Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.

This Page Intentionally Left Blank
35
Flame
Surface
Treatment
H.
Thomas
Lindland
FIym
Burtrer
Corporntion,
Nen~
Kocl~elle,
Nen'
York
1
.O
INTRODUCTION
The need for surface treatment was recognized shortly after the development of polyolefin
materials, resulting in the evolution of various treatment methods.
W.
H.
Kreidl pioneered
the process of using an oxidizing flame
on
polyolefins to produce a surface receptive to
printing and coating. At the same time, Kreidl's assistant, Kritchever, was developing the
concept of using an electrical corona to produce the same result. The two men went their
separate ways, and both methods have been widely used for the past
30
years for various
surface treating applications.
2.0
SURFACE TREATMENT
The exact mechanism of surface treatment is still unknown. In spite
of
repeated efforts,
using sophisticated instrumentation and complex laboratory methods, we still do not have
an accurate understanding of the process. Fortunately, we do not need this information to
use the process.
Solid surfaces have a surface energy specific for various materials. For a liquid drop
to spread on a given surface, the liquid surface tension must be lower than the critical
surface tension of the solid. Metal and glass exhibit a high surface energy, whereas plastics
have a low surface energy. Pretreatment increases the surface energy and therefore its
wettability. It may also eliminate
a
weak boundary layer, thus improving adhesion.
In tlame treating, the high temperature of the combustion gases causes oxygen mole-
cules
to
become disassociated, forming free, highly chemically active oxygen atoms. In
addition, because of the energy in the high temperature combustion process, oxygen atoms
may also lose electrons to become positively charged oxygen ions. Such an electrically
neutral gas made of equal amounts of positively and negatively charged particles is known
as
a plasma. Plasma may be hot or cold.
343
344
LINDLAND
In flame treating, these high speed, energetic, very reactive oxygen ions or free
oxygen atoms bombard the plastic surface and react with the molecules. This process
oxidizes the surface and requires an oxidizing flame. which is a flame with an excess
of
oxygen.
In corona treating, high voltage fields cause the oxygen molecules to break into free
atoms, which can react with the plastic. Those that do not react with the surface recombine
into molecules
of
normal diatomic oxygen or into unstable molecules of ozone-triatomic
oxygen. In addition, surface treatment involves many other complex reactions.
In many cases, the objective is to treat the surface
to
a predetermined critical surface
tension expressed in dynes per centimeter. ASTM specification
D-278-84
describes a
method of evaluating the level of surface treatment. An increase in surface energy is
usually related
to
an improved adhesion. However, sometimes a substrate may be wettable
and still not provide the desired adhesion level. Flame treatment to a consistent level is
required. This calls for maintaining the operating parameters constant and having
a
uniform
substrate surface.
3.0
BURNERS
Several burner designs are available.
3.1
Atmospheric Burners
In an atmospheric gas burner, part of the air required for combustion is premixed with
the gas prior
to
the point of ignition. The air premixed with gas is referred to as primary
air. The remaining air. called secondary air,
is
drawn from the surrounding atmosphere
at atmospheric pressure; hence the name atmospheric burner. The forerunner
of
the atmo-
spheric burner system is the well-known Bunsen burner.
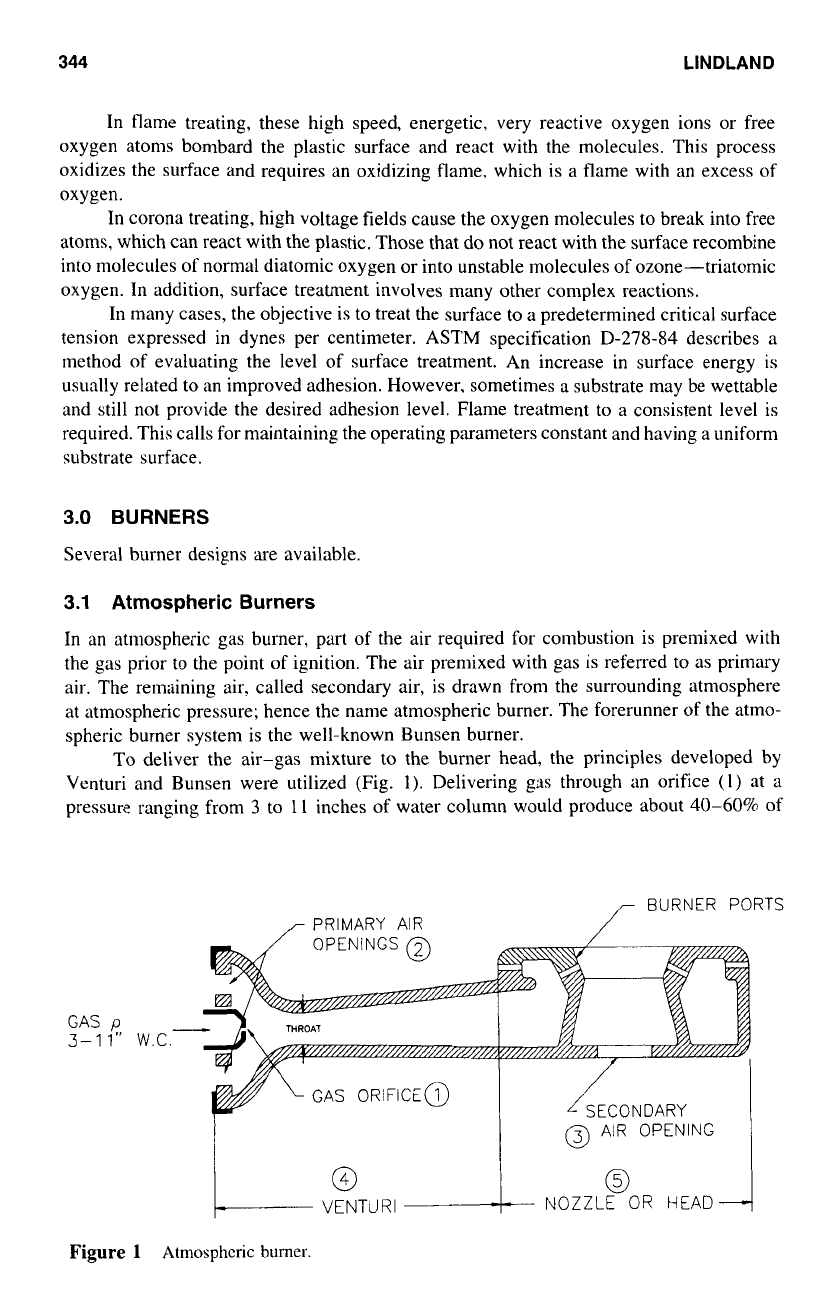
To deliver the air-gas mixture to the burner head, the principles developed by
Venturi and Bunsen were utilized (Fig.
1).
Delivering gas through an orifice
(1)
at
a
pressure ranging from
3
to
11
inches of water column would produce about
40-60%
of
r
PRIMARY AIR
/-
BURNER
GAS
p
3-1
1”
W.C
Figure
1
Atmospheric
burner.
FLAME SURFACE TREATMENT
345
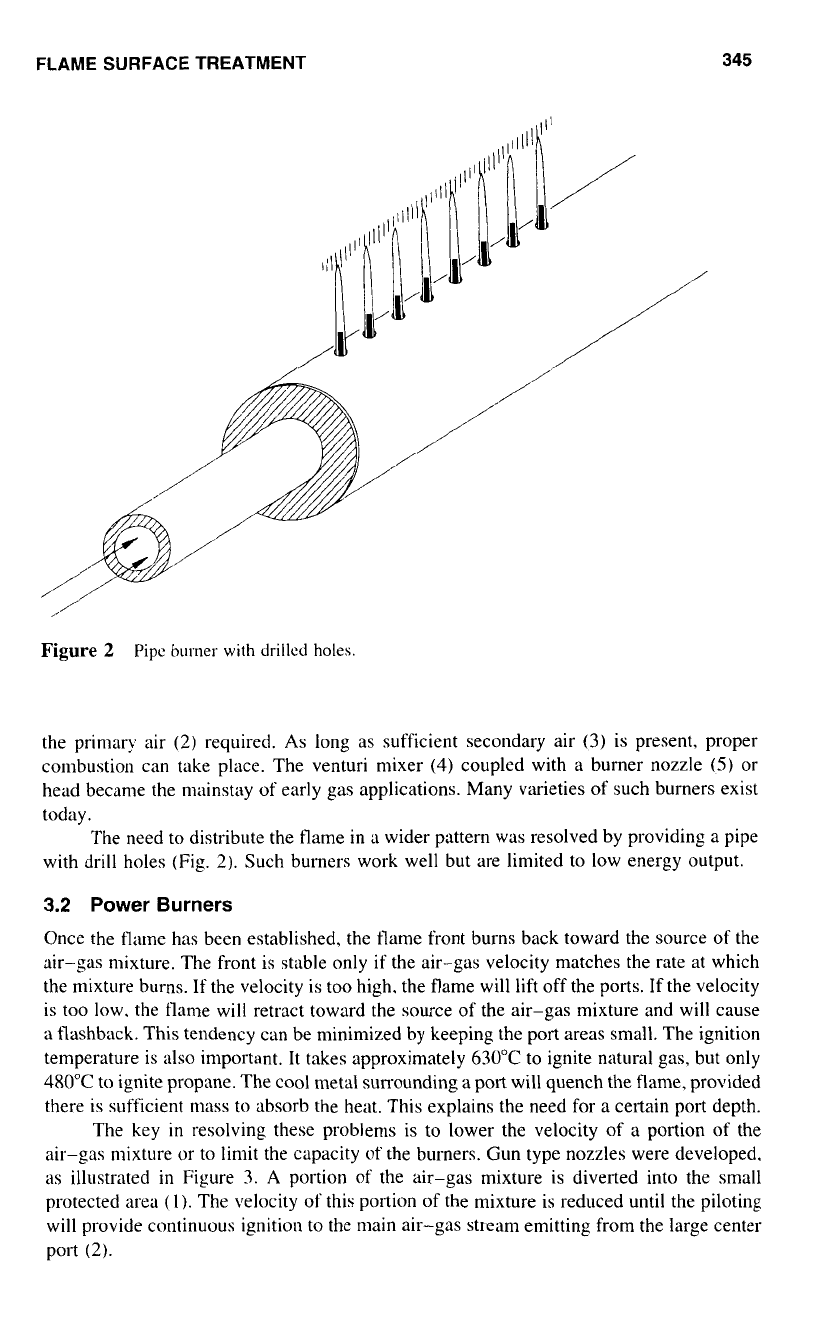
Figure
2
Pipc
burner
with
drillcd
holes
the primary air
(2)
required.
As
long as sufficient secondary air (3) is present. proper
combustion can take place. The venturi mixer
(4)
coupled with a burner nozzle
(5)
or
head became the mainstay
of
early gas applications. Many varieties
of
such burners exist
today.
The need to distribute the llame in
a
wider pattern was resolved by providing a pipe
with drill holes (Fig.
2).
Such burners work well but are limited to low energy output.
3.2
Power Burners
Once the llnme has been established, the tlame front burns back toward the source
of
the
air-gas mixture. The front is stable only
if
the air-gas velocity matches the rate at which
the mixture burns. If the velocity is
too
high. the flame will lift off the ports. If the velocity
is too low, the flame will retract toward the source of the air-gas mixture and will cause
a tlashback. This tendency can be minimized by keeping the port areas small. The ignition
temperature is
also
important. It takes approximately 630°C to ignite natural gas, but only
480°C
to
ignite propane. The
cool
metal surrounding a port will quench the flame, provided
there is sufficient mass to absorb the heat. This explains the need for a certain port depth.
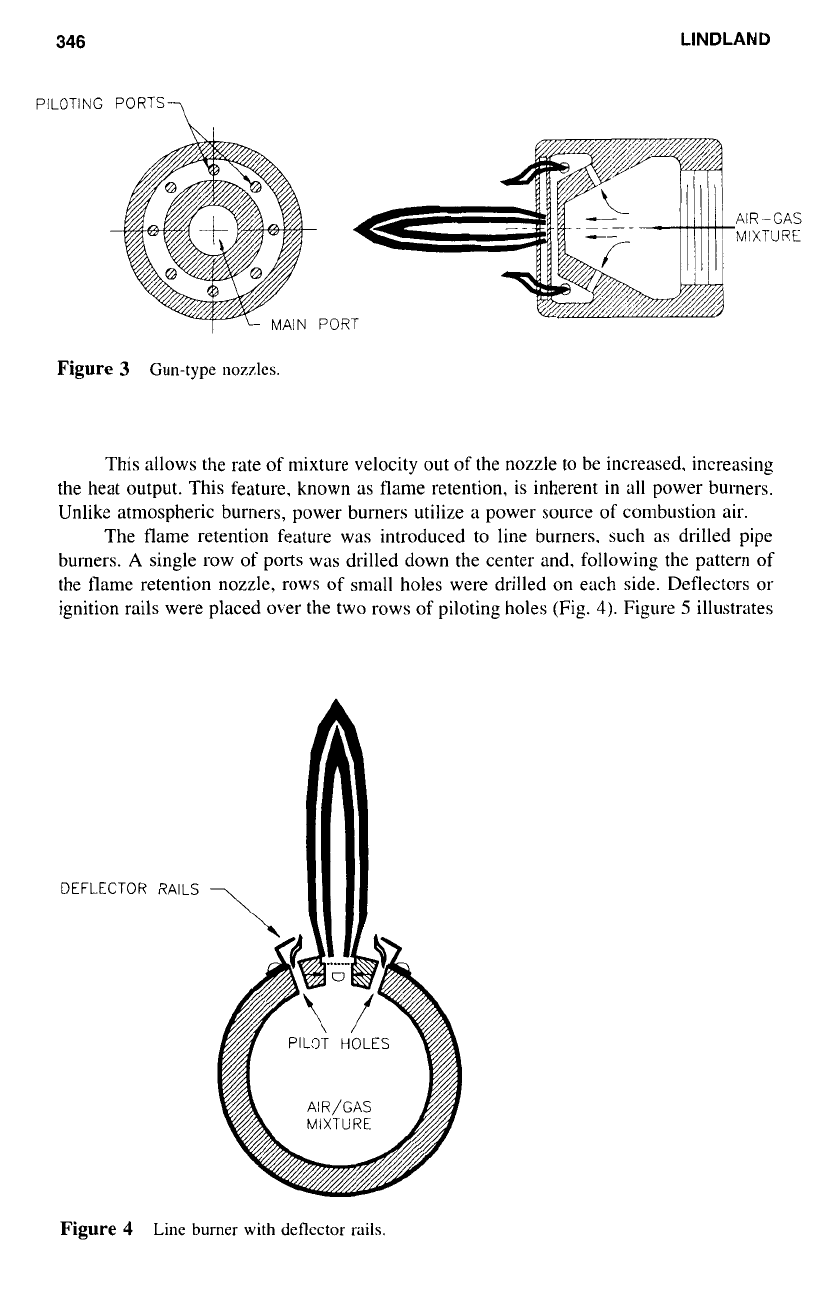
The key in resolving these problems is to lower the velocity of a portion of the
air-gas mixture or to limit the capacity
of
the burners. Gun type nozzles were developed.
as
illustrated in Figure
3.
A
portion
of
the air-gas mixture is diverted into the small
protected area
(I).
The velocity
of
this portion of the mixture is reduced until the piloting
will provide continuous ignition
to
the main air-gas stream emitting from the large center
port
(2).
346
LINDLAND
PILOTING PORTS?
\
POR
T
AIR
MIX
T
Figure 3
Gun-type nozzles.
This allows the rate of mixture velocity out of the nozzle
to
be increased. increasing
the heat output. This feature. known as flame retention, is inherent
in
all power burners.
Unlike atmospheric burners, power burners utilize
a
power source of combustion air.
The flame retention feature was introduced
to
line burners. such as drilled pipe
burners.
A
single row
of
ports was drilled down the center and, following the pattern
of
the flame retention nozzle. rows of small holes were drilled on each side. Deflectors
or
ignition rails were placed over the two rows
of
piloting holes (Fig.
4).
Figure
5
illustrates
-GAS
URE
DEFLECTOR
I
?AILS
Figure
4
Line
burner
with
deflector
rails.
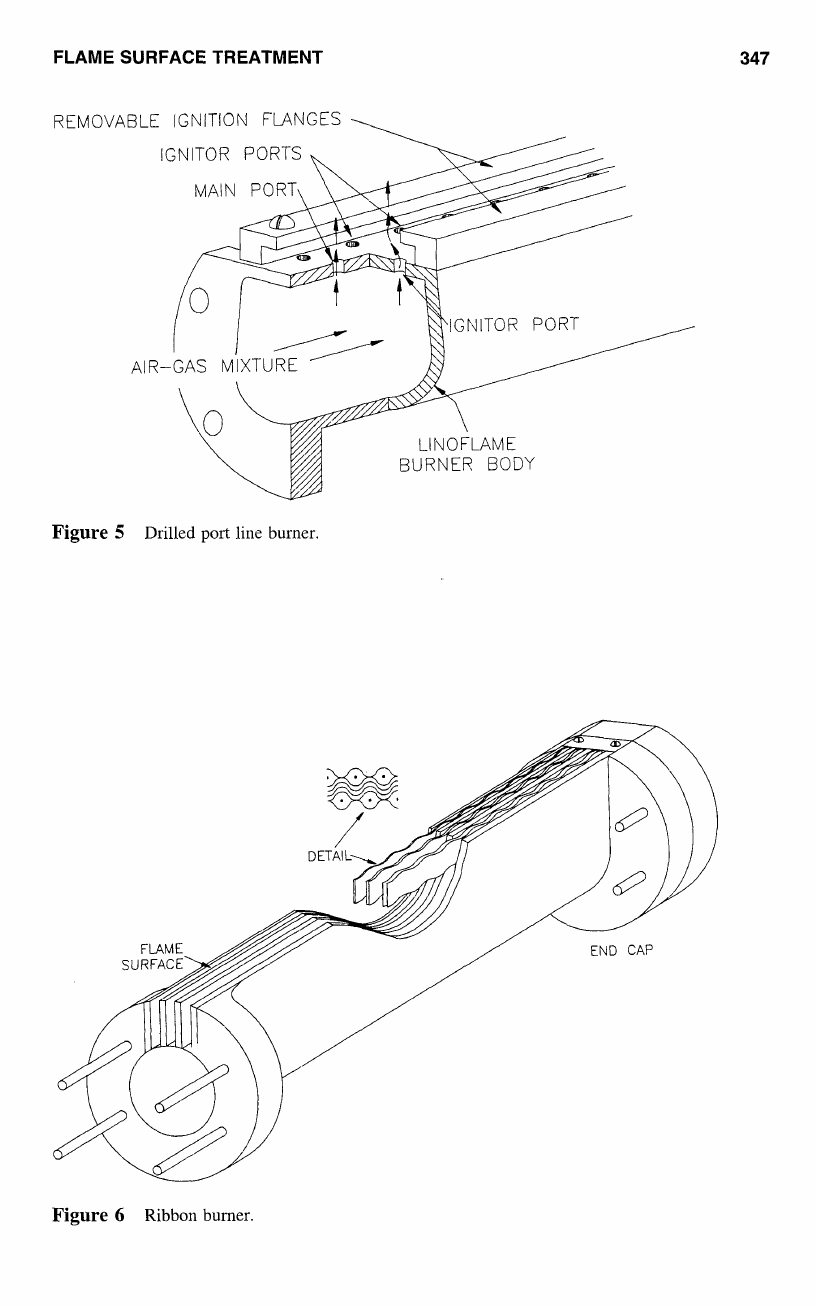
FLAME SURFACE TREATMENT
REMOVABLE IGNITION FLANGES
IGNITOR PORTS
MAIN PORT\
Figure
5
Drilled
port
line
burner.
347
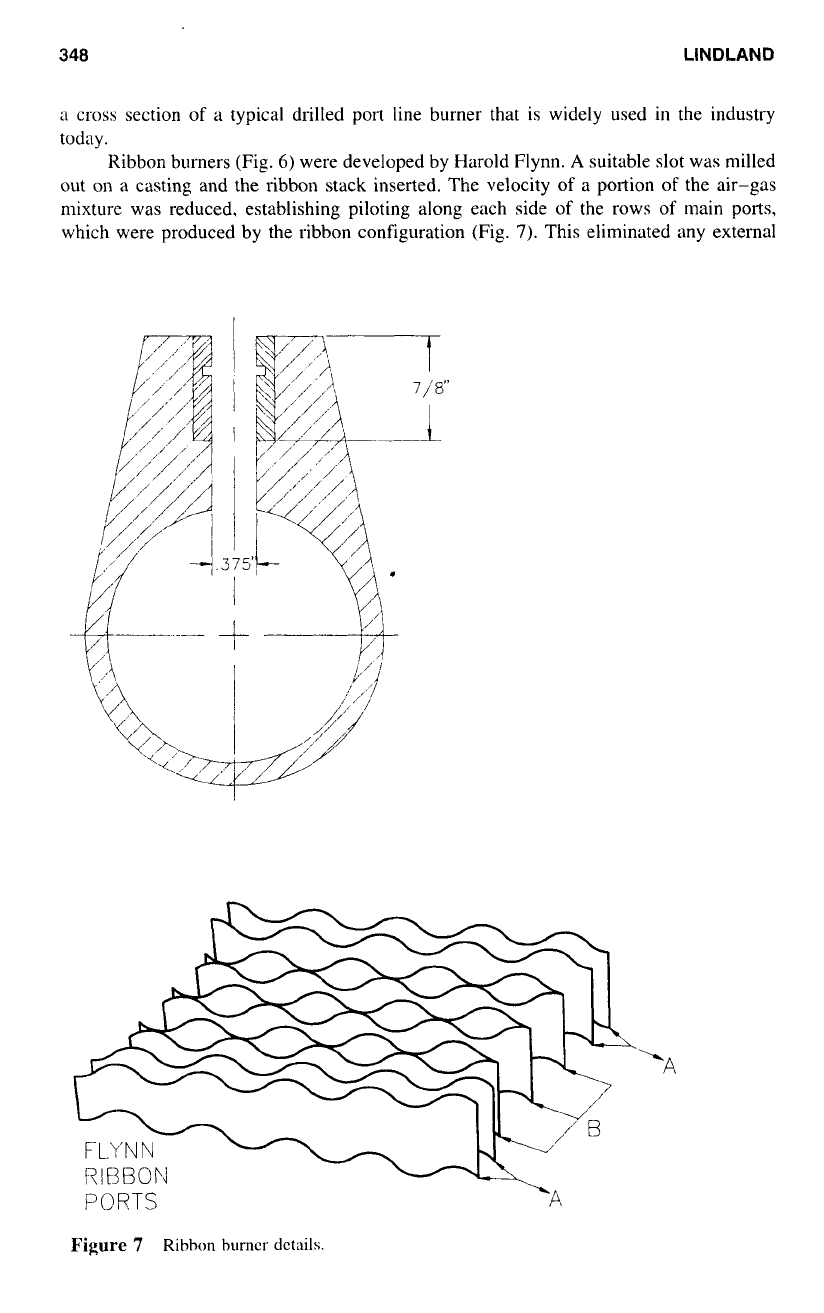
Figure
6
Ribbon burner.
348
LINDLAND
a
cross section
of
a
typical drilled port line burner that is widely used
in
the industry
today.
Ribbon burners (Fig.
6)
were developed by Harold Flynn.
A
suitable
slot
was milled
out
on
a casting and the ribbon stack inserted. The velocity
of
a portion
of
the air-gas
mixture was reduced, establishing piloting along each side
of
the rows
of
main ports,
which were produced by the ribbon configuration (Fig.
7).
This eliminated any external
Figure 7
Ribbon
bumcr
dctails.
FLAME SURFACE TREATMENT
349
deflectors or rails. Further refinements of ribbon line burners have made it possible
to
manufacture various slot widths and ribbon configurations
to
produce a variety
of
flame
patterns.
4.0
FILM TREATMENT
One
of
the problems associated with the attempt to utilize line burners for the treatment
of
polyolefin films was the requirement of increasing the flame velocity enough to penetrate
FLYNN
SMOOTH FLAME
THREE SLOT
BURNER
ASSEMBLIES
SERIES
WC-3800
WATER COOLED
WATER COOLED
THREE SLOT
FLYNN SMOOTH FLAME
RIBBON BURNER
Natural Manufactured Mixed
Use
with
all Gases
Propane
Butane
I
END VIEW
'
CATALOG
No.
SHIPPING WEIGHT
BTU
/
HR
'B'
OA.
LENGTH "A'
FLAME
SPACE
WC-3800-60 132
Ibs
900,000
62 7/6
59
3/4"
WC-3801-72
176
Ibs
1,200,000
83
3/8"
80
1/4*
WC-3802-80
155
Ibs
1,080.000
74 7/a
71 3/4.
WC-3805-84
I
84
3/6
I
87 1/T
1
1.260.000
I
203
Ibs
WC-5804-48
105
Ibs
720,000
50 3/4-
47
5/6
Figure
8
Type
3800
water-cooled
smooth flame
burner.

350
LINDLAND
4
PORT
RIBBON
PATTERN
Figure
9
Details
of
four-port
rihhon
hurncr.
the boundary air layer on the web surface. To treat the substrate with direct flame. sufficient
thermal energy had
to
be developed
to
penetrate this layer. Furthermore. the exit velocity
of the burner flame had to be adjusted
to
varying web speeds.
The principle
of
a burner designer to meet these requirements is shown
in
Figure
X.
The ribbon channels of piloting or ignition tlames firing
at
reduced exit velocities
provide a constant supply of ignition to the center ribbon channel. enabling
it
to
fire at
exit velocities far
in
excess of the normal speed
of
flame propagation. greatly increasing
the energy
output
of the burner.
Figure
9
shows a burner designed for film posttreatment application. It has a single
slot due to the lower energy output requirement. The water cooling is built into the face.
The side bars assure a smooth flame, which is required for the posttreatment process.
The capacity, tlame velocity. and flame retention capability are all related to the ribbon
configuration. By varying the number
of
ports and the width of the ribbon stack, it is
possible
to
produce a customized flame pattern for each application.

36
Plasma Surface Treatment
Stephen L. Kaplan and Peter
W.
Rose
Pltrsrlltr
Scierlce.
h(..,
Belrtlorlr.
Ctt/;/orrlitr
1
.O
INTRODUCTION
Gas
plasmas make up
99%
of our universe, existing mainly as stars. Although rare on
earth, natural plasmas include lightning, the aurora borealis, and St. Elmo's fire. Table
1
lists certain plasmas and characterizes them by particle density and temperature.
Plasmas can be produced and controlled by ionizing a gas with an electromagnetic
field of sufficient power. One useful form
of
gas plasma is made by introducing gas into
a reaction chamber. maintaining pressure between
0.1
and
IO
torr, and then applying radio
frequency (rf) energy. Once ionized. excited gas species react with surface
of
materials
placed
in
the glow discharge.
The physical and chemical properties of plasmas depend on many variables: chemis-
try, flow rate, distribution. temperature. and pressure of the gases. Additionally, rf excita-
tion frequency. power level, reactor geometry, and electrode design are equally important.
Dissociated gas molecules quickly recombine to their natural state when the plasma's
power source is shut off.
2.0
TYPES
OF
PLASMA
Plasmas occur over
a
wide range of temperature and pressures, however
all
plasmas have
approximately equal concentrations
of
positive and negative charge carriers,
so
that their
net space charge approaches zero.
In general. all plasmas fall into one of three classifications. Elements of high-pressure
plasmas. also called
l~orylannns,
are in thermal equilibrium (often at energies
>
10,OOO"C).
Examples' illustrated in Table
1
include stellar interiors and thermonuclear plasmas.
Mixed
p/cr.st?~ns
have high temperature electrons in midtemperature gas
(-
100-1000°C)
and are
formed at atmospheric pressures. Arc welders and corona surface treatment systems use
351
