Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.

352
KAPLAN
AND
ROSE
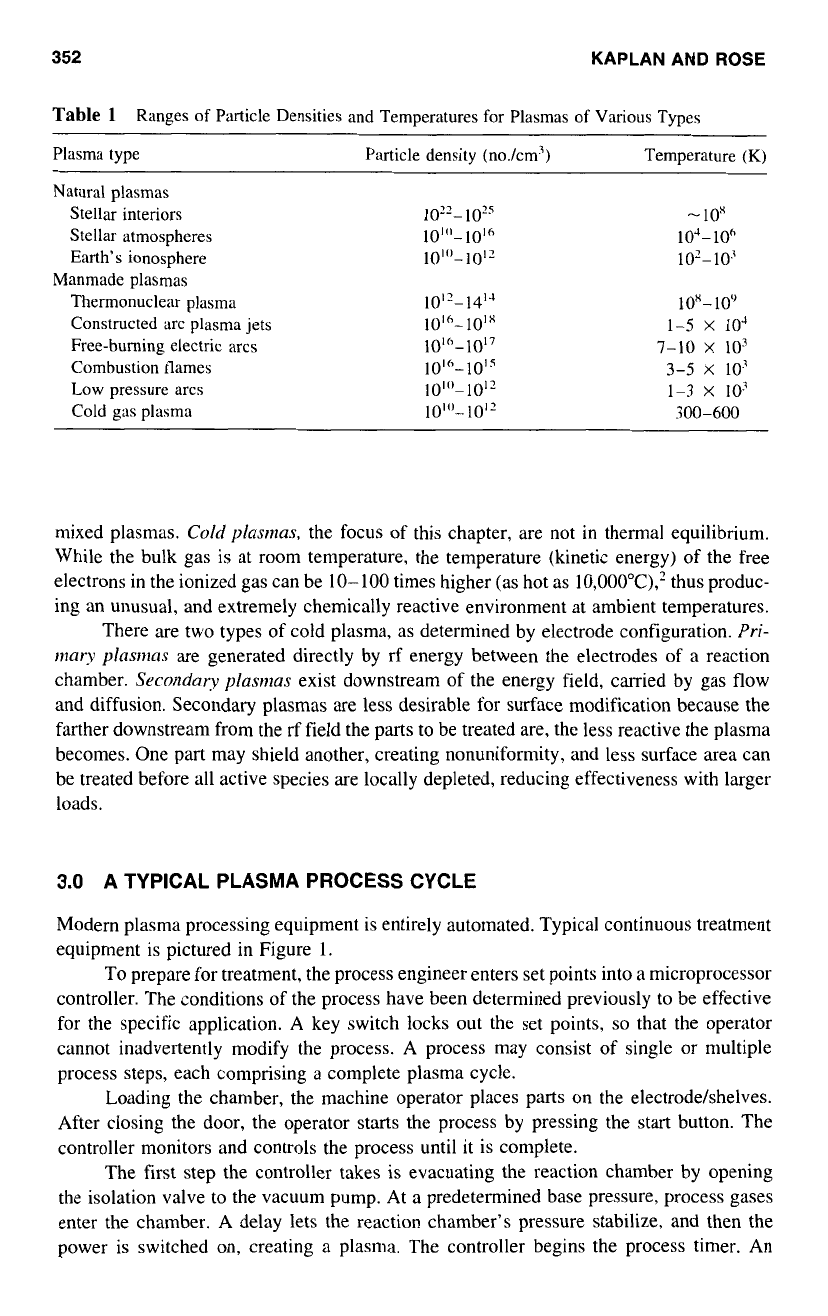
Table
1
Ranges
of
Particle Densities and Temperatures for Plasmas
of
Various Types
Plasma type Particle density (no./cm3) Temperature
(K)
Natural plasmas
Stellar interiors
Stellar atmospheres
Earth's ionosphere
Thermonuclear plasma
Constructed
arc
plasma jets
Free-burning electric
arcs
Combustion flames
Low
pressure
arcs
Cold gas plasma
Manmade plasmas
-
10%
10J-lOh
10"
1
0.?
1OX-10"
1-5
X
10'
7-10
X
10'
3-5
x
107
1-3
x
10'
300-600
mixed plasmas.
Cold
ylastnas,
the focus of this chapter, are not
in
thermal equilibrium.
While the bulk
gas
is at room temperature, the temperature (kinetic energy)
of
the free
electrons in the ionized
gas
can be 10-100 times higher (as hot as 10,OOO°C),' thus produc-
ing an unusual, and extremely chemically reactive environment at ambient temperatures.
There are two types of cold plasma,
as
determined by electrode configuration.
Pri-
ntary
plasw~as
are generated directly by
rf
energy between the electrodes of a reaction
chamber.
Secondary
plastms
exist downstream of the energy field, carried by gas flow
and diffusion. Secondary plasmas are less desirable for surface modification because the
farther downstream from the rf field the parts to be treated are, the less reactive the plasma
becomes. One part may shield another, creating nonuniformity, and less surface area can
be treated before all active species are locally depleted, reducing effectiveness with larger
loads.
3.0
A TYPICAL PLASMA PROCESS CYCLE
Modern plasma processing equipment is entirely automated. Typical continuous treatment
equipment is pictured in Figure 1.
To prepare for treatment, the process engineer enters set points into
a
microprocessor
controller. The conditions of the process have been determined previously to be effective
for the specific application.
A
key switch locks out the set points,
so
that the operator
cannot inadvertently modify the process.
A
process may consist
of
single or multiple
process steps, each comprising
a
complete plasma cycle.
Loading the chamber, the machine operator places parts on the electrode/shelves.
After closing the door, the operator starts the process by pressing the start button. The
controller monitors and controls the process until it is complete.
The first step the controller takes is evacuating the reaction chamber by opening
the isolation valve
to
the vacuum pump. At a predetermined base pressure, process gases
enter the chamber.
A
delay lets the reaction chamber's pressure stabilize, and then the
power is switched on, creating a plasma. The controller begins the process timer. An

PLASMA SURFACE TREATMENT
353
Figure
1
Typical continuous treatment equipment.
impedance-matching network continuously and automatically minimizes power mismatch
between generator and chamber as long as the
rf
power is
on.
The step ends after the process time has expired,
or
after
an
optional temperature
set point has been achieved, at which time the
rf
power and the process gases are shut
off. The vacuum pump evacuates the process gas and by-products from the chamber, and
the system repeats the entire cycle for the next step. If the last step has been completed,

354
KAPLAN AND
ROSE
the chamber vents
to
atmosphere and the controller alerts the operator that the process is
complete.
4.0
PLASMA CHEMISTRY
Three properties of the cold gas plasma-chemical dissociation, kinetic energy from ionic
acceleration, and photochemistry-make this unique environment effective for surface
treatment.
Exposing gases
to
sufficient electromagnetic power dissociates them, creating a
chemically reactive gas that quickly modifies exposed surfaces.
At
the atomic level. plasma
contains ions. electrons, and various neutral species at many different energy levels. One
of the excited species formed is the free radical, which can directly react with the surface
of organic materials. leading
to
dramatic modifications
to
their chemical structure and
properties. Modification sites also occur when ions and electrons bombarding the surface
have gained enough kinetic energy from the altering electromagnetic field to knock atoms
or groups of atoms from surfaces. Furthermore, gas phase collisions transfer en-
ergy-forming more free radicals, atoms, and ions.
Combining of dissociated species gives off photons as they are returning
to
their
ground state. The spectrum of this glow discharge includes high energy
UV
photons.
which will be absorbed on the top surface layers of the substrate. thus creating even more
active sites. The color of the glow discharge depends
on
the plasma chemistry. and its
intensity depends on the processing variables.
The plasma process modifies only several molecular layers. thus appearance and
bulk properties are usually unaffected.
In
addition, plasma changes the molecular weight
of the surface layer by scissioning (reduction in molecular length), branching, and cross-
linking organic materials. The chemistry of the plasma determines its effects
on
a polymer.
5.0
SURFACE TREATMENT APPROACHES
Like many polymer processes. plasma is a chemical process. Three types of cold plasma
treatments are used in processing polymers.
1.
Activating
plasrlzas
use
a
gas or gases that react with the product to modify its
chemistry. Such plasmas use oxygen, ammonia. air, halogens. and other gases
for cleaning surface contaminants, microablating the surface, and substituting
various chemical groups onto the polymer chain. Activating plasmas are dis-
cussed below.
2.
Grrrfiir~g
plastlla
treatment first activates the surface by exposure
to
a chemically
inert plasma. then bathes the surface
in
a vapor of an unsaturated monomer
(without plasma generation). The free radicals previously formed
on
the polymer
surface initiate grafting reactions with the reactive monomers.
3.
Plasm!
po1yneri:atinrl
utilizes plasma energy to initiate gas phase polymeriza-
tion reactions causing the deposition of organic onto surfaces within the plasma
chamber.’
6.0
PLASMA ACTIVATION OF PLASTICS
Activating plasmas have three competing molecular reactions that alter the plastic simul-
taneously. The extent of each depends on the chemistry and the process variables. They
are
as
follows.

PLASMA SURFACE TREATMENT
355
I.
Ablafion
(microetching), or removal by evaporating surface material either for
2.
Clnsslinking,
or creating covalent bonds or links between parallel long molecu-
3.
Sdxtifrrfion,
the act of replacing atom in the molecule with atoms from the
cleaning or for creating surface topography
lar chains
plasma
Ablation is an evaporation reaction in which the plasma breaks the carbon-to-carbon
bonds of the hydrocarbon polymer. As long molecules become shorter, their volatile mono-
mers or oligonlers boil off (ablate) and they are swept away with the exhaust. Ablation
is important for surface cleaning, and where desired, for surface etching. Cleaning removes
from polymer surfaces such external organic contaminants as hydraulic oils and mold
releases. Equally important is the removal
of
internal contaminants such as processing
aids and internal lubricants that have bloomed to the surface. Often an oxygen-containing
plasma is selected to facilitate rapid breakdown of the suspected contaminant into a volatile
by-product. Cleaning by plasma is more effective than cleaning by vapor degreasing or
by other methods. Plasma produces a "superclean" surface; but if gross contamination
exists. parts may be precleaned by ultrasonic cleaning, or solvent-vapor degreasing
so
that
the plasma process time is kept
to
a nlinimum and thus remains cost effective.
Once cleaned. the plasma begins ablating the top molecular layers
of
the polymer.
Amorphous. filled, and crystalline portions will be removed at different rates, giving a
technique effective for increasing surface topography with a view
to
increasing mechanical
adhesion or for removing weak boundary layers formed during molding.
Cross-linking, on the other hand, is done with an oxygen-free noble gas (argon or
helium). After the plasma has generated surface free radicals, these react with radicals on
adjoining molecules or molecular fragments to form cross-links. This process increases
the strength, the temperature resistance, and the solvent resistance of the surface.
Unlike ablation or cross-linking, substitution replaces one atom or group from the
surface with active species from the plasma. In this case, free radical sites on the surface
are free
to
react with species in the plasma, including but not exclusively free radicals.
thus altering surface chemistries by the addition
of
covalently bonded functional groups.
The selection of the process gas determines which groups will be formed on the modified
polymer. Gases or mixtures of gases used for plasma treatment of polymers include nitro-
gen, argon, oxygen, nitrous oxide, helium. tetrafluoromethane, water, and ammonia. Each
gas produces a unique plasma chemistry. Surface energy can be quickly increased by
plasma-induced oxidation. nitration, hydrolyzation. or amination.
Very aggressive plasmas can be created from relatively benign gases. For example,
an oxygen and tetratluoromethane (Freon
14)
plasma contains free radicals of fluorine.
Oxidation by fluorine free radicals is known
to
be as effective as oxidation by the strongest
mineral acid etchant solutions, with one important difference: hazardous and corrosive
materials are not used. As soon as the plasma is shut off, the excited species recombine
to their original stable and nonreactive form. In most cases treatment of the exhaust effluent
is not required.
Gases that contain oxygen are generally more effective at increasing the surface
energy. For example, plasma oxidation
of
polypropylene increases the initial surface en-
ergy of
29
dyneslcm
to
well over
73
dyneslcm in just a few seconds. At
73
dyneslcm.
the polypropylene surface is completely water wettable. Increased surface energy results
in
a plasma that yields polar groups such as carboxyl, hydroxyl, hydroperoxyl, and amino.
356
KAPLANANDROSE
A
higher energy (hydrophilic) surface translates to better wetting and greater chemical
reactivity of the modified surface
to
adhesives, paints. inks. and deposited metallic films.
providing for improved adhesion and permanency.
The enhanced surface reactivity is characterized in the laboratory by studying water
wettability. Wettability describes the ability
to
spread over and penetrate
a
surface: it
is
measured by the contact angle between the liquid and the surface. The relationship between
contact angle and surface energy is inverse-the contact angle decreases with increasing
surface energy. Wettability can easily be induced on normally nonwettable materials such
as polyolefins, engineering thermoplastics, fluoropolymers. thermosets. rubbers. and tluor-
oelastomers.
Noble gases (argon. helium, etc.) generate surface free radicals that react either with
other radicals on the surface, yielding molecular weight changes. or with the air. when
the part is removed from the chamber, thus increasing the surface energy.
Process gases such as tluorocarbons will generally provide a lower energy, or hydro-
phobic, surface by substitution of abstracted hydrogen with either fluorine or trifluoro-
methyl radicals
to
form a fluorocarbon surface. Fluorination is favored
in
some medical
applications. where
it
undesirable to have catheters be wetted by blood. The nonwettable
barrier layer also inhibits chemical penetration.
a
consideration that is important for spe-
cialty packaging.
7.0
ADHESION
Bonding in manufacturing processes is a specialized field, but generally. cleanliness and
wettability are necessary for good adhesion.
High surface energy alone does
not
guarantee better adhesion; however. the versatil-
ity of the process enables tailoring of the surface chemistry for optimal adhesion or superior
product performance. It is not uncommon to move the focus of failure from the bond line
Table
2
Typical
Bond
Improvement" Aftcr Surfacc Treatment: Solvent-Washed
Plasma
Matcrial
_______~~_____~~
~~
Vnlox (polyestcr)
522
Adh
I644
Col1
Noryl (polyphenylcnc oxide)
617
Adh
I799
CO
h
Durcl (polyarylatc)
250
Adh
2161
Coh
Vectra
(
LCP
)
939
Adh
1598
Col1
Lexan (polycarhonate)
1705
Adh
2242
C
0
h
Dclrln
(polyacetal)
I65
Adh
857
Adh
"
Lap
shear
strength
3M
Wcldbond
#3256.
"
Adh
=
adhcsive
iailurc.
Col1
=
coheslvc
failure
of
adherent.

PLASMA SURFACE TREATMENT
357
Table
3
Paint Adhesion on Polyethylene: Urethane Heat-Cured Automotive Paint
Test condition Failure Liftoff
(S)
Control (no plasma)
OB“
100
Plasma treated
5B
0
‘‘
ASTM Test Method
D3359-83.
“Measuring Adheslon
by
tape test.”
~~ ~
into the adherent or into the adhesive with a many-fold increase in the adhesion. Examples
of typical plasma improvement on
a
range of materials for epoxy bonding’.‘l and for
coating’ are shown in Tables
2
and
3.
Extremely mobile polymers. like elastomers. have shelf lives measured in minutes
or hours. The flexible molecular chains rotate the high energy functional groups into the
bulk. Once an active surface has been treated and properly coated with adhesive, the
modification is permanently tied
to
the surface. Thus priming
of
a treated elastomer fixes
the surface chemistry.
8.0
SUMMARY
Plasma surface treatment is an effective, efficient method of modifying
a
wide variety of
polymeric and elastomeric surfaces. Adhesion strength of treated materials often exceeds
that
of
the adherent. The plasma process is not operator-sensitive; its other significant
characteristics include reproducibility, cleanliness, and the ability to more consistently
provide high reliability bonds.
REFERENCES
1.
B.
Chapman,
Glow
Dischtrrge Processes,
New
York:
Wiley, 1980.
2.
J.
R. Hollahan and A.
T.
Bell,
Techniques
nrltl
Applicntiorn
(f
Plrsmn Chemistry,
New
York:
3.
S.
L. Kaplan and P. W.
Rose,
“Plasma surfacc trcatment of plastics,” presented at the SPE
4.
S.
L.
Kaplan and P. W. Rose, in
Plosfics Finislzir~g
&
Decorrrtirl<q.
D.
Satas, Ed., New
York:
5.
S.
L. Kaplan and
S.
Rines, “Plasma pretreatment for plastics,”
Prod. Finish..
January 1988.
Wiley, 1974.
46th Annual Technical Conference, Atlanta, April 1988.
Van Nostrand Reinhold, 1986.
This Page Intentionally Left Blank
Surface Pretreatment
of
Polymer
Webs by Fluorine
1
.O
INTRODUCTION
Bonding, coating laminating, painting, and printing require good substrate adherence. This
requires above
all,
surface polarity, which permits mechanical and, in particular, chemical
bonds. For this reason, polymeric materials are treated by means of oxidation processes
entailing three main groups: corona discharge, flame treatment, and chemical.
All processes are more or less disadvantageous. The chemical methods have been
proved only in narrow, limited fields of application (e.g.,
as
liquid pickling agents), or
they require high supervision and a substantial maintenance effort (e.g., ozone treatment).
State-of-the-art fluorination is troublesome because it is
a
discontinuous process and not
feasible in many cases, especially for web-shaped substances. Corona pretreatment requires
high investment and is strongly liable
to
interference. In the area
of
the dielectric material,
fires occur frequently, causing short circuits in the pretreatment station. Additionally, only
one side of the web material can be activated by the corona discharge.
This chapter describes an attractive new pretreatment method implemented by Loh-
mann GmbH, featuring the continuous fluorination of web materials.'
2.0
THE FLUORINATION PROCESS
The surface of web-shaped, polymeric substrates is subjected continuously, for a short
time, in a suitable reaction chamber, to elemental fluorine-attenuated with an inert gas.
Thus, the surface energy of the polymeric material is increased to such an extent that
excellent adherence to other polymers (e.g., lacquers and adhesive agents) is attained.'
359

360
MILKER
AND
KOCH
The fact that this fluorination technique is feasible at all,
on
an industrial scale, is
due to the astonishing chemical-technological developments realized during the past
20
years, which having made fluorine not only an important initial substance but even a
component in large-scale technical operations.
2.1
Fluorine
Fluorine, an almost colorless gas, is one
of
the strongest oxidizing agents; it is surpassed
only by a few other oxidants (e.g., chlorine fluoride, chlorine trifluoride). It reacts with
almost all organic and inorganic substances; the few exceptions include, first of all, nitrogen
and the inert gases helium, neon, and argon, plus some metal fluorides in the highest
valence state, as well as other fully fluorinated compounds (e.g., CF‘ or SF6). Fluorine’s
great reactivity can be explained by the interaction
of
the low dissociation energy
of
the
molecule itself and the very strong bond fluorine forms with other atoms. Moreover, since
the fluorine atom is rather small, the spatial relations in fluorine compounds admit high
coordination numbers of the relevant central atom.
For a long time, the extreme aggressiveness of fluorine has limited its use in industrial
applications.
Indeed, as late as 1936 a technical encyclopedia stated: “Due
to
the difficult manu-
facture and storage, fluorine has no practical importance for industry.” It was only a little
more than two decades ago that all difficulties impairing the manufacture of fluorine
on
a large scale could be considered to have been overcome.
Nowadays, elementary fluorine in the liquefied state is transported even in fuel
trucks. It is mainly used for the manufacture of the highly volatile uranium hexafluoride,
which is known to serve for the separation of the uranium isotopes and
U’”.
Thus
fluorine has become a key product for the nuclear industry.
In
Germany, Kali Chemie
AG, which has had a leading part in fluorochemistry in general, is the only manufacturer
of
this extremely reactive substance.3
2.2 Plant for Continuous Surface Fluorination
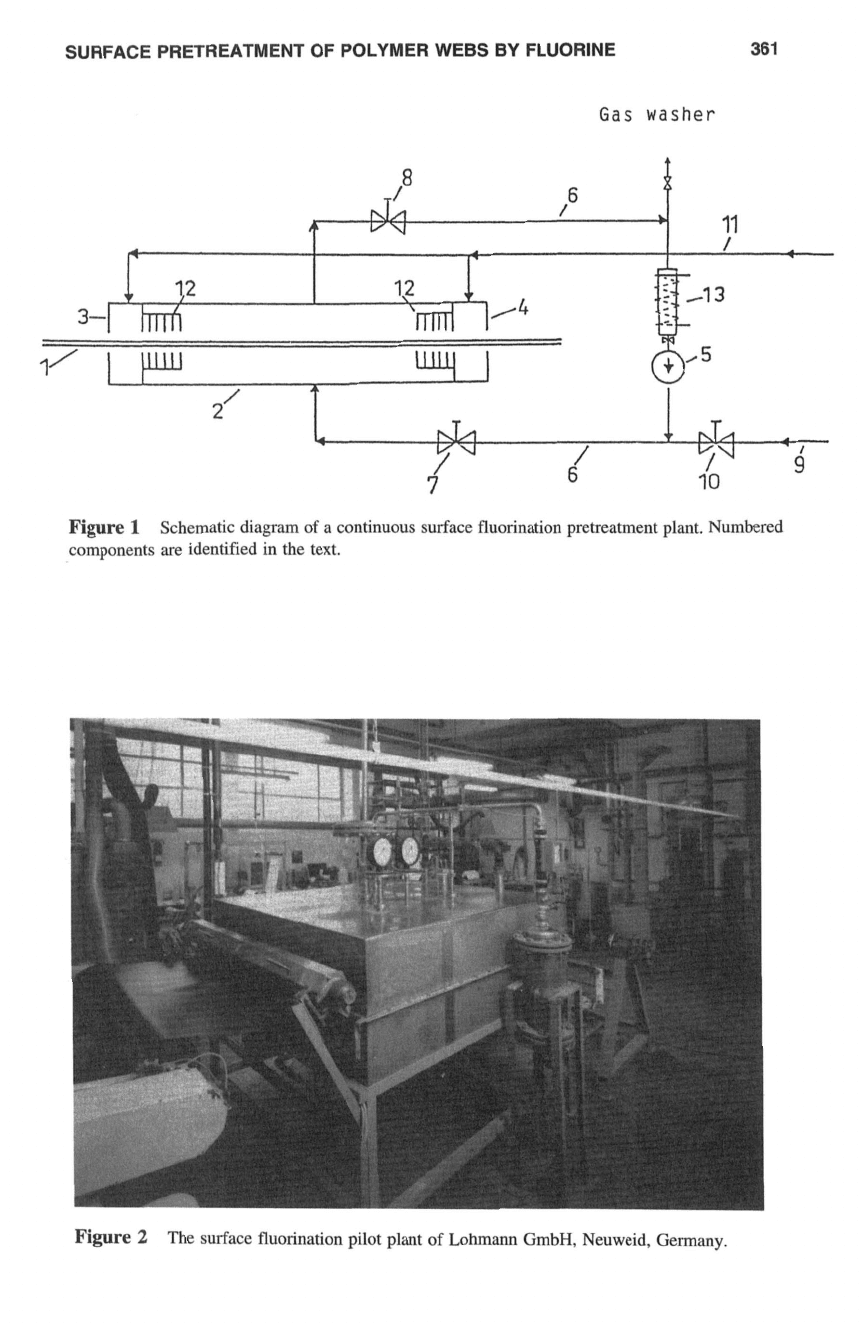
Figure
1
diagrams the construction
of
a plant for the pretreatment of web-shaped materials
with fluorine.‘ Figure
2
illustrates an actual set up. Fluorine supplied in steel pressure
bottles is attenuated to a certain concentration with inert gas (nitrogen, rare gases, and
compressed air) in a corresponding dose discharge devise. This fluorine-inert gas com-
pound is available
for
the system via the line 9
in
Figure
1.
The attenuation step can be
dispensed with by connecting instead
to
a steel pressure bottle filled with a fluorine-nitro-
gen compound.
The fluorine delivery amounts for a
10
vol% standard compound-due to the higher
filling pressure-to
50%
of
that obtained with the pressure bottle containing elementary
fluorine. After the reaction chamber
(2:
Fig.
1)
has been filled with attenuated fluorine,
the fluorine-inert gas compound is circulated through reaction chamber
2
by the pump
(5)
via pipe 6 and the two stop valves
(7
and
8).
The web-shaped material to be pretreated
is fed into chamber
2
via the intake airlock (3) and leaves it via the outlet airlock
(4).
The parameters time
of
contact and concentration of fluorine influence the surface
energy of the polymer. The surface effect attainable depends additionally on the chemical
quality of the polymer. Because
of
the extreme reactivity of the elemental fluorine, all
polymeric materials disposing of substitutable hydrogens can, in principle, be activated.
Airlocks 3 and
4
are supplied with inert gas via pipe
11.
The airlocks, as well as the
SURFACE PRETREATMENT
OF
POLYMER WEBS BY FLUORINE
361
Gas
washer
a
6
A
/
1,2
\
l
p4
2’
A
;4
Y
6
/
‘4
10
7
Figure
1
Schematic diagram
of
a continuous surface fluorination pretreatment plant. Numbered
components
are
identified in the text.
,
l
l
Figure
2
The surface fluorination pilot plant
of
Lohmann GmbH, Neuweid, Germany.
