Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.


362 MILKER
AND
KOCH
series-connected flow resistors
(12)
prevent, to a great extent. the escape of tluorine and
hydrogen fluoride (HF) into the ambient air. Such a system
of
airlocks is described in
details
in
Ref.
S.
From pipe
9
and via dosing valve
10,
the escaped portion of tluorine is
replaced, but a small quantity of it is
lost
as HF by virtue of the chemical conversion with
the reactant. Since a partial fluorination causes surface activation, which is sufficient for
many purposes, the quantity
of
fluorine actually consunled or the resulting quantity of HF
is rather small.
The low portions of HF in the system are removed by means of a hydrogen fluoride
absorber
(13
in Fig.
1).
Such a cleaning is also necessary
if
the fluorine is to be dosed by
a glass rotameter. The absorber comprises a pipe made of Monel. nickel, or steel (diameter,
50
mm; length,
450
mm), and filled with granular, porous sodium fluoride. Welded to
both sides of the pipe are caps that carry supply and drain pipes. The absorber can be
heated up to
300°C
by means of an electric tube furnace. The HF absorption ensues at
room temperature. For regeneration, the absorber is heated
in
the nitrogen flow to
+
300°C.
A
suitable porous sodium fluoride can be produced by heating grained or pelleted sodium
bifluoride to
250-300°C
in a nitrogen flow.
If
required, the entire system can be rinsed via a gas washer: the
F7
and HF portions
in the rinsing gas are absorbed
in
counterflowing diluted potash lye and are made innocu-
ous. The exhaust air is completely free from pollutants.
2.3
Safety Precautions
The hazard associated with the gases fluorine and hydrogen fluoride being delivered
through the airlock to the open air is comparable to the one caused by ozone released.
which occurs inevitably as a result of the corona pretreatment. The safety measures known
and approved for ozone apply also to fluorine and hydrogen fluoride. For these gases, a
threshold limit value of
0.1
ppm in air was fixed, as for ozone. Biological assays have
shown, that the toxicity of fluorine and hydrogen fluoride is many times less than that of
ozone.
Since fluorine is perceptible by its smell rather like heavily chlorinated water, even
in
rather low concentrations, cases of poisoning are extremely rare.
On-the-job safety is safeguarded by installing a chemical detector giving an acoustic
alarm and interrupting the fluorine supply if the threshold contents
of
fluorine admitted
to the air is exceeded.
3.0
PRETREATMENT
WITH
FLUORINE: APPLICATION EXAMPLES
Extensive serial tests on a pilot plant as described in Section
2.2
illustrate that:
A
concentration of
5-
10
vol% fluorine in the plenum chamber is fully sufficient to
raise the surface energy of the polymer above
S0
mN/m.
Average fluorine consumption is only
2.5
kg for the pretreatment of approximately
100,000
m2
of polymer surface. (The fluorine costs are thus practically negligible.)
All
tests described below were performed with a working width of
1
m; the reaction
chamber to which the fluorine-nitrogen was admitted had a length of
1
m and a height
of
30
cm. for a volume of
300
liters.

SURFACE PRETREATMENT
OF
POLYMER WEBS BY FLUORINE
363
Table
1
Adhesive Bonding Versus Pretreatment
Foamed plastic Surface tension (mN/m) Pebra test" Force test' (N/62S mm')
~~~~~ ~
Not
pretreated
<3o
3
sec 99
Corona pretreated
so
>2 min 129- 149
Fluorine pretreated
S4
>2 min
153
3.1
Polyethylene-Vinyl Acetate Copolymer Foam
A closed-cell foam,
1
mm thick, is
to
be pretreated on both sides at a web speed of
S
m/
min.
After having passed the fluorine atmosphere, the surface tension of the foam material
increases from 30 mN/m to 54 mN/m. If the foam material is used as, for example,
supporting material for double-sided, pressure-sensitive adhesive tapes, the adhesive bond
is
as
good as with the corona-pretreated material. This is shown in Table
1.
Electron spectroscopy for chemical analysis (ESCA) researchX indicates that activa-
tion of the foam selected in this example is predominantly based on the partial fluorination
of the terminal methyl group of the comonomeric vinyl acetate. As with the corona pretreat-
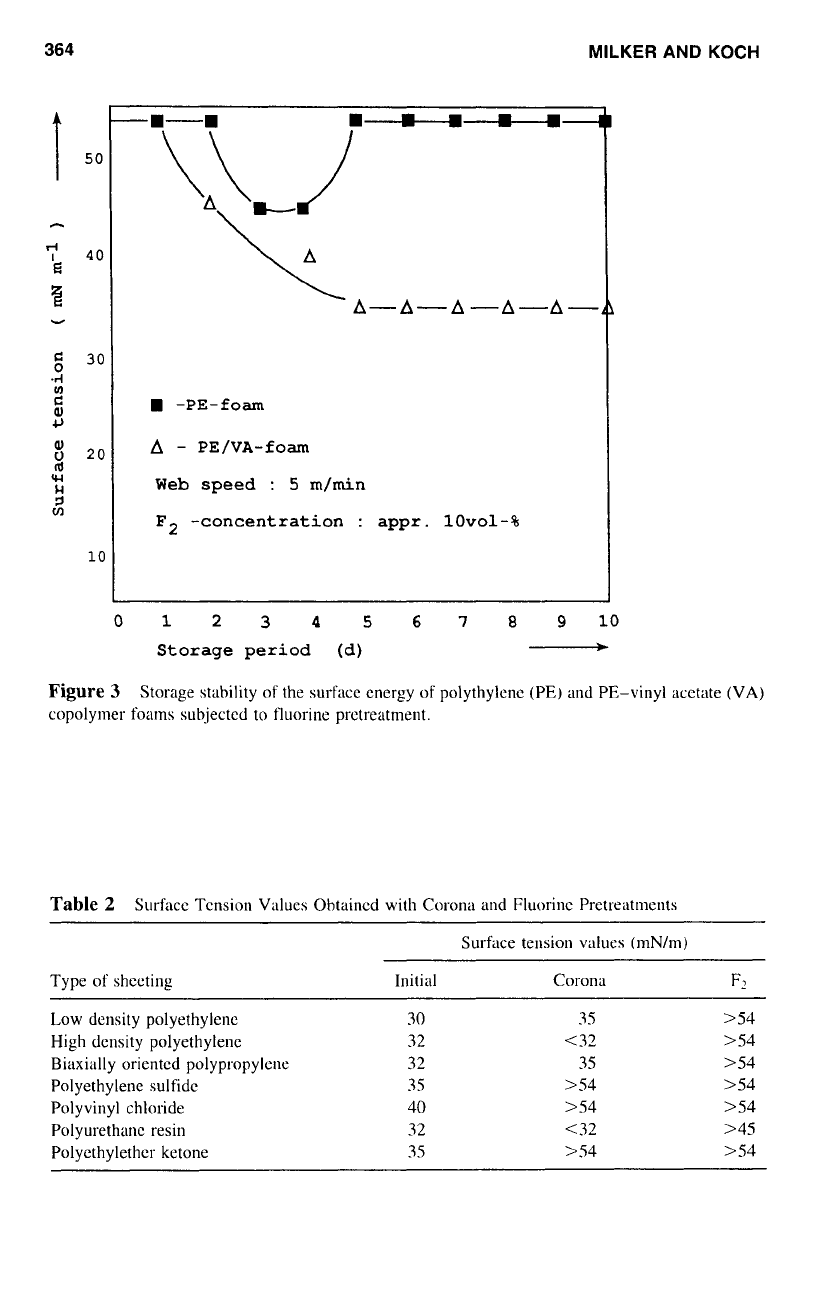
ment, the surface tension degrades gradually, but
it
stabilizes on a higher level, as indicated
in Figure 3. Pure polyethylene foam, however, maintains for weeks the conferred surface
tension of more than S0 mN/m.
3.2
Plastic Sheeting
With the maximum web speed
of
24
mhin
(F2
concentration
S
vel%)
attained
in
the pilot
plant, common film or sheeting qualities of differing widths were successfully pretreated
on one or two sides,
as
shown
in
Table 2. The pretreatment effect remained constantly
high throughout an observation period of
6
weeks.
3.3
Air Cushion Sheeting
For certain applications an adhesive agent coating that is applied on the burl side has to
be firmly bonded to the surface of the sheeting or film, even
in
the interstices. Here. the
fluorination technique is in fact predestined
to
be a solution for problems. As expected, such
a polyethylene air cushion sheeting or film can be pretreated as well as other polyethylene
sheetings.
3.4
Terpolymer Rubber
Ethylene propylene rubber monomer (EPDM) belongs to the materials that will be used
to
an ever-increasing extent in the future-in particular in the automobile industry.
The negative side of this versatile, almost indestructible polymer body is its obstinate
pretreatment behavior.
So,
after the conveyance
of
EPDM sectional cuts
on
an endless
belt through the fluorination chamber, only 45 mN/m is measured. This surface energy,
however, guarantees satisfactory substrate adherence.
MILKER
AND
KOCH
-m". m"+
-PE-fo=
A
-
PE/VA-fo=
Web
speed
:
5
m/rnin
F2
-concentration
:
appr.
lOvol-8
012345678910
Storage period (d)
___)
Figure
3
Storage stability of the surface energy
of
polythylcne
(PE)
and PE-vinyl acetate
(VA)
copolymer
foams
subjected to fluorine pretreatment.
Table
2
Surface Tension Values Obtaincd with Corona and Fluorine Pretreatments
Surface tension values
(mN/m)
Type
of
sheeting Initial Corona
F?
Low density polyethylene
30
35
High density polyethylenc
32
<32
Biaxially oriented polypropylene
32
35
Polyethylene sulfide
35
>S4
Polyvinyl chloride
40
>S4
Polyurethane resin
32
<32
Polyethylether ketone
35
>S4
>54
>54
>S4
>54
>54
>45
>S4

SURFACE PRETREATMENT
OF
POLYMER WEBS BY FLUORINE
365
4.0
ADVANTAGES OF SURFACE PRETREATMENT WITH FLUORINE
Compared with other pretreatment processes, the fluorination method not only features
a
wide spectrum of applications but unlike corona pretreatment and ozonization, it does not
require electrical apparatus, which is susceptible
to
interference and requires intensive
maintenance. Additionally, the basic investment for fluorine fittings (discharge station)
and for
a
reaction chamber is lower than the prime cost of
a
corona pretreatment station.
A particular advantage of continuous fluorination can be seen in the application
to web-shaped bodies, which-when wound in
a
compact roll-are not accessible for
fluorination by present state-of-the-art means. Such materials can be continuously surface
activated now from roll to roll, independent
of
roll length.
Another very important argument for the fluorination technique is the fact that the
surface treatment effect, unlike corona pretreatment, is long-lasting-at best, irreversi-
ble-on both sides. This is of enormous importance for practical applications in industry,
since subsequent surface refining processes need not follow immediately the surface activa-
tion.
Furthermore, the fluorination process provides effective pretreatment of the follow-
ing materials:
Burled, embossed, or otherwise irregularly formed surfaces of sheetings or films
Narrow fabrics
Foams wider than
2
mm
Biaxially stretched polypropylene sheeting of any width
5.0
SUMMARY
Now that fluorination technique has become controllable, it offers promising and beneficial
prospects for the pretreatment of polymeric, web-shaped materials. It cannot and will not
displace corona pretreatment, which has proved its usefulness for decades, but is surely
an attractive solution for
a
number of special applications.
REFERENCES
1.
R. Milker,
Coaling,
11,
294-298 (1985).
2.
EP
0 214 635.
3.
KC-Fluor, Kali-Chemie AG.
4.
G
87 03 823.4.
5.
DOS
30-38-741.
6.
Test instruction AW
14,
Pebra.
7.
Test instruction
0400,
Lohmann GmbH, Neuwied, West Germany.
8.
Dr. Ruppert, Chemical Institute
of
the University
of
Bonn, personal communication.
This Page Intentionally Left Blank

Calendering
of
Magnetic Media
1
.O
INTRODUCTION
Calendering is
a
continuous web process: two or more cylindrical rolls are nipped together
under mechanical load and driven via
a
system motor. This chapter discusses the calen-
dering
of
coatings in the manufacture of magnetic media products.
2.0
CALENDERING MAGNETIC MEDIA PRODUCTS
In
the magnetic tape industry, the calendering process, relates to improving performance
of the manufactured product. Improvements to the surface
of
the applied coatings
on
the
substrate, usually polyester film, can be varied depending
on
the end use of the magnetic
media product; they include:
Improved surface finish
Densified coating
Improved aesthetics
Reduced product thickness
Improved product electrical output
Reduced recorder head wear
Promotion
of
coating durability
Some of the product improvements listed are interrelated and can be optimized.
As
an example, improvement of the surface finish will be achieved by the calendering process
and the coating will be densified. Iron oxide in the coating binder system must be main-
tained at the coating surface and
not
caused
to
migrate toward the base film. The surface
can become very smooth
if
a
percentage of binder system is caused to migrate to the
coating surface, displacing the iron oxide particles away from the eventual recording head.
367

368 MCCLENATHAN
Figure
1
Nip
definition.
The condition of “too smooth” can also be achieved, and the lack of surface boundary
lubrication air will allow contact between the tape and recording head, resulting in exces-
sive drag on the tape.
The surface conditions required
on
the magnetic media products are critical. The
hydrodynamic lift clearances are
5-10
microinches (pin.) (0.12-2.5 pm),
as
compared
to a coated surface roughness (after calendering) of 0.6-1 pin. (0.014-0.025 pm) for the
magnetic media products. To achieve this smooth finish, the coating to be calendered is
placed
in
contact with the polished surface of
a
steel hot roll. The basic nip area, as shown
in Figure
1,
is the physical contact area between two rolls. One roll is the compliant roll
and has a lower modulus
of
elasticity than the second roll, which is usually steel. Calen-
dering results from the process, which consists of a controlled nip pressure and controlled
heat applied to the product. Optimization
of
these two basic parameters will, in most cases,
cause product quality improvements.
The most noted factors
to
be controlled during calendering are
as
follows.
1.
Variable and controlled nip pressure.
The calenders are usually hydraulically
loaded to achieve loadings from 500
to
4000
PLI,
where
PLI
is pounds per inch
of roll face, (89-714 kgkm).
2.
Steel (or kor) roll surfcrcefinish.
Roll surfaces are hardened and chrome plated
to provide optically perfect surfaces
of
better than 0.5 pin. (0.012 pm).
3.
Temperufure
sf
the product.
The temperature of the hot steel roll and the com-
pliant roll surfaces must be controlled. The steel roll is heated internally by
water, oil, or electricity and controlled to
?
1°C.
4.
Cleanliness.
Compliant rolls must not be a source of particle contaminants. Any
foreign particles or dirt will mark the product when passed through the high
pressure nip area.
5.
Rolls
must
be ofgoodphysical design.
Deflection must be minimum. Roll runout
of
0.001
in. (0.025 mm) TIR
or
better must be maintained. The face surfaces
on
all
rolls must be straight. Tolerances depend on the compliant roll material’s
ability to conform to the steel
roll
surface.
6.
Cornpliant
roll.
A variety of compliant roll materials with different surface
finishes and hardnesses are available and include the following: paper filled-cot-
tons, polyester, nylon, polyimide, rubber, steel, and epoxy-glass. The hardness
range of most of the compliant rolls (excluding steel) that are used is 80-90
Duro Durometer “D.”
CALENDERING
OF
MAGNETIC MEDIA
369
7.
Tenlperuture.
Control of the web temperature during passage through the calen-
der must be held at an acceptable level
to
provide plasticity
to
the coating binder
system without distorting the base film.
Roll
diameters, number of nips, web
speed, web thickness, and the web wrap angles on the rolls provide controllable
variables for calendering success.
3.0
CALENDER
DESIGN
Calender configurations vary from
a
simple two-roll configuration to a multiroll stack of
eleven rolls. Unsupported compliant rolls require larger diameters
to
provide high nip
loading capability. This results
in
the most common arrangement, featuring the two external
rolls made of steel (not compliant) to provide the required nip stiffness. This concept
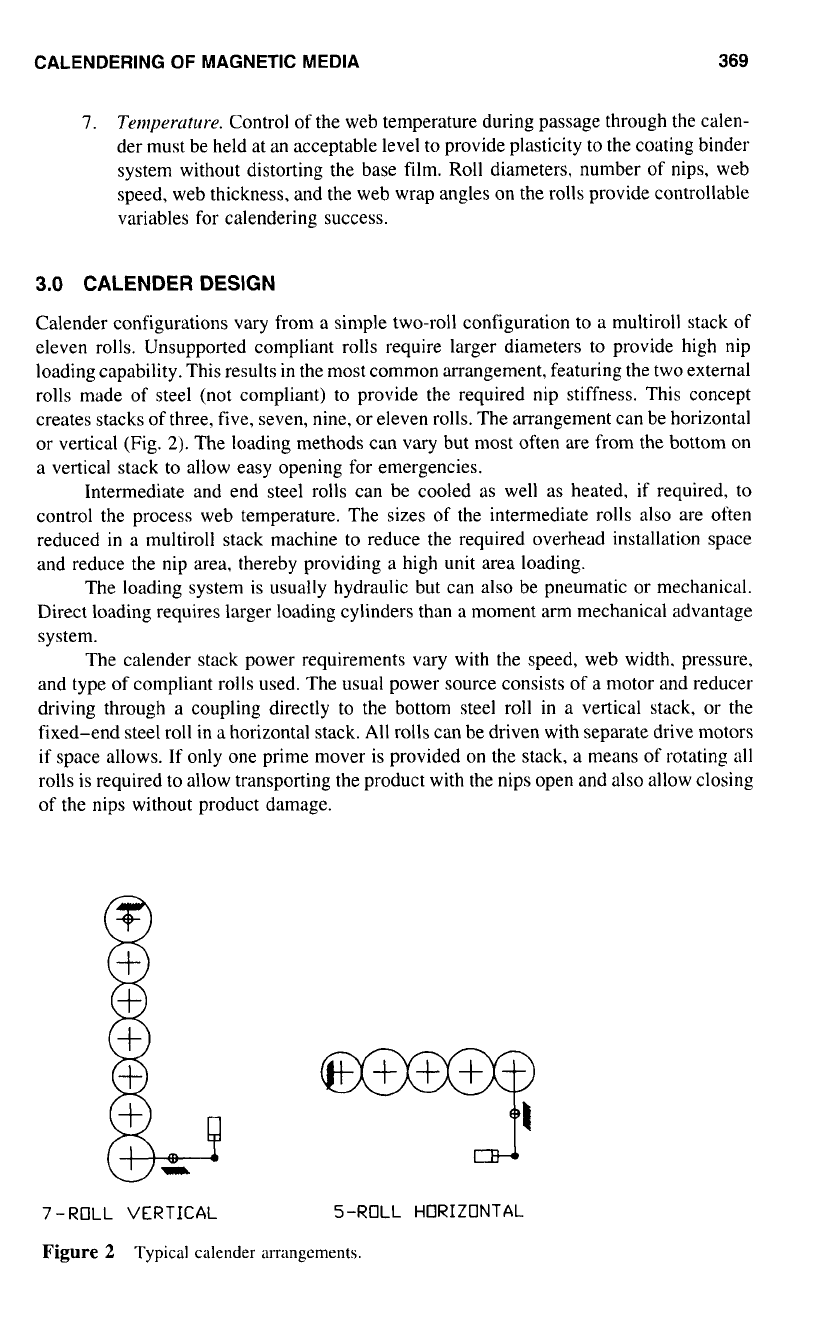
creates stacks of three. five, seven, nine, or eleven rolls. The arrangement can be horizontal
or
vertical (Fig.
2).
The loading methods can vary but most often are from the bottom on
a
vertical stack
to
allow easy opening for emergencies.
Intermediate and end steel rolls can be cooled
as
well
as
heated,
if
required,
to
control the process web temperature. The sizes of the intermediate rolls
also
are often
reduced in
a
multiroll stack machine
to
reduce the required overhead installation space
and reduce the nip area, thereby providing
a
high unit area loading.
The loading system is usually hydraulic but can
also
be pneumatic
or
mechanical.
Direct loading requires larger loading cylinders than
a
moment arm mechanical advantage
system.
The calender stack power requirements vary with the speed, web width, pressure,
and type of compliant rolls used. The usual power source consists of
a
motor and reducer
driving through
a
coupling directly
to
the bottom steel
roll
in
a
vertical stack,
or
the
fixed-end steel roll in
a
horizontal stack.
All
rolls
can be driven with separate drive motors
if space allows. If only one prime mover is provided on the stack,
a
means of rotating
all
rolls is required to allow transporting the product with the nips open and
also
allow closing
of
the nips without product damage.
7
-ROLL VERTICAL 5-ROLL HORIZONTAL
Figure
2
Typical calender arrangements.

370
MCCLENATHAN
Calenders are provided with emergency stop cables, nip guards,
and
drive stop
interlocks
to
open all nips.
REFERENCES
1.
J.
J.
Brondijk, P.
E.
Lvierenga,
E.
E.
Feekes, and
W.
J.
J.
M. Sprangers, “Roughness and
deformation aspects in calendering
of
particulate magnetic tape,”
IEEE
Trtrns.
Mngn.
MAG,
23
l),
January
1987.
2.
Alex
N.
Tabenkin, “The growing importance of surface finish specs,”
Mochitze
Design,
Sept.
20,
1984.
3.
J.
A. McClenathan,
The
Design
ofa
Magnetic
Tup
Cderzder,
Birmingham, AL: IMD, Feb.
4.
Harold Bredin, “Tribology and the design of magnetic storage system.”
Meck.
Eq.,
March
5.
A. J. Holloway, “Polyester film surface definition and control,” presented at the Symposium
17, 1970.
1985.
of
Magnetic Media Manufacturing Methods, Hawaii, May
1983.

Embossing
1
.O
GENERAL
Embossing is a method by which a web is textured by the use of a pattern roll pressing
against a backup roll under controlled conditions. One can emboss both thermoplastic and
nonthermoplastic webs by choosing the proper roll arrangement to deform
the
web.
To emboss thermoplastic materials, the web is deformed by preheating and pressing
it
with a cooled embossing (pattern) roll to set the pattern and cool the web to retain that
pattern.
The degree
of
preheating to soften the web must be carefully controlled
so
that no
melting or degrading
of
the web will take place. To make the heat removal process as
efficient as possible,
no
more heat should be applied than is needed to satisfactorily emboss
the product.
To emboss
a
nonthermoplastic web such as paper, textile, or
foil,
one must apply
pressure that exceeds the elastic limit
of
the substrate and imparts the pattern. This type
of embossing involves the use of male and female rolls. either two rolls with matched
patterns made
of
steel or other metal, or a steel pattern roll that comes in contact with a
filled backup roll, which takes
a
permanent deformation for a given pattern by running
the steel etnbossing roll
in
contact with the backup roll during a “running
in”
period. In
some cases, special rubber-covered rolls can be used; their behavior eliminates the need
for ”running in.”
2.0
THERMOPLASTIC WEBS
Embossing thermoplastic webs is achieved by using an engraved metal roll pressing against
a rubber-covered backup roll. The metal roll is cooled with a refrigerated solution to
remove heat from the product and to set
in
the embossed pattern. The backup roll is
internally cooled, mainly to increase the life
of
the rubber covering. The roll may also be
371
