Salby M.L. Fundamentals of Atmospheric Physics
Подождите немного. Документ загружается.

560
17 The Middle Atmosphere
z ~ .
I
I "1
I '.
%
"',,,2
- I
-C +C
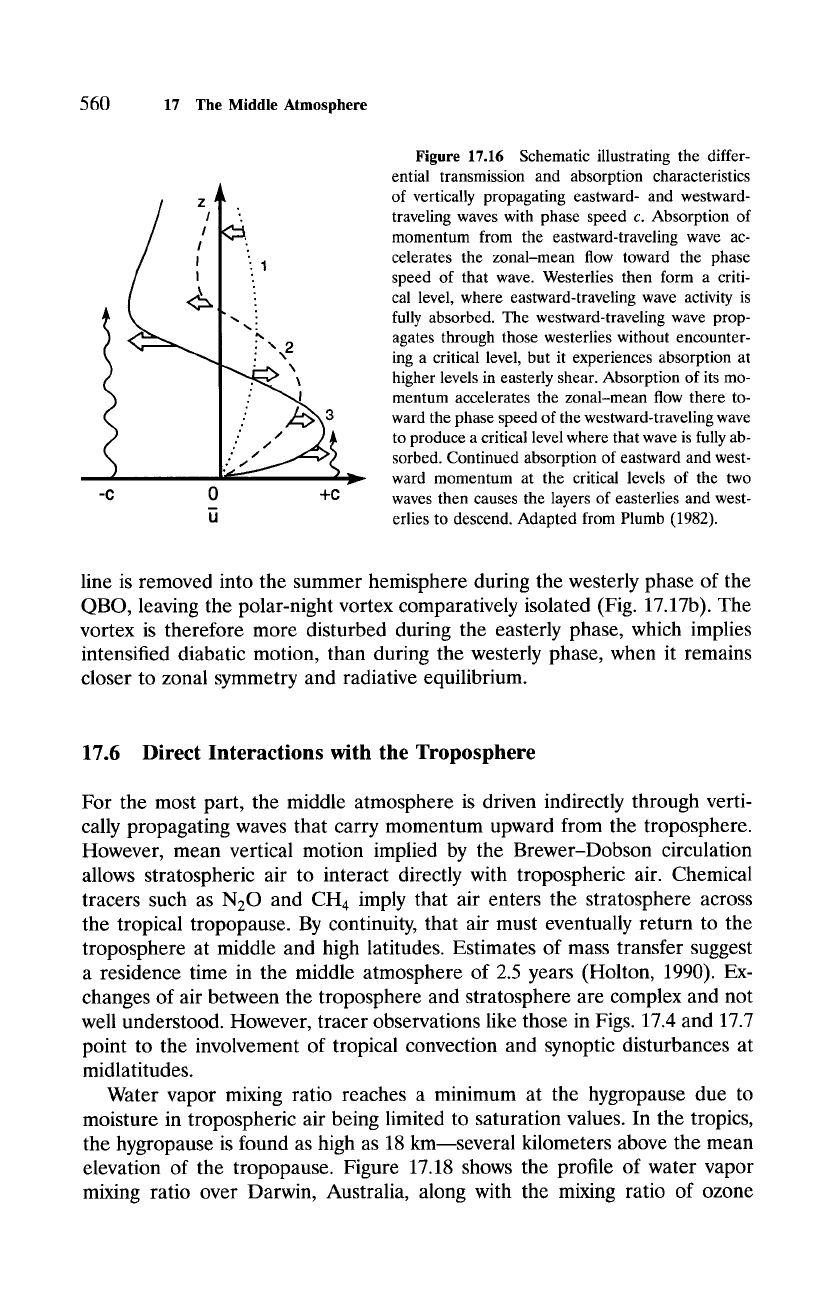
Figure 17.16
Schematic illustrating the differ-
ential transmission and absorption characteristics
of vertically propagating eastward- and westward-
traveling waves with phase speed c. Absorption of
momentum from the eastward-traveling wave ac-
celerates the zonal-mean flow toward the phase
speed of that wave. Westerlies then form a criti-
cal level, where eastward-traveling wave activity is
fully absorbed. The westward-traveling wave prop-
agates through those westerlies without encounter-
ing a critical level, but it experiences absorption at
higher levels in easterly shear. Absorption of its mo-
mentum accelerates the zonal-mean flow there to-
ward the phase speed of the westward-traveling wave
to produce a critical level where that wave is fully ab-
sorbed. Continued absorption of eastward and west-
ward momentum at the critical levels of the two
waves then causes the layers of easterlies and west-
erlies to descend. Adapted from Plumb (1982).
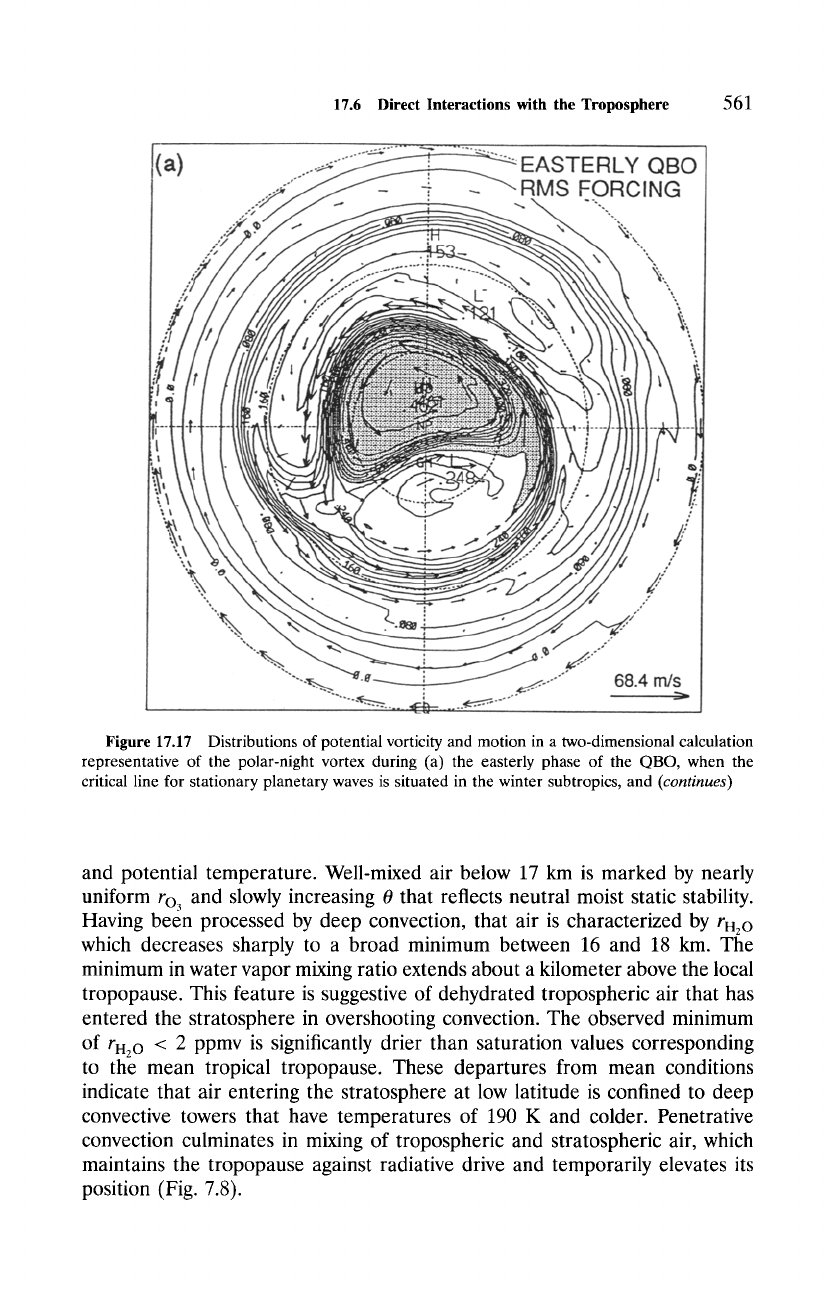
line is removed into the summer hemisphere during the westerly phase of the
QBO, leaving the polar-night vortex comparatively isolated (Fig. 17.17b). The
vortex is therefore more disturbed during the easterly phase, which implies
intensified diabatic motion, than during the westerly phase, when it remains
closer to zonal symmetry and radiative equilibrium.
17.6 Direct Interactions with the Troposphere
For the most part, the middle atmosphere is driven indirectly through verti-
cally propagating waves that carry momentum upward from the troposphere.
However, mean vertical motion implied by the Brewer-Dobson circulation
allows stratospheric air to interact directly with tropospheric air. Chemical
tracers such as N20 and CH 4 imply that air enters the stratosphere across
the tropical tropopause. By continuity, that air must eventually return to the
troposphere at middle and high latitudes. Estimates of mass transfer suggest
a residence time in the middle atmosphere of 2.5 years (Holton, 1990). Ex-
changes of air between the troposphere and stratosphere are complex and not
well understood. However, tracer observations like those in Figs. 17.4 and 17.7
point to the involvement of tropical convection and synoptic disturbances at
midlatitudes.
Water vapor mixing ratio reaches a minimum at the hygropause due to
moisture in tropospheric air being limited to saturation values. In the tropics,
the hygropause is found as high as 18 km~several kilometers above the mean
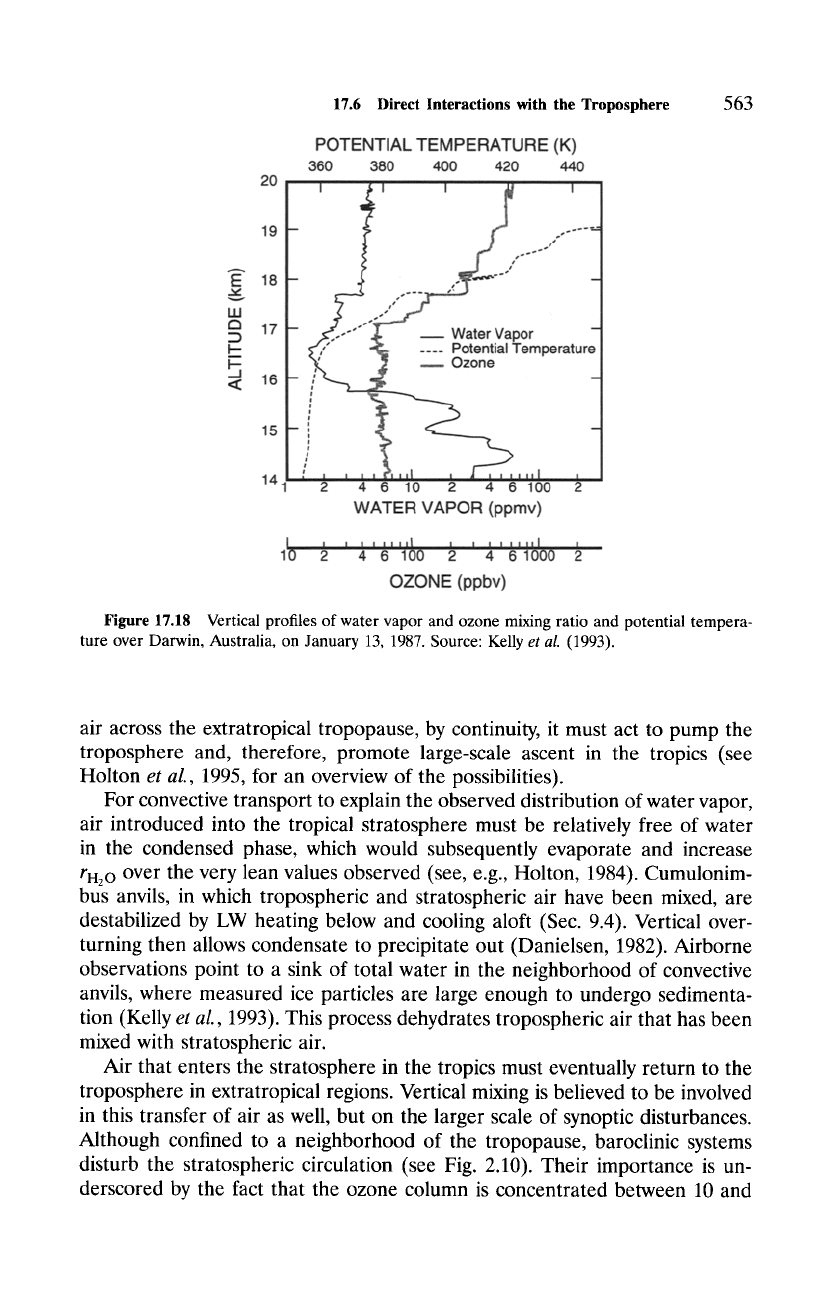
elevation of the tropopause. Figure 17.18 shows the profile of water vapor
mixing ratio over Darwin, Australia, along with the mixing ratio of ozone
17.6
Direct Interactions with the Troposphere
561
~a)
EASTERLY QBO
RMS FORCING
9 ,~,. -o . ~
-- .-- .~/
.... .~.....
"-e.o~ ~
.... 68.4 m/s
9
.... .~....
~
__..~.... ...... --_
Figure 17.17 Distributions of potential vorticity and motion in a two-dimensional calculation
representative of the polar-night vortex during (a) the easterly phase of the QBO, when the
critical line for stationary planetary waves is situated in the winter subtropics, and
(continues)
and potential temperature. Well-mixed air below 17 km is marked by nearly
uniform ro3 and slowly increasing 0 that reflects neutral moist static stability.
Having been processed by deep convection, that air is characterized by rH~ o
which decreases sharply to a broad minimum between 16 and 18 km. The
minimum in water vapor mixing ratio extends about a kilometer above the local
tropopause. This feature is suggestive of dehydrated tropospheric air that has
entered the stratosphere in overshooting convection. The observed minimum
of rH2o < 2 ppmv is significantly drier than saturation values corresponding
to the mean tropical tropopause. These departures from mean conditions
indicate that air entering the stratosphere at low latitude is confined to deep
convective towers that have temperatures of 190 K and colder. Penetrative
convection culminates in mixing of tropospheric and stratospheric air, which
maintains the tropopause against radiative drive and temporarily elevates its
position (Fig. 7.8).
562
17 The Middle Atmosphere
(b)
WESTERLY QBO
RMS FORCING
,',,3 r/, ~_,. f
9 ,~,~ ', \ - ~ ]
, /
~ ,o
"% ~ ,7
9 ,,o
:- N ~ j-"
""-:-" .----'" 59.5 m/s
: t- "~ ~._
""'r j 1 _f.,...... - "'--
.... ..~.7. .... ~ __ --
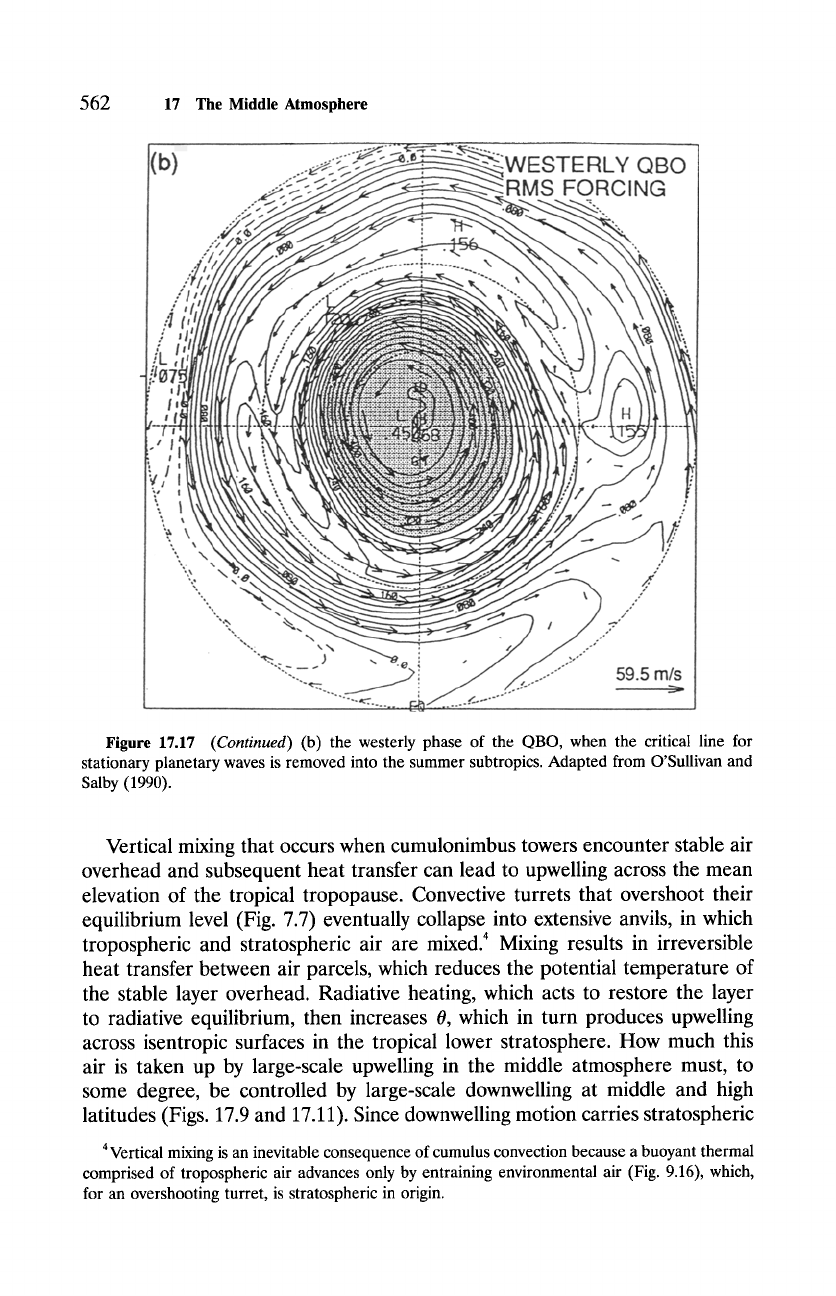
Figure 17.17
(Continued)
(b) the westerly phase of the QBO, when the critical line for
stationary planetary waves is removed into the summer subtropics. Adapted from O'Sullivan and
Salby (1990).
Vertical mixing that occurs when cumulonimbus towers encounter stable air
overhead and subsequent heat transfer can lead to upwelling across the mean
elevation of the tropical tropopause. Convective turrets that overshoot their
equilibrium level (Fig. 7.7) eventually collapse into extensive anvils, in which
tropospheric and stratospheric air
are mixed. 4
Mixing results in irreversible
heat transfer between air parcels, which reduces the potential temperature of
the stable layer overhead. Radiative heating, which acts to restore the layer
to radiative equilibrium, then increases 0, which in turn produces upwelling
across isentropic surfaces in the tropical lower stratosphere. How much this
air is taken up by large-scale upwelling in the middle atmosphere must, to
some degree, be controlled by large-scale downwelling at middle and high
latitudes (Figs. 17.9 and 17.11). Since downwelling motion carries stratospheric
4 Vertical mixing is an inevitable consequence of cumulus convection because a buoyant thermal
comprised of tropospheric air advances only by entraining environmental air (Fig. 9.16), which,
for an overshooting turret, is stratospheric in origin.
17.6
Direct Interactions with the Troposphere
563
20
19
E 18
LU
a 17
:)
< 16
15
14
POTENTIAL TEMPERATURE (K)
360 380 400 420 440
I I I I
f
:" -- Water V~por -
~ oO~gt, a emperature
/,, -
1 2 4 6 10 2 4 6 100 2
WATER VAPOR
(ppmv)
I
, , , ..... I , , ]bl00
2
10 2 4 6 100 2 ~l~l I
OZONE (ppbv)
Figure
17.18 Vertical profiles of water vapor and ozone mixing ratio and potential tempera-
ture over Darwin, Australia, on January 13, 1987. Source: Kelly
et al.
(1993).
air across the extratropical tropopause, by continuity, it must act to pump the
troposphere and, therefore, promote large-scale ascent in the tropics (see
Holton
et aL, 1995, for an overview of the possibilities).
For convective transport to explain the observed distribution of water vapor,
air introduced into the tropical stratosphere must be relatively free of water
in the condensed phase, which would subsequently evaporate and increase
rH2 o over the very lean values observed (see, e.g., Holton, 1984). Cumulonim-
bus anvils, in which tropospheric and stratospheric air have been mixed, are
destabilized by LW heating below and cooling aloft (Sec. 9.4). Vertical over-
turning then allows condensate to precipitate out (Danielsen, 1982). Airborne
observations point to a sink of total water in the neighborhood of convective
anvils, where measured ice particles are large enough to undergo sedimenta-
tion (Kelly
et aL, 1993). This process dehydrates tropospheric air that has been
mixed with stratospheric air.
Air that enters the stratosphere in the tropics must eventually return to the
troposphere in extratropical regions. Vertical mixing is believed to be involved
in this transfer of air as well, but on the larger scale of synoptic disturbances.
Although confined to a neighborhood of the tropopause, baroclinic systems
disturb the stratospheric circulation (see Fig. 2.10). Their importance is un-
derscored by the fact that the ozone column is concentrated between 10 and

564 17 The Middle Atmosphere
20 km (Fig. 1.17). It is also suggested by daily variations of Eo3, which are as
large as seasonal variations.
The daily distribution of total ozone (Fig. 1.19a) is punctuated by anoma-
lies of high Eo3 that circumscribe the poles. Comparison with the 500-mb
circulation in Fig. 1.9a reveals that those anomalies are positively correlated
with midlatitude cyclones. According to the pressure on the 375 K isentropic
surface, which is contoured in Fig. 1.19, midlatitude cyclones coincide with
depressions of 0 surfaces in the lower stratosphere. Isentropic surfaces are
deflected downward to greater pressure over mature baroclinic systems, in
which static stability is reduced. Ozone-rich air descending along those sur-
faces undergoes compression, which magnifies Eo3 locallymby as much as
100%. Analogous behavior occurs on planetary dimensions during a strato-
spheric warming, when air is advected meridionally along isentropic surfaces
(refer to Fig. 17.14). Conversely, expansion and adiabatic cooling when air
ascends isentropically over an anticyclone have been found to support the for-
mation of extensive polar stratospheric clouds (PSCs) (Jones
et al.,
1990; Pitts
et al.,
1990).
Meteorological analyses suggest that isentropic surfaces undergo vertical de-
flections of a couple of kilometers. However, airborne measurements record
anomalous concentrations of stratospheric species as low as 700 mb. Strato-
spheric air enters the troposphere inside
tropopause folds,
in which isopleths
of stratospheric tracers are overturned and drawn downward into the tropo-
sphere along frontal zones that intensify during the development of baroclinic
systems. Their impact is manifested in the zonal-mean ozone mixing ratio
(Fig. 1.17), which dips downward and enhances ozone number density at the
poleward edge of the subtropical jet--precisely where baroclinic systems de-
velop (Sec. 15.2).
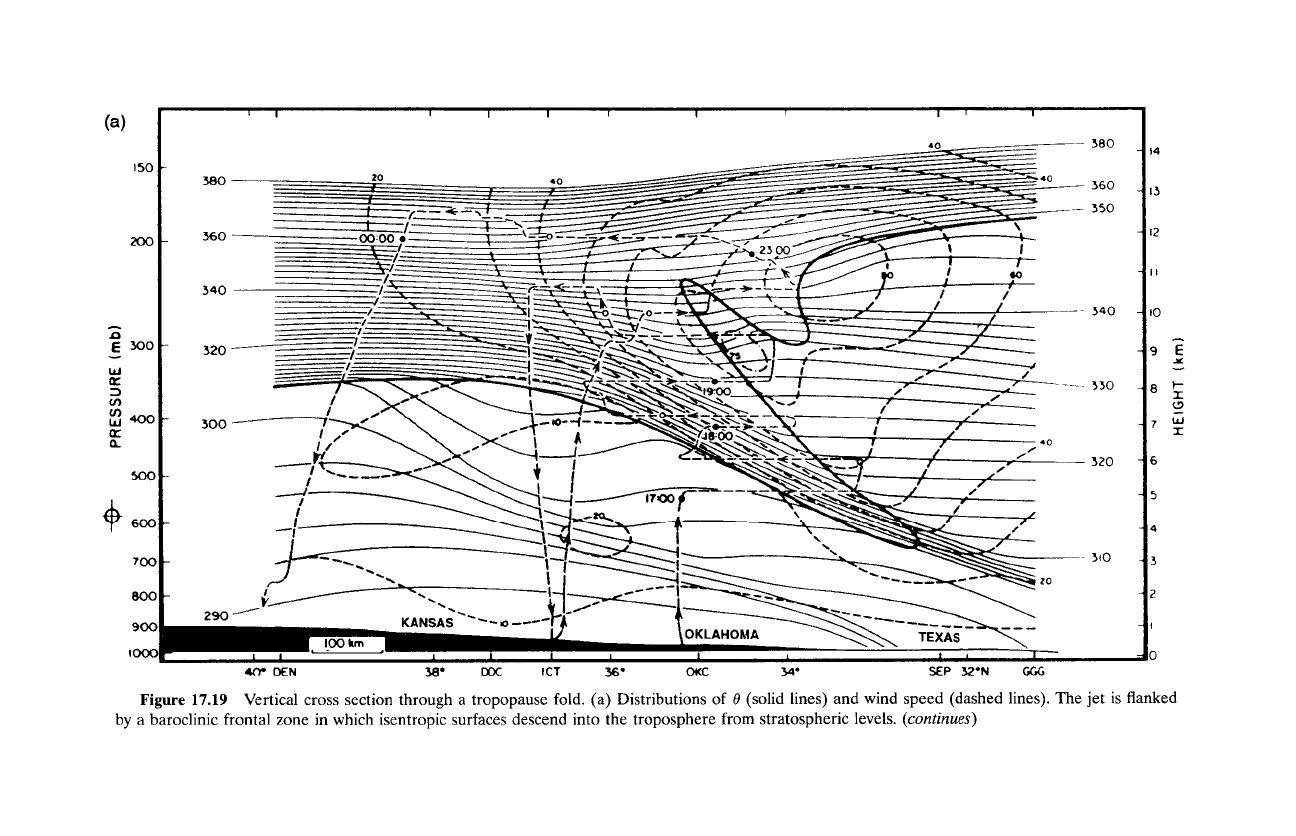
Figure 17.19 shows a cross section of a tropopause fold. Isentropic sur-
faces (Fig. 17.19a), which reflect stratospheric air in strong vertical stability
(e.g., large
30/3z),
have been drawn downward and concentrated into a tilting
frontal zone that separates cold and warm air. Strongly baroclinic, that region
develops from deformations accompanying the amplification of a baroclinic
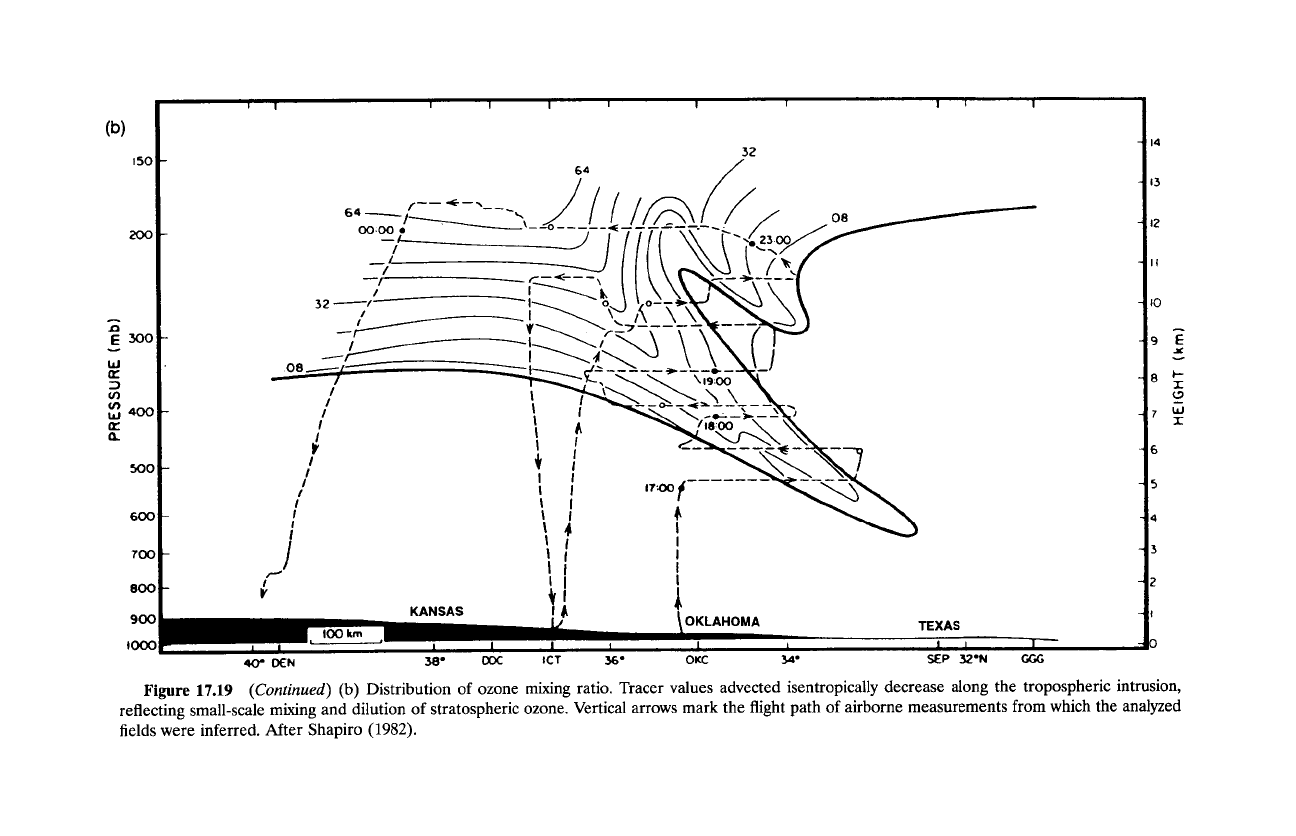
disturbance (Hoskins, 1982). According to the distribution of ozone mixing
ratio (Fig. 17.19b), stratospheric air drawn into the frontal zone is diluted by
small-scale mixing that diminishes
I'O3
along the intrusion. Once it has entered
the troposphere, the water-soluble species 0 3 quickly encounters convective
systems, in which it is absorbed, precipitated to the surface, and destroyed
through oxidation processes.
17.7 Heterogeneous Chemical Reactions
The chemical reactions considered in Secs. 17.1 and 17.2 involve species only in
gas phase. During the 1970s and 1980s, these and other elements of gas-phase
300
360
350
340
330
320
310
14
13
I2
ll
10
-
35
-
0:
7L;I
2
6
5
4
3
2
I
3
Figure
17.19
Vertical cross section through
a
tropopause fold. (a) Distributions of
0
(solid lines) and wind speed (dashed lines). The jet is flanked
by a baroclinic frontal zone in which isentropic surfaces descend into the troposphere from stratospheric levels.
(continues)
(w~)
1HO13H
(qw)
3~IN553~Id
64
i'
14
-
I3
-
I2
11
-
I0
-9
-I
-6
-5
4
-3
-2
go
lo00
0
W
DEN
38'
Mx
ICT
36.
OKC
34'
Figure
17.19
(Continued)
(b)
Distribution
of
ozone mixing ratio. Tracer values advected isentropically decrease along the tropospheric intrusion,
reflecting small-scale mixing and dilution
of
stratospheric ozone. Vertical arrows mark the flight path
of
airborne measurements from which the analyzed
fields were inferred. After Shapiro
(1982).

17.7 Heterogeneous Chemical Reactions
567
photochemistry were the focus of intensive investigation. Although an impact
from anthropogenic species was suggested, it was not expected to emerge
clearly before the twenty-first century and even then it was expected to reduce
the column abundance of ozone by only about 5%. The timing and magnitude
of anticipated changes were set by two considerations: (1) Reactive species
had to increase to relatively large concentrations before catalytic destruction
of ozone via (17.10) and (17.13) became sufficiently fast. (2) Those reactions
were favored at higher altitudes, where only a small fraction of the ozone
column is affected.
For these reasons, the discovery of the Antarctic ozone hole in 1985
caught much of the scientific community by surprise. Moreover, the order-
of-magnitude greater depletions observed (e.g., Fig. 1.19b) were found at
latitudes where ozone was thought to be photochemically inert~because UV
fluxes there are small. The key ingredient not considered in earlier investiga-
tions was the presence of solid phase in the stratosphere, which is normally
excluded by very low mixing ratios of water vapor.
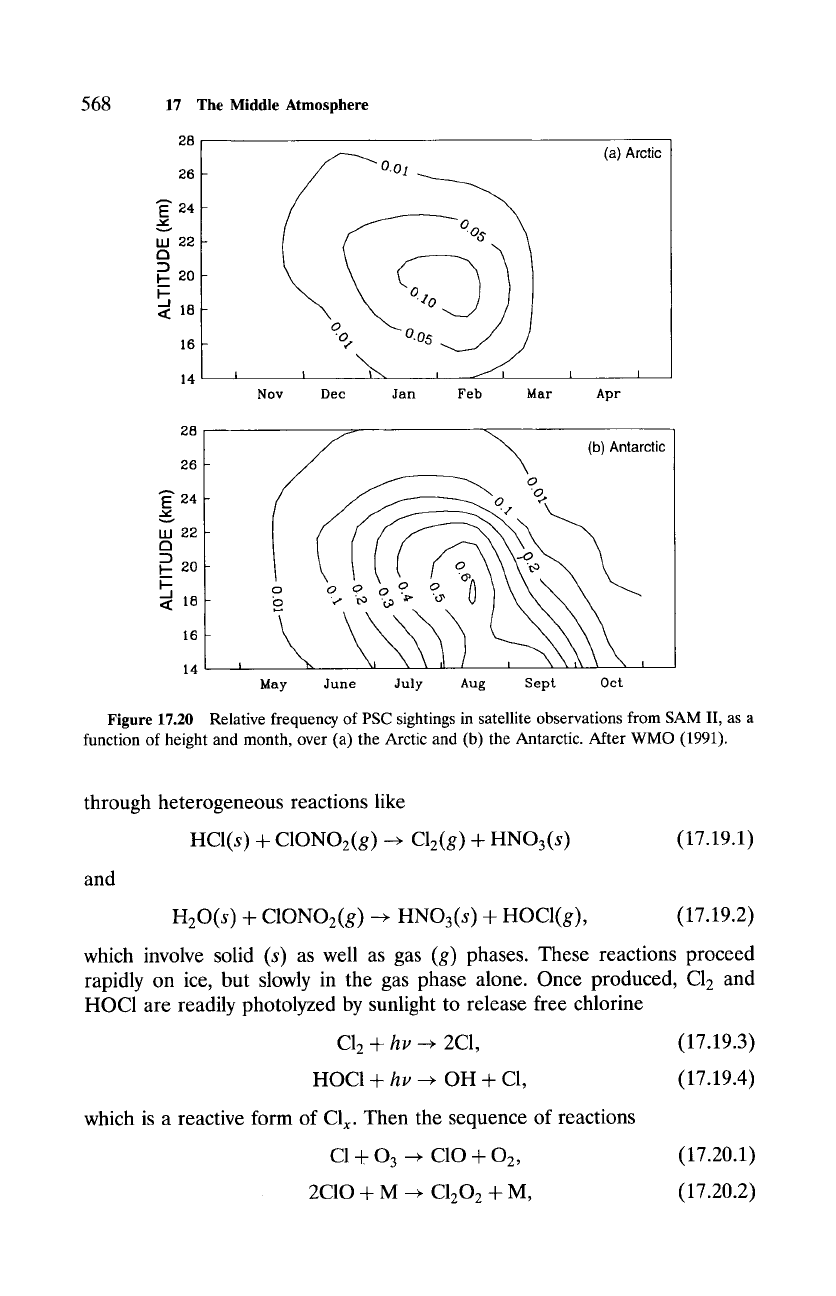
PSCs (Sec. 9.3) were already recognized to form over the Antarctic due
to its very cold temperatures, but they were regarded largely as a curiosity.
PSCs appear far more frequently in the Antarctic stratosphere than in the
warmer Arctic stratosphere (Fig. 17.20). During late Austral winter, when
temperatures are coldest, fractional coverage over the Antarctic exceeds 50%,
chiefly by very tenuous clouds in the PSC I category. It is now widely accepted
that PSCs provide the surfaces on which certain reactions proceed much faster
than they can in gas phase alone. Moreover, the presence of PSCs shifts
catalytic destruction of ozone from the upper stratosphere, where only a small
fraction of the ozone column resides, to the lower stratosphere, where Eo3 is
concentrated.
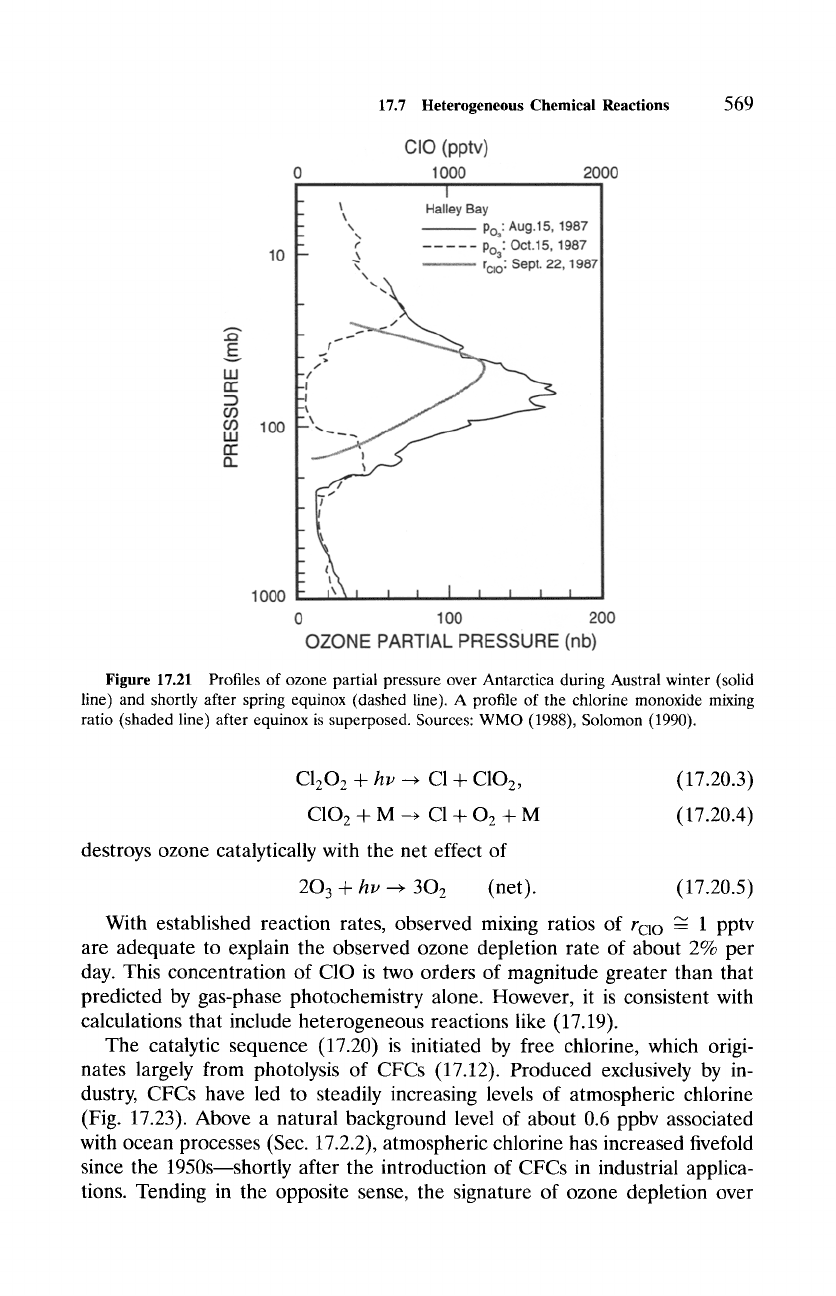
Figure 17.21 shows profiles of ozone concentration over Antarctica dur-
ing Austral winter (solid line) and shortly after equinox (dashed line), when
the sun rises above the horizon. A marked reduction of ozone has occurred
between 10 and 20 km, where most of the ozone column normally resides.
Decreases in Eo3 of 50% are observed at this time year, with column abun-
dances as low as 100 DU having been recorded. Superposed in Fig. 17.21 is
the profile of C10 (shaded line), which is produced by destruction of ozone
(17.13). Consistent with the observed ozone depletion, rcl o maximizes be-
tween 10 and 25 km~precisely where PSCs are sighted (Fig. 17.20). A cor-
respondence between reduced 03 and increased C10 is also apparent across
the edge of the polar-night vortex (Fig. 17.22). Ozone decreases sharply and
chlorine monoxide increases sharply where temperature becomes colder than
196 K, which is close to the threshold temperature for the formation of
type I PSCs.
The reactions now recognized to be primarily responsible for the ozone
losses in Figs. 1.19b, 17.21, and 17.22 involve two stages: First, inactive chlorine
species such as HC1 and C1ONO2 are converted to reactive forms of C1 x
568
17 The Middle Atmosphere
28
26
"E 24 -
v
Ill 22-
a
:D
I-- 20
-
._1
< 18-
16-
14
(a) Arctic
~
0.01 __
~
Nov Dec Jan Feb Mar Apr
28
26
~" 24
~e
v
w 22
a
20
..J
<
18
16
14
(b) Antarctic
i
May June July Aug Sept Oct
Figure 17.20 Relative frequency of PSC sightings in satellite observations from SAM II, as a
function of height and month, over (a) the Arctic and (b) the Antarctic. After WMO (1991).
through heterogeneous reactions like
HCl(s) + C1ONOz(g) --+ CI2(g) + HNO3(s)
and
(17.19.1)
HOC1 are readily photolyzed by sunlight to release free chlorine
C12 4-
hv --~
2C1,
HOC1 4-
hu ~
OH + C1,
which is a reactive form of CI x. Then the sequence of reactions
C1 + 0 3 ~ C10 +
02,
2C10 + M ~ C120 2 4- M,
(17.19.3)
(17.19.4)
(17.20.1)
(17.20.2)
HzO(s) 4- C1ONOz(g) --+ HNO3(s) 4- HOCI(g), (17.19.2)
which involve solid (s) as well as gas (g) phases. These reactions proceed
rapidly on ice, but slowly in the gas phase alone. Once produced, C12 and
17.7 Heterogeneous Chemical Reactions
569
..Q
E
v
w
n-"
w
CE
s
10
100
1000
CIO (pptv)
) 1000 2000
I
Halley Bay
- \
- \ ~ Po3: Aug.15, 1987
- ~ po 3 Oct.15, 1987
m \
....................... rc~o: Sept. 22, 1987
\
_//
-I
-t
-
: i I i I i ! i i
100 200
OZONE PARTIAL PRESSURE (nb)
Figure 17.21 Profiles of ozone partial pressure over Antarctica during Austral winter (solid
line) and shortly after spring equinox (dashed line). A profile of the chlorine monoxide mixing
ratio (shaded line) after equinox is superposed. Sources: WMO (1988), Solomon (1990).
C1202 4- ht, --+ C1 + C102,
(17.20.3)
C10 2 + M --+ C1 +
0 2
-t- M
(17.20.4)
destroys ozone catalytically with the net effect of
203 + hv ~ 302 (net). (17.20.5)
With established reaction rates, observed mixing ratios of rc10
~
1 pptv
are adequate to explain the observed ozone depletion rate of about 2% per
day. This concentration of C10 is two orders of magnitude greater than that
predicted by gas-phase photochemistry alone. However, it is consistent with
calculations that include heterogeneous reactions like (17.19).
The catalytic sequence (17.20) is initiated by free chlorine, which origi-
nates largely from photolysis of CFCs (17.12). Produced exclusively by in-
dustry, CFCs have led to steadily increasing levels of atmospheric chlorine
(Fig. 17.23). Above a natural background level of about 0.6 ppbv associated
with ocean processes (Sec. 17.2.2), atmospheric chlorine has increased fivefold
since the 1950s~shortly after the introduction of CFCs in industrial applica-
tions. Tending in the opposite sense, the signature of ozone depletion over
