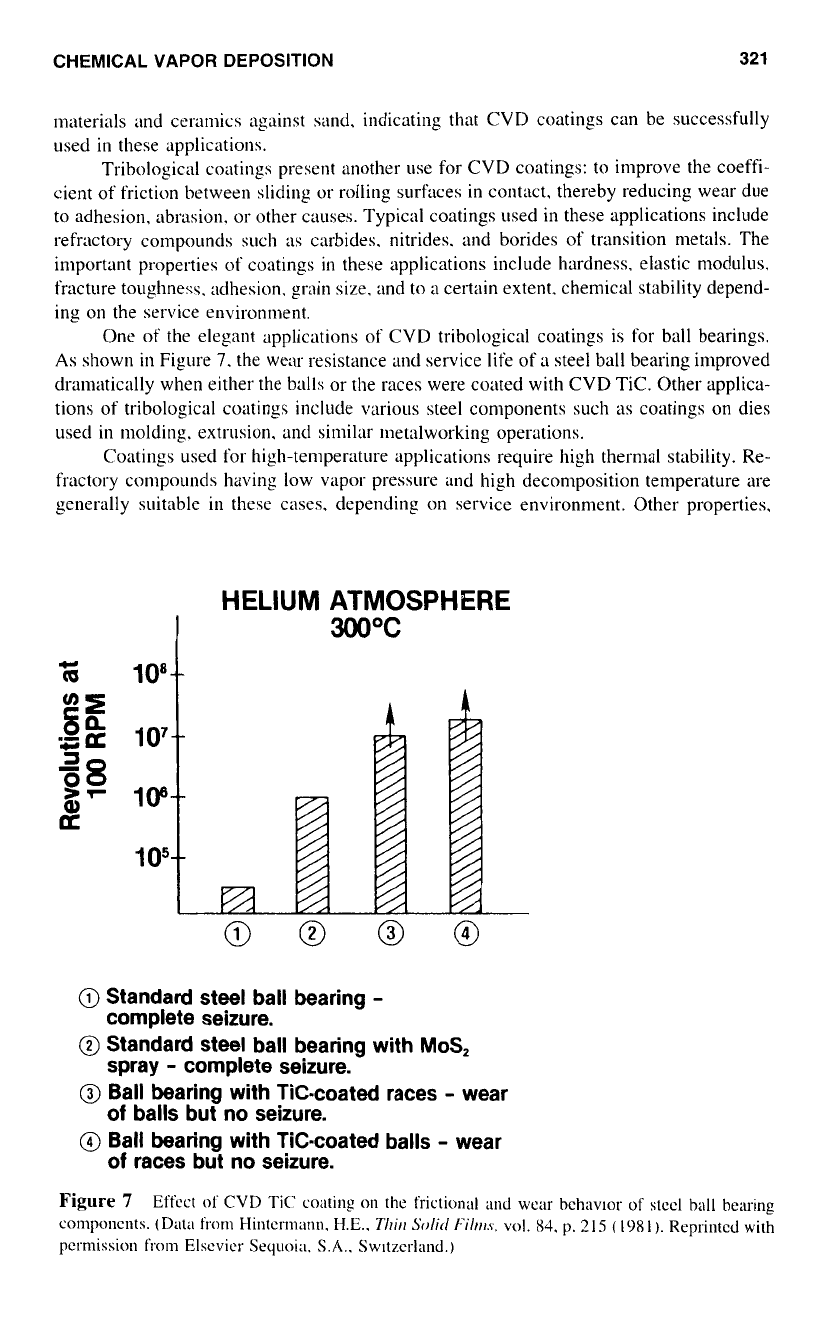
One
of
the elegant applications of CVD tribological coatings is for
ball
bearings.
As
shown
in
Figure
7.
the wear resistance
and
service life of
;I
steel ball bearing improved
dranlatically when either the balls or the races were coated with CVD TIC. Other applica-
tions of tribological coatings include various steel components such
as
coatings on dies
used
in
molding. extrusion. and similar metalworking operations.
Coatings used for high-temperature applications require high thermal stability. Re-
fractory compounds having low vapor pressure and high decomposition temperature are
generally suitable
in
these cases, depending
on
service environment. Other properties.
HELIUM ATMOSPHERE
300°C
c
z
IO8
105
I
4
@
Standard steel ball bearing
-
@
Standard steel ball bearing with
MoS,
@
Ball bearing with Tic-coated races
-
wear
@
Ball bearing with TiC-coated balls
-
wear
complete seizure.
spray
-
complete seizure.
of
balls but
no
seizure.
of
races but no seizure.
