Satas D., Tracton A.A. (ed.). Coatings Technology Handbook
Подождите немного. Документ загружается.

262
PRANEVICIUS
growth rate and the characteristics. If
the
ion bombardment energy is high. surface reaction
mechanisms and film properties may be influenced by
it.
The quantitative analysis of heterogeneous reactions
on
the substrates immersed in
reactive plasma becomes complex if we include different stages
of
reactions. Let us con-
sider the sequence of processes of adsorption and chemical reaction
LIS
;I
one-step process
introducing kinetics terms in the form
where
c,
and
cil
are the partial surface concentrations
of
substrate atoms
B
and reaction
products
BA,,;
R;''
and
R::
are the frequency probabilities of the reactions
B
+
nA
=
BA,,
and
BA,,
-,
B
+
IIA,
and
A
is the reactive atoms in plasma. The parameter
R:"
depends on the concentration of reactive species in plasma
[AJ
and the type of reaction.
It
can be presented in the form
where
K
is the reaction rate.
The oxidation reactions include different stages and may be presented
as
M
+
O-+MO R?''
=
Kl[O]
MO
+
0
-+
MO-
R?''
=
KzlOl
M
+
20
+
MO?
RC,"
=
K.3[0]'
Thus the surface consists of three constituents:
M.
MO.
and
MOz.
which are removed by
sputtering with sputtering rates
11'~,
\I,?,
and
II~~.
respectively.
The balance equations for each component on the surface
if
dissociation reactions
are neglected can be written in the form
The steady-state target etching rate is expressed as
where
R"
=
R?".
11-
=
II*?
in
the case of reaction
M
+
0
-
MO
and
R'"
=
R"'
q
,
11'
=
t1-3
in the case of reaction
M
+
20
-,
MO?.
It
corresponds
to
the case when the surface consists of atoms
of
the target material
and of the ultimate reaction products which are removed with corresponding sputtering
rates equal to
\1l1
and
~9~.
respectively.
If
the reaction rate of reactive species is high
REACTIVE PLASMA: DEPOSITION AND ETCHING
263
(F"
+
\I'~
and
R'"
B
the etching rate is limited by the removal rate of reaction
products; and if
R'"
<
11'~
and
R'"
4
\v2,
the etching rate approaches the sputtering rate
of
the target material. When the reaction rates are high and reaction products are volatile,
the removal rate of reaction products is expressed according
to
Eq.
(6).
and
it
depends
on temperature. With the decrease of the reaction rate. the prevailing mechanism of the
removal
of
target material atoms is physical sputtering.
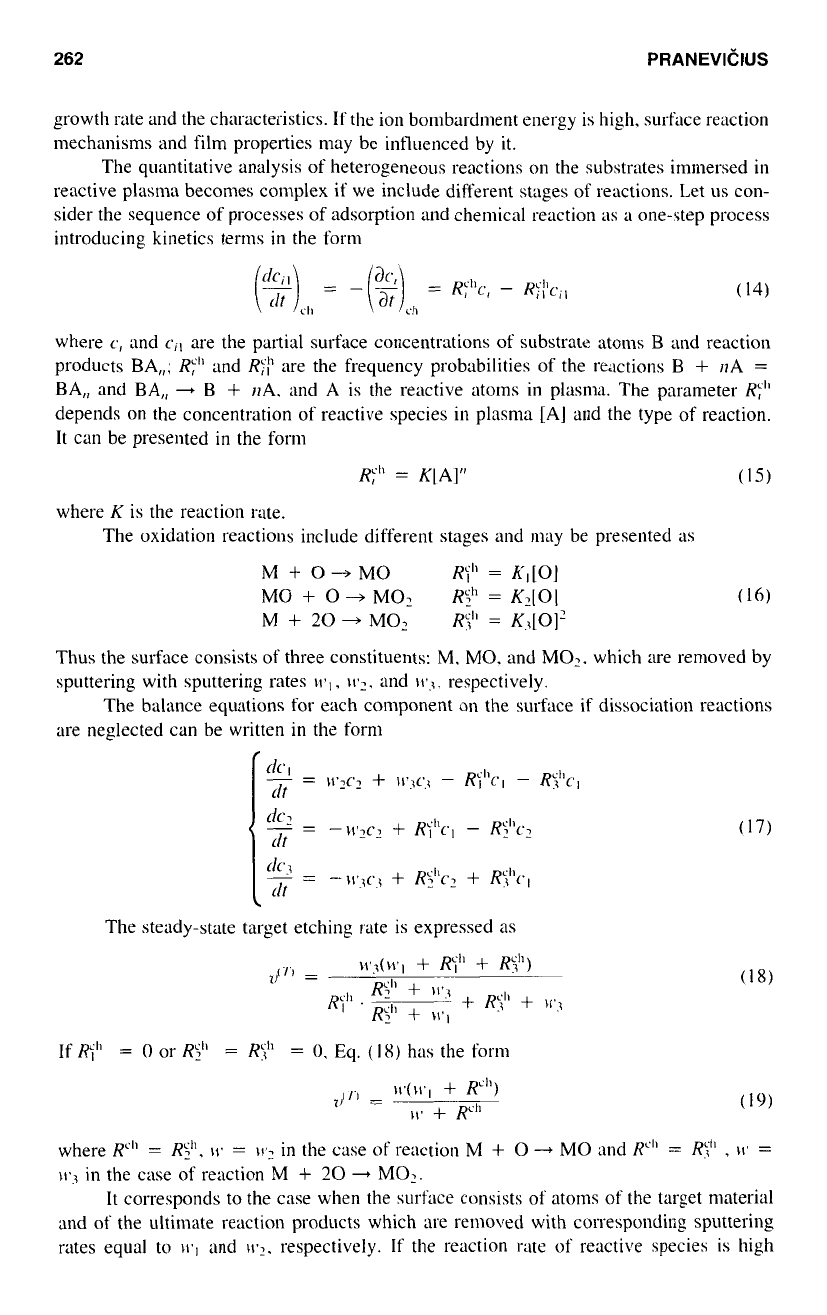
The influence
of
the intermediate reaction rates
on
the reactive etching rate is illus-
trated
in
Figs. 6a. b. and c. Figure 6d includes dependences
of
etching rate
on
the oxygen
concentration calculated for different values of the ratio of sputtering rates
H,~/H-~.
The
values
of
parameters follow: Fig.
6a.
\I-,
=
1
.0.
=
=
0.2.
K3
=
1
.0
for
K,
=
0.
0.5.
1
.O,
2.0,
5.0.
and
10.0
(curves
1-6,
respectively): Fig. 6b,
\Ill
=
1
.O.
11'~
=
0.4,
=
0.2.
KI
=
1.0.
K3
=
10"
for
K.
=
0.
10-j.
10".
IO",
and
10
(curves
1
-5,
respectively); Fig. 6c.
K,
=
1.0.
K2
=
0.
\I,,
=
I
.O,
=
0.4,
I!'~
=
0.4
for
K>
=
0,
IO-', IO-',
IO-',
1.
and
10
(curves
1-7,
respectively); Fig. 6d,
K,
=
K3
=
1
.O,
K?
=
0.
=
1.0.
~'3
=
0.2
for
\1~2/\113
=
0.1,
0.25, 0.5, 1.0, 2.0, 5.0,
and
10.0
(curves
1-7,
respectively). The values of intermediate reaction rates affect the transition between
"metallic" and "oxide" sputtering regimes.
The increase
in
K,
and
K3
decreases the steepness
of
the dependences of the etching
rate
on
the oxygen concentration. The preferential sputtering
of
the intermediate reaction
10.'
10:
IO'
1
(a) Oxygen concentration. r. LI.
10"
IO"
10
10'
10'
(b)
Oxygen concentration. r.
U.
1
.o
5
0.8
2
0.6
6
c
30
.-
f:
G
3
0.4
v!
0.2
0.0
F
I
I
1
I
I
In'
to'
10
IO'
IO'
IO1
10.'
IO'
1
IO
10:
(c) Oxygen concentration. r.
U.
(d)
Oxygen concentration, r.
11.
Figure 6
The calculated dependences
of
the sputtering
rate
on
the
cotlccntratim
of
oxygcn.

264 PRANEVICIUS
product MO
in
comparison with
MO2
makes curves more steep with the appearance of a
minimum in the case of low values
of
w2h3
(Fig. 6d).
2.3 Reactive Sputtering with the Formation
of
Volatile Compounds
For substrate etching applications, the surface atoms of substrate are constantly reacted
with chemicals supplied from the plasma phase reactions. Reaction products are volatile
and instantaneously removed. The role of
ion
bombardment
in
the mechanism of reactive
etching is not well understood.
There are at least four kinds of
thin
film materials that are deposited from plasma
etching processes: carbon. polymers. silicon, and silicon oxide. These reactions happen
in common etching conditions with conventional feed gases. Fluoro-, chlorofluoro-, and
hydrotluoro-carbon gases, such as CFJ. CF3C1, CF2C12. C2F,. and CHF3, are commonly
used in the plasma etch processes. For special cases, other gases, such as CHJ and acetone,
are used. They are carbon sources during
the
etch. Except
in
a few special cases, carbon
is actively involved
in
both plasma reactions and surface reactions
of
an etching process.”
Carbon residues are identified in many cases.
Let us consider, for illustration, silicon etching in plasma including fluorocarbon
gases. Many authors suggest that the formation
of
volatile SiFJ is the result
of
the reac-
tionsI3
Si
+
F
-+
SiF
SiF
+
F
-+
SiFl
SiF2
+
F
+
SiF3
SiF3
+
F
+
SiFJ
t
(20)
The rate of each heterogeneous reaction is proportional to the surface concentration
of atomic tluorine atoms and can be presented
as
RP‘‘
=
KJF]
(21
1
where index
j
indicates the reaction step in
Eq.
(20).
Simultaneously with the processes
of heterogeneous reactions. the deposition of atomic carbon arriving from plasma takes
place. Thus six components have to be considered on the surface: Si, SiF, SiF,. SiF3.
SiF,, and
C.
Let us assume that the arriving carbo! is adsorbed only by silicon atoms.
Denoting the relative surface concentration as
cl
(ci_
I
c,
=
l),
respectively, the system
of
kinetics equations for each component
on
the surface has the form
The steady-state surface composition can be easily obtained it’ we introduce the new
notation
(23
1
REACTIVE PLASMA: DEPOSITION AND ETCHING
265
The steady-state surface concentrations of the six components are expressed as
To
simplify the analysis of the results obtained let us assume that all intermediate
reaction rates and sputtering rates
of
reaction products determined according Eq.
(20)
are
equal
(&l1
=
p’’;
=
\byl.
i
=
1,
2,
3,
4). The silicon stationary etching rate can be
presented as
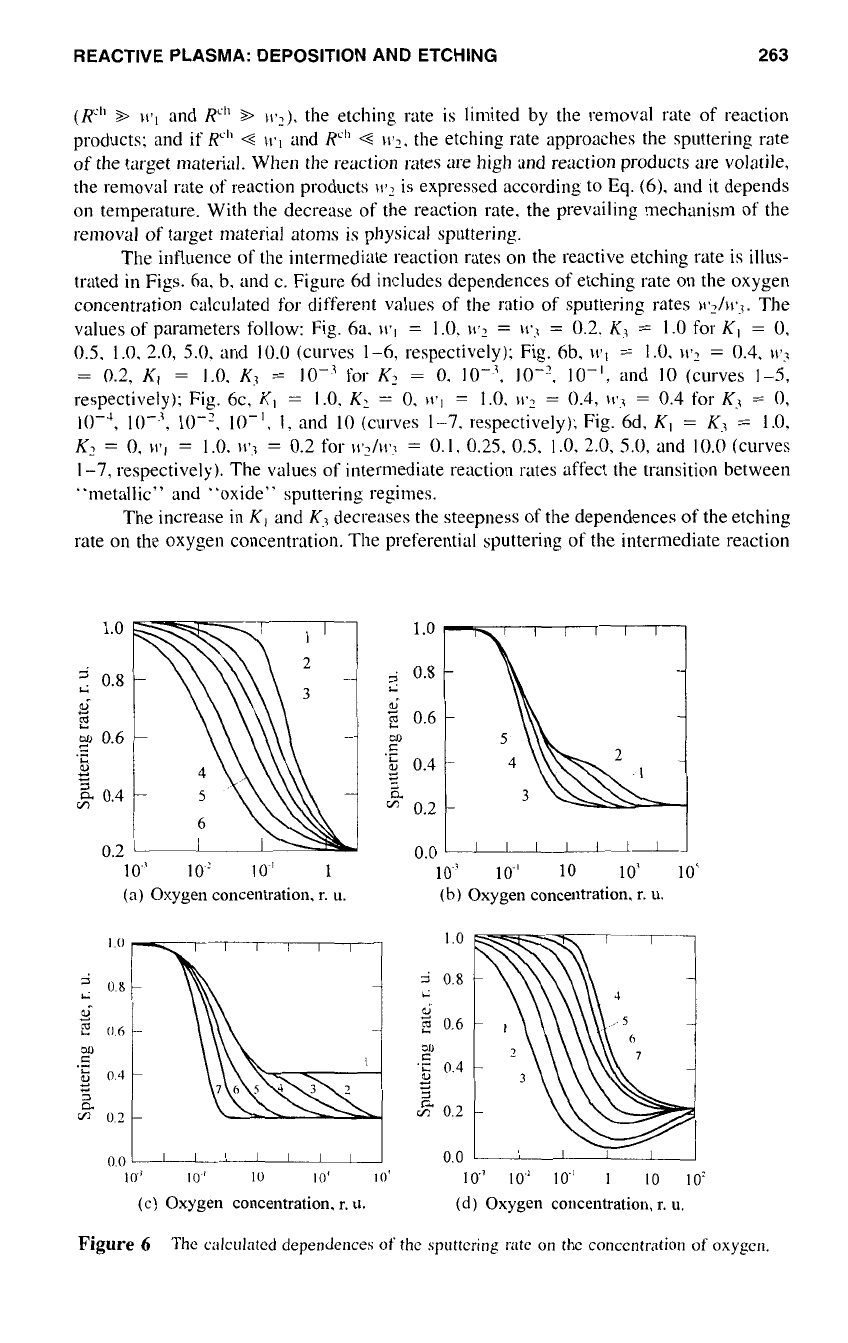
Figure
7
illustrates calculated dependences of the etching rate
of
silicon in depen-
dence on the concentration of fluorine atoms in plasma for different values
of
the ratio
K/w~,
where
K
is the carbon adsorption rate and
W(,
is the carbon sputtering rate. The
values of parameters used: [F]
=
[C],
ws/wI
=
IO,
w&I
=
0.1,
R/w,
=
0.1
for
K/W~
=
0,
0.2,
0.5,
and
1
.O
(curves 1-4, respectively). Solid lines correspond to the reaction
Si
+
4F
=
SiFl
1
and dotted lines to the reactions defined by eqs.
(20).
If we make the similar assumption that the reaction rates presented by Eq.
(20)
are
very fast and can be changed by one reaction Si
+
4F
=
SiF
T,
the number of constants
determining the etching rate decreases. In this case, the surface consists
of
three compo-
nents: Si, SiF,, and
C.
The steady-state etching rate is expressed as
7j71
=
+
RC‘’
I
+
(K/\t>3)
+
(RCh/\\’?)
(26)
The dependences of the steady-state etching rate of silicon
on
the concentration of
fluorine atoms
in
plasma for different values
K/MJ~
are presented in Fig.
7
(solid lines). It
is reasonable to assume that the above-considered case is more probable at high substrate
0.2
1
l
I
l
10”
IO’
1
10’
IO’
Concentration
of
reactwe atoms,
r.
U.
Figure
7
The dependences
of
the
etching
rate
of
silicon
on
the concentration
of
fluorine atoms.

266
PRANEVICIUS
temperatures because reaction rates are temperature dependent and sputtering rates are
practically temperature independent.
It follows that adsorption of carbon, which is removed from the surface by physical
sputtering, significantly affects etching kinetics. To decrease the effect of the adsorbed
carbon
on
the process of reactive etching in plasma, the new components are introduced
in plasma reacting chemically with carbon on the surface with the formation of volatile
compounds. For example, it is well known that carbon deposits found on the surface after
reactive etching do not exist if oxygen is present in the mixture
of
working gases.'4 The
carbon is mainly removed in the form of volatile
CO2.
The formation of
CO,
may be
introduced in the general scheme of surface reactions adding new terms to the balance
Eq.
(22).
2.4
The influence of the Heterogeneous Reactions on the
Concentration of Reactive Atoms in Plasma
In the above-considered deposition/etching processes it was assumed that the partial pres-
sure (concentration) of reactive species
in
plasma is a controllable parameter. By control-
ling the partial pressure
of
the reactive
gas
in the region of the target, it is possible
to
maintain the required arrival rate of gas atoms.
In
reality the controllable parameter is the flow of the reactive gas. When the reactive
gas
is
added to the process chamber during sputtering, it reacts with the substrates, chamber
walls, and target. The sputtering rate from the compound on the target is less than from
the pure metal surface. When this transition occurs, the overall sputtering rate drops rapidly.
If the reactive gas is supplied at a constant rate, the partial pressure of reactive gas rises
rapidly
as
the sputtering rate drops because less
gas
is being consumed in the process. If
the flow of reactive
gas
is reduced after the partial pressure has risen
on
the high level,
the partial pressure will not follow the same trajectory on the way down as
it
did on the
way up, and a reactive sputtering hysteresis loop is formed." The hysteresis effect makes
it impossible to produce certain compositions if the mass flow control
of
the reactive
gas
is used.
The severity of the hysteresis effect can be reduced by increasing the pumping speed
of the system
so
that the quantity
of
gas removed by the pumps is much greater than
consumed chemically.
In
this way, the destabilizing pressure swings at the target are
greatly reduced when the target changes from the metallic
to
the compound mode.'"
A
solution to the instability can be obtained by providing control of the partial
pressure of the reactive gas with rapid feedback. In many cases this can best be done by
using an observation of the spectral emission of the
gas
or the sputtered metallic atoms
to control the admission of the reactive gas. Through partial pressure control of the reactive
gas it is possible all material compositions in spite of the hysteresis effect. The other
controllable parameters are power to the sputtering target and flows of the reactive gas
admitted into the chamber and pumped out. If the reactive
gas
partial pressure is held
constant at the same time that the power to the sputtering target is held constant, a balance
between consumption and availability of the reactive gas is maintained. If there is process
disturbance, such as an arc on the target, a partial pressure controller will momentarily
reduce flow to maintain constant partial pressure.
It
follows that the analysis of the interdependences between the flow of reactive
atoms and their partial pressure during reactive deposition/sputtering is complex and of
great importance. Few attempts have been made to analyze the hysteresis effect, but they
REACTIVE PLASMA: DEPOSITION AND ETCHING
267
have
a
qualitative character and are aimed to explain the hysteresis phenomena in depen-
dence on the properties
of
reactive species and the material
of
the target.
Heterogeneous reactions use the reactive species and their concentrations in plasma
decreases.
It
may seem that assumption of the constant concentration
in
plasma is fulfilled
if the flow of reactive atoms is high. There may be the illusion that in the case of high
flow of reactive species we have effective removal of reaction products from the reaction
zone and
so
high
a
supply of reactive atoms that the changes in their concentration as the
result of heterogeneous processes are negligible. However, it
is
important to remember
that the processes of dissociation of molecules and formation of reactive atomdradicals
in plasma have finite time. In the case of
a
high flow of reactive atoms they are passing
the reaction zone without dissociation and do not take part in the heterogeneous processes.
E~perimentally".'~ it is observed that with the increase in flow of CF4 species the silicon
etching rate decreases. Another experimentally observed fact is that with increase in the
surface area of treated samples the etching rate decreases. It is explained that with the
increase of surface area affected by heterogeneous reactions the concentration of reactive
species decreases
as
their consumption increases (loading effect).
Such experiments require measurements
of
component concentrations in plasma.
Usually it is not
a
simple experimental problem because in many cases the life time of
active reactive species is short and the composition of components measured by the mass
analyzer at some distance from the reaction zone does not correspond to the plasma
composition in the reaction zone. Experimental results concerning the dependence of the
composition of reactive species
in
a
reaction zone on the flow
of
oxygednitrogen are not
found in the literature. Some results exist in studies of silicon etching in C2F, plasma.
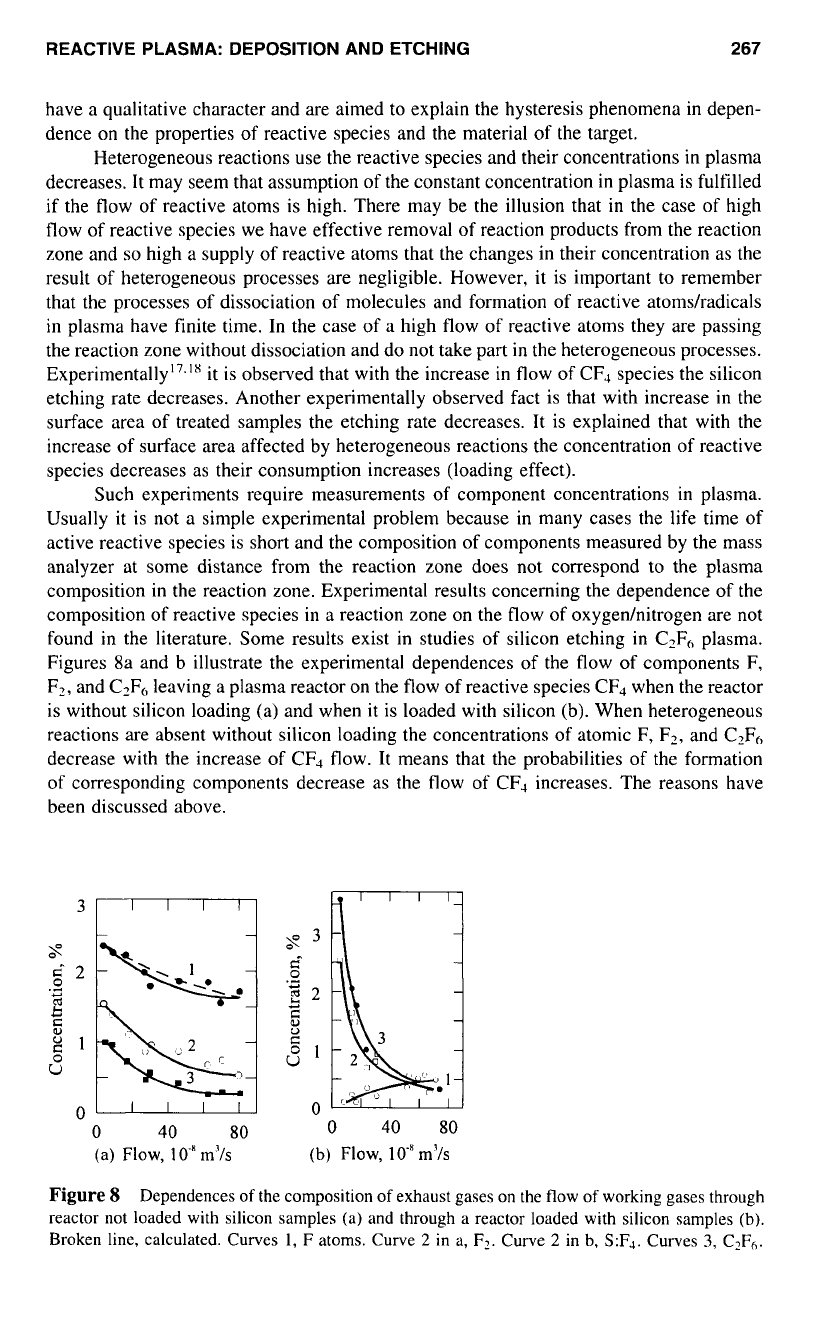
Figures 8a and b illustrate the experimental dependences of the flow of components F,
F?, and CIF(, leaving
a
plasma reactor on the flow of reactive species CF., when the reactor
is without silicon loading (a) and when it is loaded with silicon (b). When heterogeneous
reactions are absent without silicon loading the concentrations of atomic F,
F?,
and CzFh
decrease with the increase
of
CF, flow. It means that the probabilities of the formation
of corresponding components decrease as the flow of CF4 increases. The reasons have
been discussed above.
0
40
80
0
40
80
(a)
Flow,
10"
m'/s
(b)
Flow,
IOn
m'/s
Figure
8
Dependences
of
the composition
of
exhaust gases on the flow
of
working gases through
reactor not loaded with silicon samples
(a)
and through
a
reactor loaded with silicon samples
(b).
Broken line, calculated. Curves
1,
F
atoms. Curve
2
in a,
F?.
Curve
2
in
b,
S:F,.
Curves
3,
CzF6.

268
PRANEVICIUS
In the presence of heterogeneous reactions the concentration of atomic F leaving
the reactor
in
the range of low flow of CF, is about zero, and with an increase in CF.,
flow the concentration of
F
slowly increases. The concentrations of other components
sharply decrease. As the concentration of atomic F increases, the concentration of reaction
products SiF, sharply decreases (Fig. 8b). This indicates that atomic F determines the
etching rate and that heterogeneous and homogeneous processes
in
plasma have
to
be
considered simultaneously with heterogeneous reactions.
Consideration of homogeneous processes
in
plasma is complex and
not
the goal
of
the present work. There are many possible reactions
in
reactive plasma taking place with
different cross-sections and different lifetimes
of
reaction products.18 However, the most
important ones are related with the formation and recombination
of
atomic F.
In
the plasma of a mixture of CF,
+
Ar, the reaction
e
+
CF,
-
CF2
+
F
+
e
(27)
is the typical dissociation reaction with the formation of atomic F.
Recombination of atomic
F
takes place mainly on the surfaces of chamber wall:
CF3
+
F
+
M
CF,
+
M
(28)
where
M
is the reactor wall or other particle.
Other recombination reactions, e.g., F
+
F
+
M
-
F?
+
M
and CF2
+
CF3
-
C?F,
+
M,
are possible. However, their reaction rates are lower than the rate of the
reaction
(28).
The following consideration will be done assuming that there are three components
in
the plasma: CF.,, F. and CF3. Let us denote the dissociation rate as
kD
and recombination
rate as
k,;
6
is the tlow of working gas,
no
the initial concentration
of
CF.,, and V,, the
volume of the reactor.
The system of balance equations
for
each component
in
plasma then may be pre-
sented as
where the function T([F], c,) determines the consumption rate of atomic F on the surface
of the reacting solid. In the case of a heterogeneous reaction, Si
+
4
F
-
SiF.,
T
as
T([F].
c,)
=
K[FI4
.
(’1
(30)
In
the case
of
the heterogeneous reactions defined according
Eqs
(201,
the function R[F],
c,)
has the form
REACTIVE PLASMA: DEPOSITION AND ETCHING
269
Let us introduce the notation
all
=
kl,
[e]V,,,
=
kRnOVl,,
and
(P
=
[F]/no. The steady-
state solutions of Eqs.
(29)
are expressed as
and the concentration of atomic tluorine atoms
in
plasma
in
relative units is found as the
solution of the quadratic equation
aR(P2
+
(6
+
6,,)(p
-
y61,
=
0
(33)
where
y
is a dimensionless coefficient, determining the consumption rate of atomic flourine
on the surface
of
silicon:
y
=
V/(V
+
V,)
and
V,
=
S.C.T
([F], c,)/[F].
If
heterogeneous reactions are included, the coefficient
y
=
1,
and the relative
concentration
of
atomic flourine atoms
in
plasma is the solution
of
the equation
+
*D)'
+
46116~
-
(6
+
61))
cp=
W<
(34)
If recombination does not take place
(aR
-
O),
Eq
(34)
takes the form
cp
=
6[,/(6
+
all)
and thus, with the increase in
aR,
(P
increases and approaches
1
when
aI1
>>(B
If we include heterogeneous reactions on the target material,
Eq.
(33)
becomes
nonlinear. Let us consider the formation
of
SiF4 in four steps according Eq.
(20).
In
this
case the etching rate is equal
to
+
"81,).
where
Rhh
is the reaction rate
if
[F]
=
no
and
V,)
is the volume
of
the gas, which is on
the surface of solids,
c/;
=
RZ"
(P/(
\vi
+
Rh"
(P)
and
D
=
1
+
dl
+
dl&
+
c/,c/?d3.
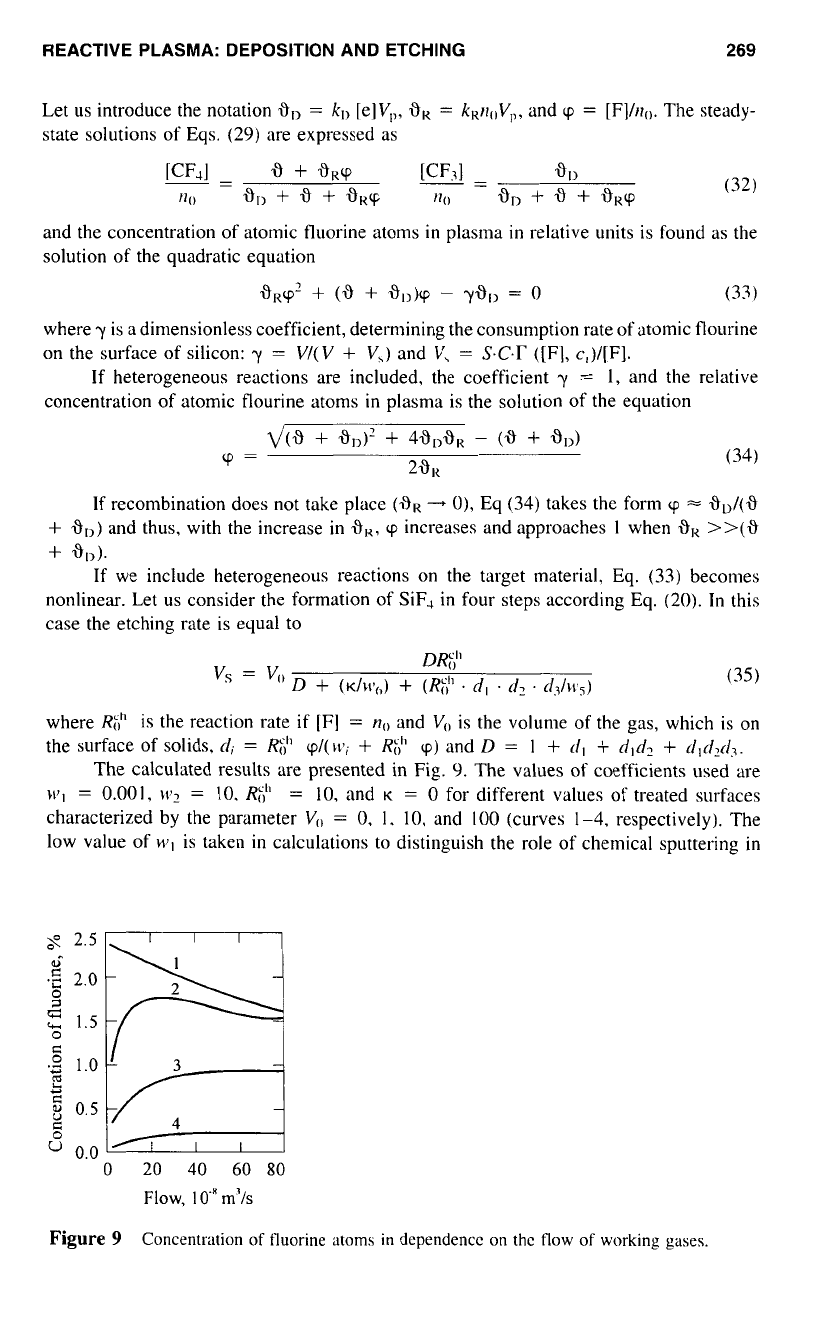
The calculated results are presented in Fig.
C).
The values
of
coefficients used are
wl
=
0.001,
\v1
=
IO.
Rh"
=
10,
and
K
=
0
for different values of treated surfaces
characterized by the parameter
V,,
=
0,
1.
10.
and
100
(curves
1-4,
respectively). The
low value
of
wI
is taken
in
calculations to distinguish the role of chemical sputtering
in
2.5
2.0
!5
2
1.5
ai
3
0
E
.S
1.0
g
0.5
c)
E
E
C
0
0.0
0
20
40
60
80
Flow,
10"
m'/s
Figure
9
Concentration
of
fluorine atoms in dependence on the
flow
of
working
gases.
270
PRANEVICIUS
5
0.05
i
0.04
c
Lu
0.03
C
g
0.02
G
0.01
0
IO”
IO”
1
10 10’
10’
Surface area,
r.
U.
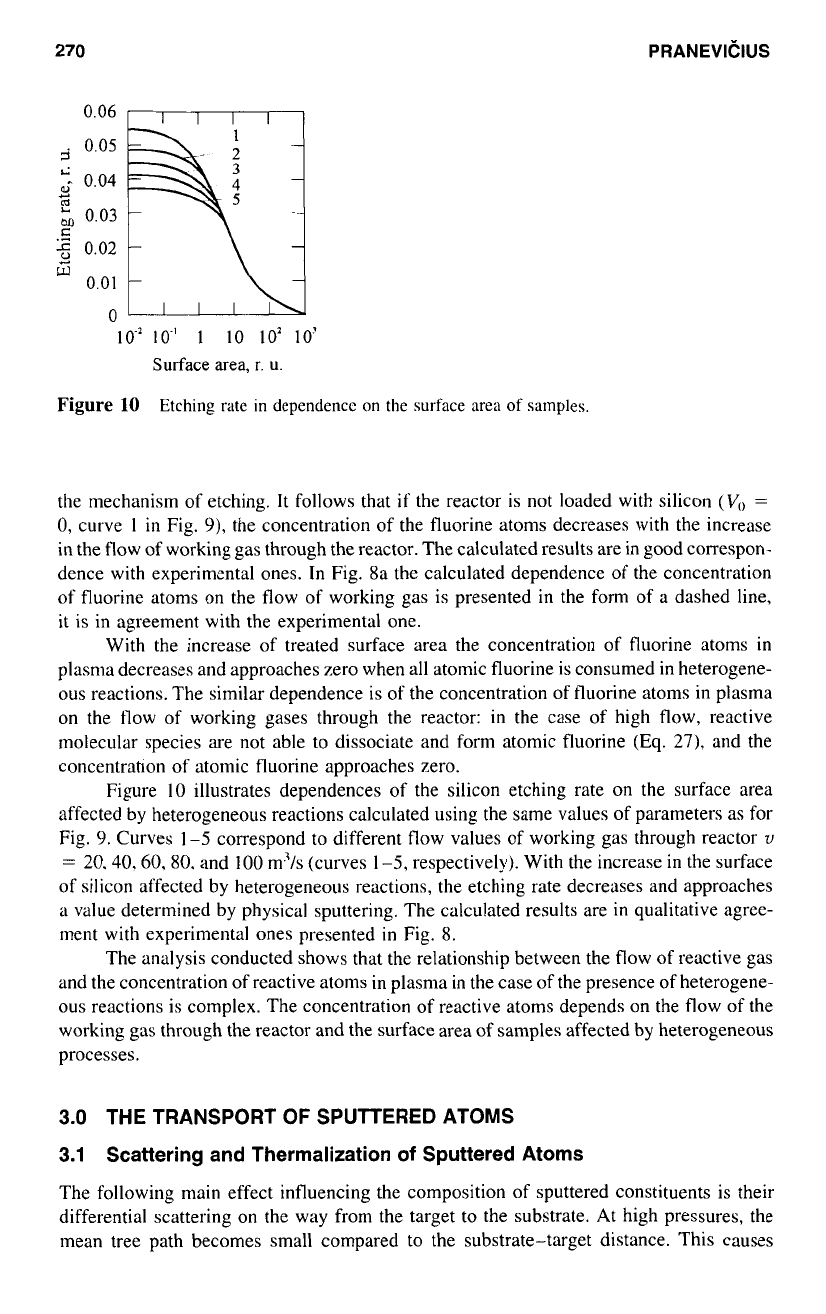
Figure
10
Etching rate
in
dependencc
on
the surface area of samples.
the mechanism of etching. It follows that
if
the reactor is not loaded with silicon
(V,,
=
0,
curve
1
in Fig.
9),
the concentration of the fluorine atoms decreases with the increase
in the flow of working gas through the reactor. The calculated results are in good correspon-
dence with experimental ones. In Fig. Sa the calculated dependence of the concentration
of fluorine atoms on the flow of working gas is presented in the form of a dashed line,
it is in agreement with the experimental one.
With the increase
of
treated surface area the concentration of fluorine atoms
in
plasma decreases and approaches zero when all atomic fluorine is consumed in heterogene-
ous reactions. The similar dependence is
of
the concentration
of
fluorine atoms in plasma
on the flow
of
working gases through the reactor: in the case
of
high flow, reactive
molecular species are not able to dissociate and form atomic fluorine (Eq.
27),
and the
concentration of atomic fluorine approaches zero.
Figure
10
illustrates dependences
of
the silicon etching rate on the surface area
affected by heterogeneous reactions calculated using the same values of parameters as for
Fig.
9.
Curves
1-5
correspond to different flow values of working gas through reactor
U
=
20.40.60.80.
and
100
m3/s (curves
1-5,
respectively). With the increase
in
the surface
of silicon affected by heterogeneous reactions, the etching rate decreases and approaches
a value determined by physical sputtering. The calculated results are
in
qualitative agree-
ment with experimental ones presented
in
Fig.
8.
The analysis conducted shows that the relationship between the flow
of
reactive gas
and the concentration of reactive atoms in plasma
in
the case of the presence of heterogene-
ous
reactions is complex. The concentration of reactive atoms depends on the flow of the
working gas through the reactor and the surface area of samples affected by heterogeneous
processes.
3.0
THE TRANSPORT
OF
SPUTTERED ATOMS
3.1
Scattering and Thermalization
of
Sputtered Atoms
The following main effect influencing the composition of sputtered constituents is their
differential scattering
on
the way from the target
to
the substrate. At high pressures, the
mean tree path becomes small compared to the substrate-target distance. This causes
REACTIVE PLASMA: DEPOSITION AND ETCHING
271
sputtered particles to undergo several scattering processes with particles of the working
gas before they reach a substrate. Therefore the original fluxes
of
components at the target
surface are smeared out, and the composition of the particles reaching the substrate cannot
be described by
Eqs.
(1
2)
and
(1
3).
The flux of i-type components at the surface of substrate
may be presented asfi
=
Afro
exp(
-LA,),
where
L
is the distance between substrate and
target and
X,
is the mean free path'" which is equal to
where
N,
=
fi/i,
and
i,
is the thermal velocity of atoms.
dl
and
rfA
are diameters
of
i-type
sputtered and working gas atoms, and
M,
and
MA
are the masses
of
sputtered atoms and
working gas atoms, respectively.
p
is the working gas pressure.
The quantitative analysis of the variations of the tlux values and
flux
compositions
in
dependence on the working gas pressure shows that if
LA,
<
0.05,
then less than
5%
of particles ejected from the target are scattered. and the trajectories of particles
in
the
gap between substrate and target can be considered as straight lines.
If
LA,
>
3,
then
95%
of
sputtered particles are scattered, and the mass-transfer
mechanism may be considered as the process of enhanced diffusion. The flux of i-type
atoms at the surface of the substrate can be evaluated as
It is important to note that the scattering process not only decreases the tlux
of
particles arriving but also changes the composition. In the case of two-component tlux.
the ratio of fluxes of arriving atoms can be evaluated as
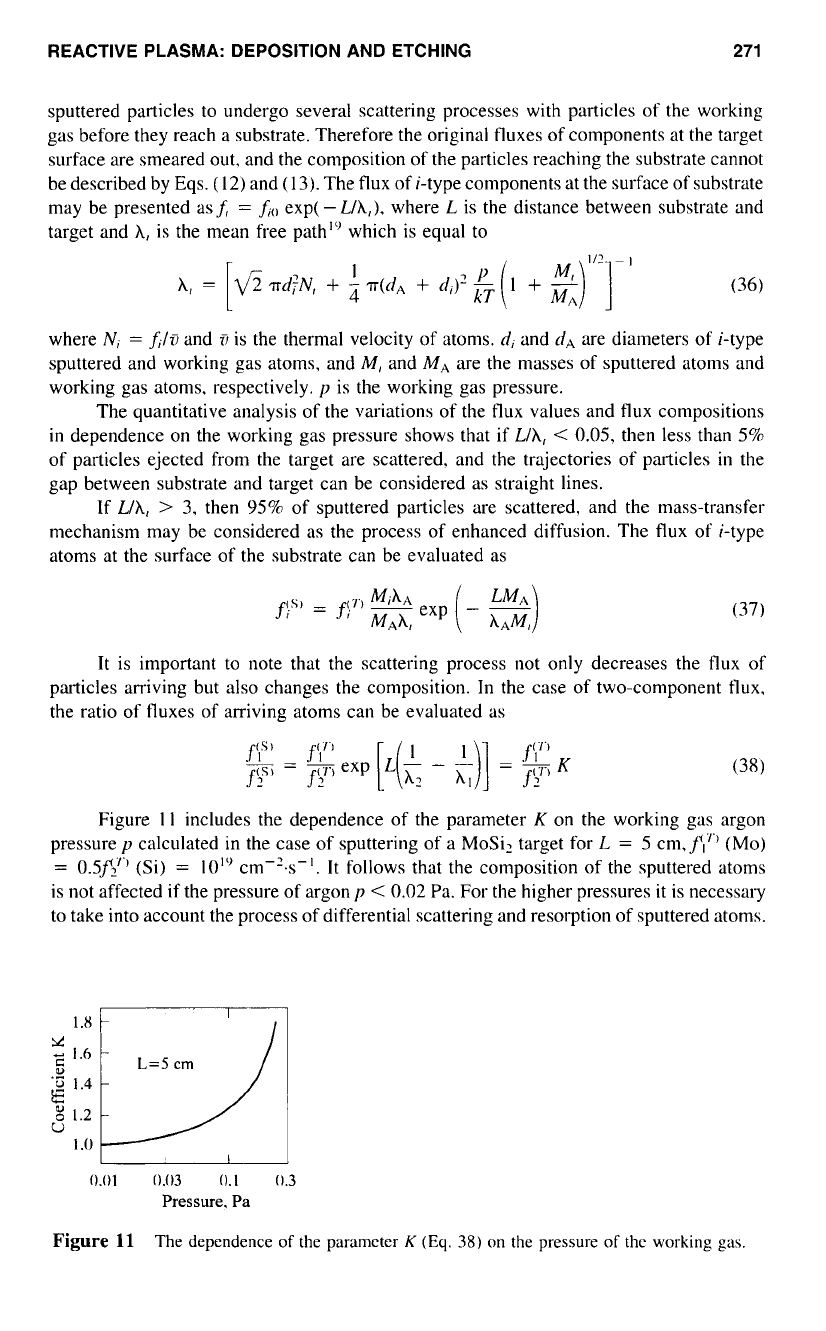
Figure
1 1
includes the dependence of the parameter
K
on the working gas argon
pressure
y
calculated in the case of sputtering of a MoSi, target for
L
=
5
cm,fI7" (MO)
=
0.5f2"'
(Si)
=
10'"
cm-2.s-'.
It
follows that the composition
of
the sputtered atoms
is not affected if the pressure of argon
p
<
0.02
Pa. For the higher pressures it is necessary
to
take into account the process of differential scattering and resorption of sputtered atoms.
I
l
1
0.01
0.03
0.1
0.3
Pressure.
Pa
Figure
11
The dependence
of
the parameter
K
(Eq.
38)
on the pressure
of
the working
gas.
