Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.

PRANEVICIUS
Pressure,
Pa
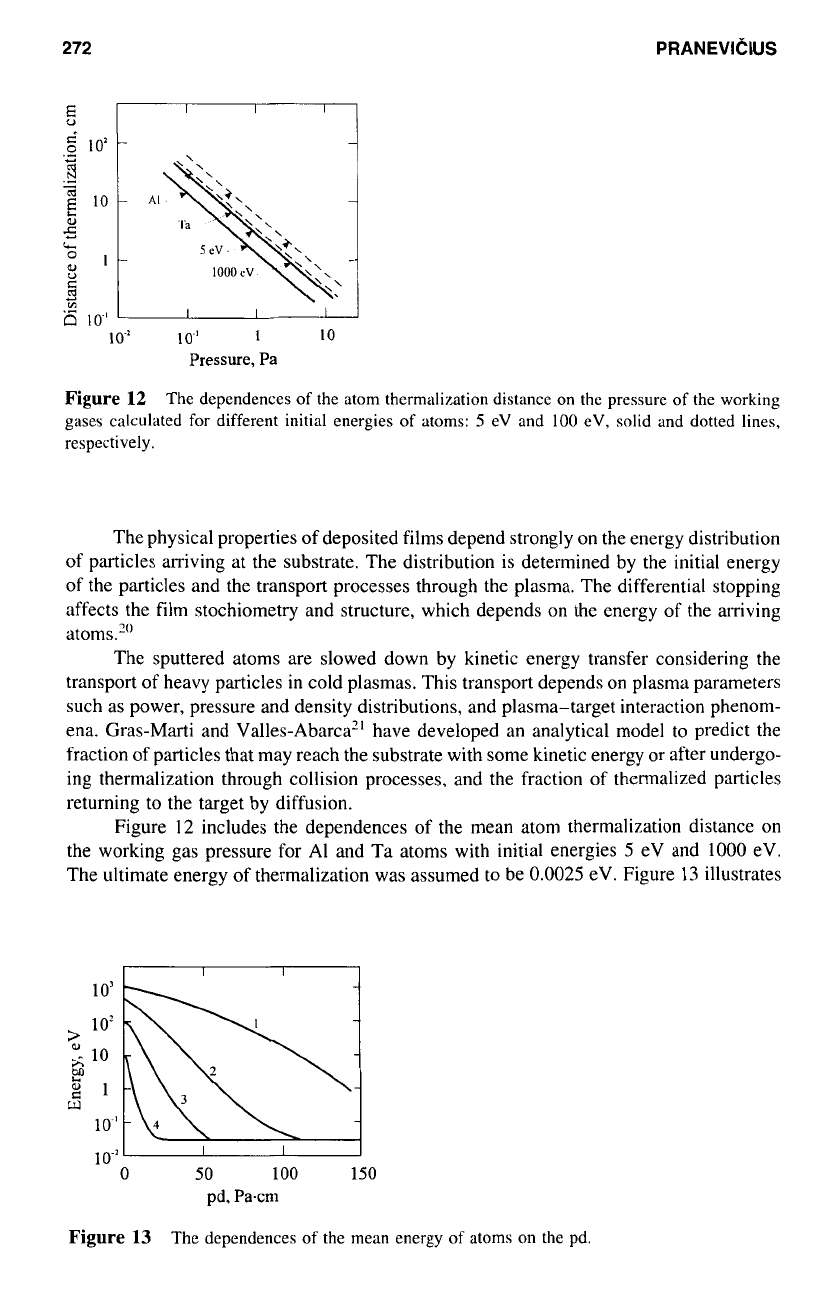
Figure
12
The dependences of the atom thermalization distance on the pressure
of
the working
gases calculated
for
different initial energies
of
atoms:
5
eV and
100
eV, solid and dotted lines,
respectively.
The physical properties of deposited films depend strongly
on
the energy distribution
of
particles arriving at the substrate. The distribution is determined by the initial energy
of
the particles and the transport processes through the plasma. The differential stopping
affects the film stochiometry and structure, which depends
on
the energy of the arriving
atoms.”’
The sputtered atoms are slowed down by kinetic energy transfer considering the
transport of heavy particles in cold plasmas. This transport depends
on
plasma parameters
such as power, pressure and density distributions, and plasma-target interaction phenom-
ena. Gras-Marti and Valles-Abarca’l have developed an analytical model to predict the
fraction
of
particles that may reach the substrate with some kinetic energy or after undergo-
ing thermalization through collision processes, and the fraction of thermalized particles
returning to the target by diffusion.
Figure
12
includes the dependences of the mean atom thermalization distance
on
the working
gas
pressure for AI and Ta atoms with initial energies
5
eV and
1000
eV.
The ultimate energy of thermalization was assumed
to
be
0.0025
eV. Figure
13
illustrates
I
I
l
I
1
o-z
0
50
100 150
pd,
Paan
Figure 13
The dependences of the mean energy of atoms on the pd.
REACTIVE PLASMA:
DEPOSITION AND
ETCHING
273
the calculated mean energy of atonis
in
dependence on pd with initial energies equal to
10
eV,
100
eV,
500
eV. and
1000
eV (curves
1-4.
respectively). Figures 12 and
13
are
used
in
Ref.
22.
The microstructure and composition of sputtered deposited multicomponent thin
films is sensitive
to
the transport mechanism
of
sputtered particles. and the pressure plays
a fundamental role in the collision processes, determining the fraction and the energy of
the extracted atoms arriving at the substrate.
3.2 Resorption
of
Sputtered Atoms
High sputtering rates are. unfortunately. difficult to achieve
in
conventional triode deposi-
tion systems. It is possible
to
increase them by increasing the working gas pressure.
However, for the practical target-to-substrate distance of
5-20
cm. pressure
0.5
nlTorr
and less, the sputtered flux is predominantly in a line-of-sight mode. The majority of the
sputtered atoms pass through the background gas and arrive at the substrate without scatter-
ing and with their
full
emitted energy. At this low pressure, the triode deposition system
operates poorly. With the increase of pressure all
of
the sputtered atoms have undergone
dozens
of
gas-phase collisions.
As
a result. part
of
the sputtered atoms return to the target
and are resorbed.
i;”
=
\t’;c,.
Part of the sputtered atoms scattered
on
the way from the target to substrate
returns back to the sut-face. It depends
on
the working gas pressure as was considered
above.
Let us define that the probability for the i-type sputtered atom to return back to the
target is equal to
pi.
The resorption rate
of
sputtered i-type atoms can be presented as
K,~
=
aij
p,
)vi
c,.
If the resorption of sputtered atoms takes place simultaneously with the
process
of
adsorption of atoms from the surrounding ambient. the resulting
flux
of i-type
atoms sticking to j-type atoms is presented as
K,,
=
aii
(ii
+
pi
N.;
c,).
The resorption process taking place during simultaneous deposition and sputtering
may be included
in
the general scheme of processes as the additional conlponent originated
from the sputtered atoms
to
the tlux
of
arriving atoms
to
the surface.
The flux of i-type sputtered atoms leaving the target
in
relative units is equal to
0.5
a
F”
0.4
L
....
0
4-
E
2
0.3
0
U
0.2
0
S
IO
15
20
1
me, r.u.
-.
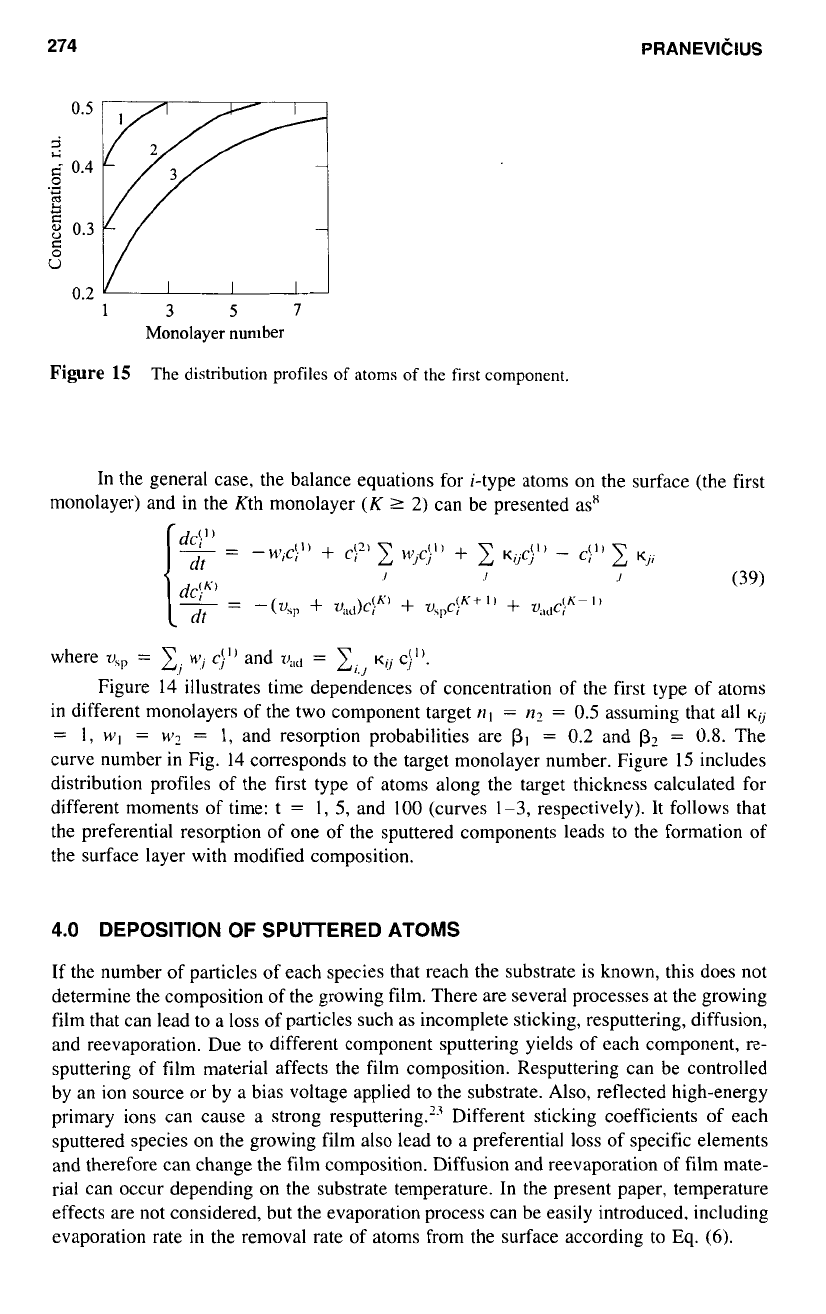
Figure
14
The time dependences of
the
surface concentration
of
the first component in different
subsurface
monolayers. The curve
number
corresponds
to
the
monolayer
number.
274
PRANEVICIUS
0.2
1
3
5
7
Monolayer
number
Figure
15
The
distribution
profiles of
atoms
of
the
first
component.
In the general case, the balance equations for i-type atoms
on
the surface (the first
monolayer) and in the Kth monolayer
(K
2
2)
can be presented
asx
where
7lSp
=
cj
wi
c)’)
and
=
C,,,
cjl).
Figure
14
illustrates time dependences
of
concentration of the first type of atoms
in different monolayers of the two component target
111
=
n2
=
0.5
assuming that
all
K~
=
1,
WI
=
w2
=
1,
and resorption probabilities are
PI
=
0.2
and
Pz
=
0.8.
The
curve number in Fig.
14
corresponds
to
the target monolayer number. Figure
15
includes
distribution profiles of the first type of atoms along the target thickness calculated for
different moments of time: t
=
I,
5,
and
100
(curves
1-3,
respectively). It follows that
the preferential resorption of one of the sputtered components leads to the formation of
the surface layer with modified composition.
4.0
DEPOSITION
OF
SPUlTERED ATOMS
If the number of particles
of
each species that reach the substrate is known, this does not
determine the composition of the growing film. There are several processes
at
the growing
film that can lead to
a
loss
of
particles such
as
incomplete sticking, resputtering, diffusion,
and reevaporation. Due to different component sputtering yields of each component, re-
sputtering of film material affects the film composition. Resputtering can be controlled
by an
ion
source or by a bias voltage applied to the substrate.
Also,
reflected high-energy
primary ions can cause a strong resputtering.23 Different sticking coefficients of each
sputtered species on the growing film also lead to
a
preferential
loss
of specific elements
and therefore can change the film composition. Diffusion and reevaporation of film mate-
rial can occur depending on the substrate temperature. In the present paper, temperature
effects are not considered, but the evaporation process can be easily introduced, including
evaporation rate in the removal rate of atoms from the surface according to
Eq.
(6).

REACTIVE PLASMA: DEPOSITION AND ETCHING
275
4.1
Multielement Adsorption
of
Sputtered Atoms
Reactive gas arriving at the substrate consists of two fluxes: the first, which consists of
sputtered target material atoms and can be calculated according
to
Eq.
(37),
and the second,
which consists of the atoms arriving from the working gas and is defined by the reactive
gas partial pressure and can be evaluated according to Eq.
(1).
In the following calculations.
the flux of arriving reactive species from plasma
to
the substrate will be neglected. The
reasons for this are the following: the degree
of
ionization and dissociation of oxygen is
small in the low-temperature plasmas. This means that oxygen arriving
to
the substrate
from the working gas is mainly in the molecular (nonactivated) state with energies approxi-
mately equal to
kT.
The sputtered oxygen arriving at the substrate is mainly in the atomic
(activated) state with high kinetic energies (the mean energy of sputtered atoms is equal
to
9-20
eV). Thus the reaction rates
of
sputtered atomic oxygen with metal atoms on the
substrate are significantly higher than the reaction rates of the molecular oxygen arriving
from the surrounding. Without the external irradiation of the substrate, the dissociation
rate
of
the molecular oxygen is low, and its role in the oxidation of the deposited metal
atoms is small.
Assuming that thin film growth is defined only by the arriving sputtered atoms, the
balance equations for each component in the growing film are
l
where
~r)
is the adsorption rate of the sputtered i-type atoms from the target to the
j-
type atoms on the surface
of
the substrate and is equal to
where
ai[’
is the corresponding sticking coefficient on the substrate and
c:,T’
is the surface
concentration
of
the i-type atoms
on
the target. Inserting Eq.
(40)
into Eq.
(41),
we obtain
the needed equations to calculate the composition and film growing rate
in
dependence
on the sputtering deposition parameters and the partial pressure of oxygen.
To simplify analysis let
us
assume that sticking coefficients
of
the arriving sputtered
atoms are equal to one. Thus the steady-state surface concentration of constituents
on
the
substrate is equal
to
The steady state film growing rate is equal to
zp’
=
c
K;i”c:.”’
1.J
(43)
and taking into account the above assumptions the film growth rate on the substrate is
equal to the sputtering rate of the target
(J.“
=
JT’).
The stoichiometry of the growing film
in
dependence
on
the partial pressure of the
reactive gases is obtained from the analysis of the composition of the growing film. When
sticking probabilities of arriving components are different and
aZ2
=
0,
the steady-state
composition of the growing film can be calculated as
276
PRANEVICIUS
For practical applications it is important to know the composition
of
growing film
not in dependence on the phenomenological parameters
K~
and
W?
but
in
dependence
on
technological parameters such as
ion
beam current density to the target and the partial
pressure
of
reactive species in working gas.
Introducing the notation
y
=
K~/u’~
and taking into account Eqs.
(1)
and (6), we
get
y
=
p/(pZo),
where
p
=
(Y2/a$y)
.
VmT.
The steady-state concentration of constituent components
in
the film can be written
as
(45
1
where
U
=
all/a12
and
b
=
w2/wI.
The steady-state film growth rate is expressed as
It is important to note that both the composition
of
film, Eq.
(45),
and the film
growing rate eq. (46), depend on the ratio of the ion beam current density to the partial
pressure. In the case
y
-
0
(low partial pressure
of
reactive species or high ion beam
current density), the growing film mainly consists of metal atoms
(cI
-
1)
and the film
growing rate mainly depends on the values
of
the parameters
aI
I
and
wI.
With the increase
in partial pressure of oxygen, the concentration
of
oxygen in the growing film increases,
and the film growth rate decreases, approaching zero.
Qualitatively the same results are obtained
if
we include into consideration the
existence of the activated atoms on the target surface. In this case the surface concentration
of
reactive species
in
the growing film can be presented as
U21.b’~
f
Gz(M’J
-\V?)
cy
+
cy)
=
b1
U,b?Wl
+
U,bI).I’Z
+
Glb?(bl’3
-
M’,)
+
Gd7l(wl
-
M’?)
(47
1
Figure 16 illustrates the dependences of the surface concentrations of reactive species
Prcssurc.
r.
U.
Figure
16
The dependences
of
the concentration
of
oxygen
in
the
growing
film
on
the
partial
pressure
of
oxygen
in
plasma: curve
I,
\vl
=
\v2
=
2,
by3
=
4,
and
W.,
=
3;
curve
2,
\vl
=
4,
\v2
=
2,
\v3
=
8,
and
\v4
=
4.
The values
of
other parameters:
=
I,
=
2,
az3
=
3,
=
4,
RI
=
R?
=
0.1,
and
GI
=
IO.
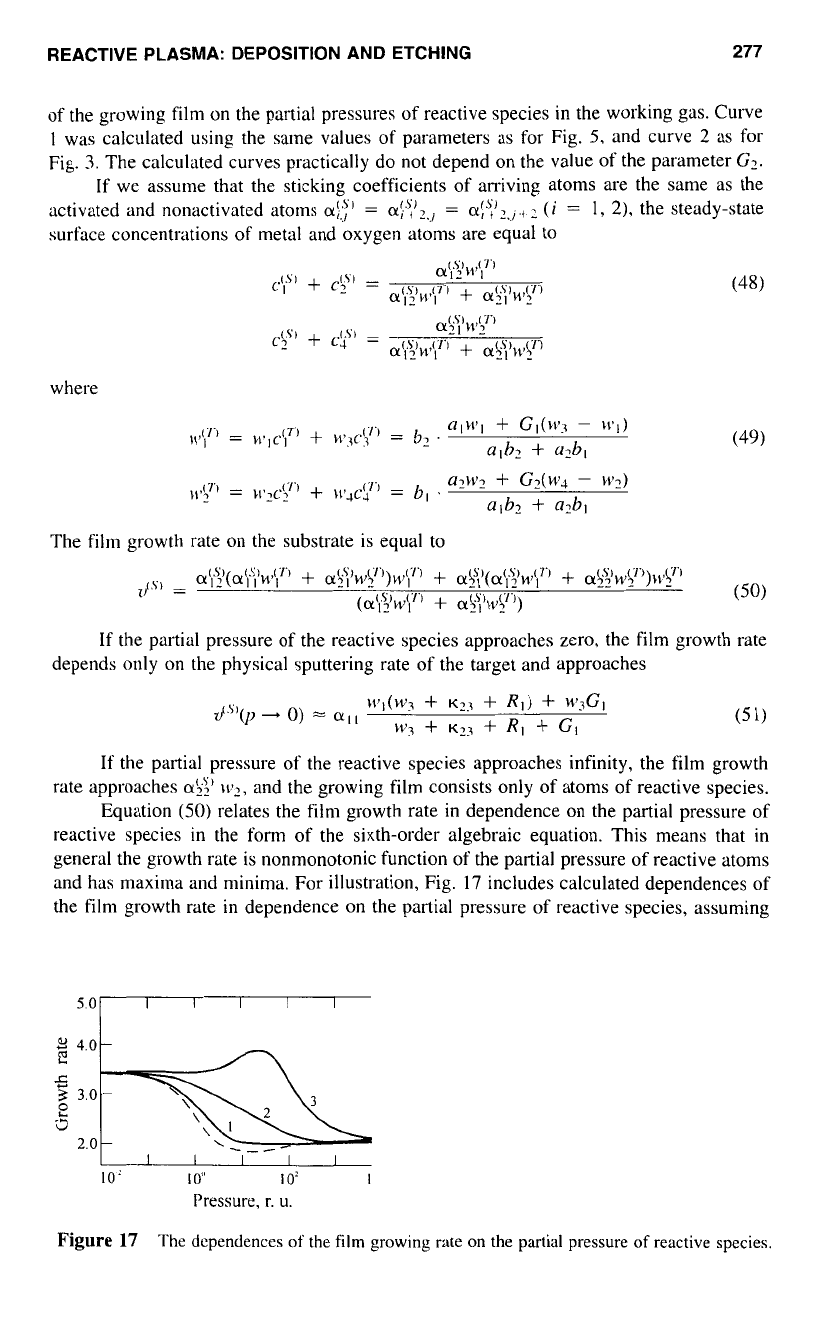
REACTIVE PLASMA: DEPOSITION AND ETCHING
277
of the growing film
on
the partial pressures of reactive species in the working gas. Curve
1
was calculated using the same values of parameters as for Fig.
5,
and curve
2
as for
Fig.
3.
The calculated curves practically do not depend
on
the value of the parameter
G?.
If we assume that the sticking coefficients of arriving atoms are the same
as
the
activated and nonactivated atoms
a!;)
=
l
l
-..I
-
-
(i
=
I,
2),
the steady-state
surface concentrations
of
metal and oxygen atoms are equal
to
where
The film growth rate
on
the substrate
is
equal to
If the partial pressure
of
the reactive species approaches zero, the film growth rate
depends only
on
the physical sputtering rate of the target and approaches
If the partial pressure of the reactive species approaches infinity, the film growth
rate approaches
ai;'
W?,
and the growing film consists only of atoms of reactive species.
Equation
(50)
relates the
film
growth rate
in
dependence on the partial pressure of
reactive species
in
the
form
of
the sixth-order algebraic equation. This means that
in
general the growth rate is nonmonotonic function of the partial pressure of reactive atoms
and has maxima and minima. For illustration, Fig.
17
includes calculated dependences of
the film growth rate in dependence
on
the partial pressure of reactive species, assuming
3.0
2.0
IO"
I
0'
I
Pressure,
r.
U.
Figure
17
The dcpendences
of
the
film
growing rate on
the
partial pressure
of
reactive species.

278
PRANEVICIUS
10”
10”
10’
Pressure,
r.
U.
Figure
18
The concentration of reactive species in the growing film in dependence on the partial
pressure of reactive species in plasma.
that
aZ1
=
az3
=
I,
= =
2,
\vi
=
kt’?
=
2,
w3
=
4,
\tIJ
=
6,
GI
=
10,
RI
=
1,
2,
and
3,
respectively). The broken curve corresponds
to
G,
=
0.
The steady-state concentration of the reactive species incorporated in the growing
film in dependence on their partial pressure (Fig.
18)
has been calculated according to
Eq.
(20)
using the same values of parameters as for Fig.
17.
The steady state composition
of
a
growing film practically does not depend
on
the value
of
the parameter
G?.
In Ref. 24, the sputtering of Ti was studied
in
the plasma of working gases of argon
and methane. Figure
19
includes experimental results
of
the concentration of carbon
in
the film deposited
on
the substrate
of
the triode system in dependence on the partial
pressure of methane. The experimental results are
in
good qualitative agreement with
calculated ones. Similar experimental dependences have been obtained
in
Refs.
9
and
10,
where deposition of oxides and nitrides has been studied by sputtering metal targets in
reactive plasma.
However, for many metal systems, the behavior for sputtering in dependence on the
partial pressure of nitrogen
is
significantly different from the parallel case with oxygen.
With oxygen, most oxides are formed spontaneously, and the reactions are exothermic: no
additional energy is required. The transition from the “metallic” to the “oxide” sputtering
regime is sharp. For the case
of
reactive sputter deposition with nitrogen, the nitride
formation often requires additional energy to form. In addition, the sticking probability
R
-
=
0
1
&(.S)
-
(S)
=
&(.S)
-
1
7-
.
1
II
-
&I?
22
-
,
=
0.5,
and
G,
=
I,
IO,
and
100
(curves
2
10”
10-5
10-4
10-3
10”
”
Methane
partial
pressure,
Torr
Figure
19
The concentration of carbon in growing film deposited
by
Ti sputtering in the working
gas
of
argon and methane in dependence on the partial pressure of methane.’.‘

REACTIVE PLASMA: DEPOSITION AND ETCHING
279
$.,
8.0
2
4.0
00
*
c
E
2.0
0
10
20
30
40
Pressure
of
nitrogen.
lnTorr
Figure
20
The sputtering
rate
of
In
in
dependence on
the
partial prcssurc
of
nitrogen.
of the nitrogen. even under the best of circumstances, is considerably less than
1.0.
The
result is that the transitions are less abrupt from the metallic to the compound state (Fig.
20
for sputtering of
In
in nitrogen and Fig. 4a in oxygen plasmas). The additional energy
enhancing the formation
of
the compounds can be obtained biasing the substrate to a
negative voltage during deposition. The bias causes ions from the plasma
to
be accelerated
to the sample. depositing additional energy
in
the near surface region. The required level
of ion bombardment scales with the deposition rate. It is difficult to achieve high levels
of bias current with the conventional triode deposition system.
A
solution has been the
development of the magnetically unbalanced magnetrons by
B.
Windows.’5
Ion irradiation of the substrate and growing film during deposition is used
to
affect
sticking probabilities
of
the arriving atoms, heterogeneous reaction rates, nucleation and
growth kinetics. and interdiffusion rates at heterojunction interfaces.’”
-”
The film growth
kinetics and the composition of the growing films grown during simultaneous deposition
and ion sputtering is considered in Ref.
8.
Low-energy ion irradiation during film growth
modifies the morphology, microstructure, preferred orientation, state of stress, and physical
properties
of
deposited layers. It is used to densify and increase the internal strength of
the layers as well as to increase fildsubstrate adhesion.
5.0 CONCLUSIONS
Phenomena and processes taking place
in
solids immersed in reactive plasma are not
completely understood. However, progress in understanding can be achieved through the
conclusions of computer simulations of the variety
of
processes simultaneously taking
place on the surface and in subsurface layers of plasma-affected solids, by making compari-
sons
with experimental results, in some cases the prevailing processes can be distinguished.
The general system of balance equations for the first monolayer
of
the multielement
solid immersed in plasma, including the processes
of
sputtering, adsorption, and a variety
of
heterogeneous reactions. can be presented as
where
Rji
=
R$
+
K;~,
and
C:”
is the partial concentration of i-type atoms in the second
monolayer. For a determination
of
C:,’),
an additional system of equations is needed.x

280
PRANEVICIUS
However, if the etching rate is high and the mixing process between monolayers can be
neglected
c!”
=
ni,
where
ITi
is the partial bulk concentration
of
i-type atoms. In this case,
Eq.
(52)
becomes linear, and the solutions are expressed as sums
of
exponents. The analysis
of
the matrix
nlrt’l
-
M’;
tt1\t32
RI,?
...
tI]M’/,
+
R1.k
n2wI
+
tt?bt’2
-
rt’?
...
n7wh
+
i
(53)
...
...
...
...
nk\t’l
+
Rk.1
ItA\t’l
+
RL.1
...
tTkM‘k
-
M(
where
bt(
=
\vi
+
xi
Rii,
gives the roots of the characteristic equations. The solutions
of
Eq.
(52)
determine the fluxes
of
the sputtered atoms. The obtained values of fluxes are
used to calculate the growth rate and composition
of
the growing film on the substrate
according
Eq.
(52).
REFERENCES
1.
2.
3.
4.
5.
6.
7.
8.
9.
IO.
11.
12.
13.
14.
IS.
16.
17.
18.
19.

REACTIVE PLASMA: DEPOSITION AND ETCHING
281
20.
21.
22.
23.
24.
25.
26.
27.
28.
