Salby M.L. Fundamentals of Atmospheric Physics
Подождите немного. Документ загружается.

570
17
The Middle Atmosphere
1200
1000
I I I
I
I
I
I
I
I
I
210
~" -" %
-
205
800-
0 :.~
LU
~__ ",, IT
< '-,,
IT 600 - "" <~
(.9 \./~'-',, T
:~
- 200
,._0 ,.
0 , IT
Z ~ "~,,,',..,",[ \
:~.,.,
~, r
ELI
_ '; /a i =
x :.:.; ~:"',, ,..' i,
400 -
- ~
LU
O
195
0 190
-62 -64 -66 '68 -70 -72
200
>
E
t'~
v
O
2~
<
IT
(.9
Z
X
1
O
LATITUDE (Degs)
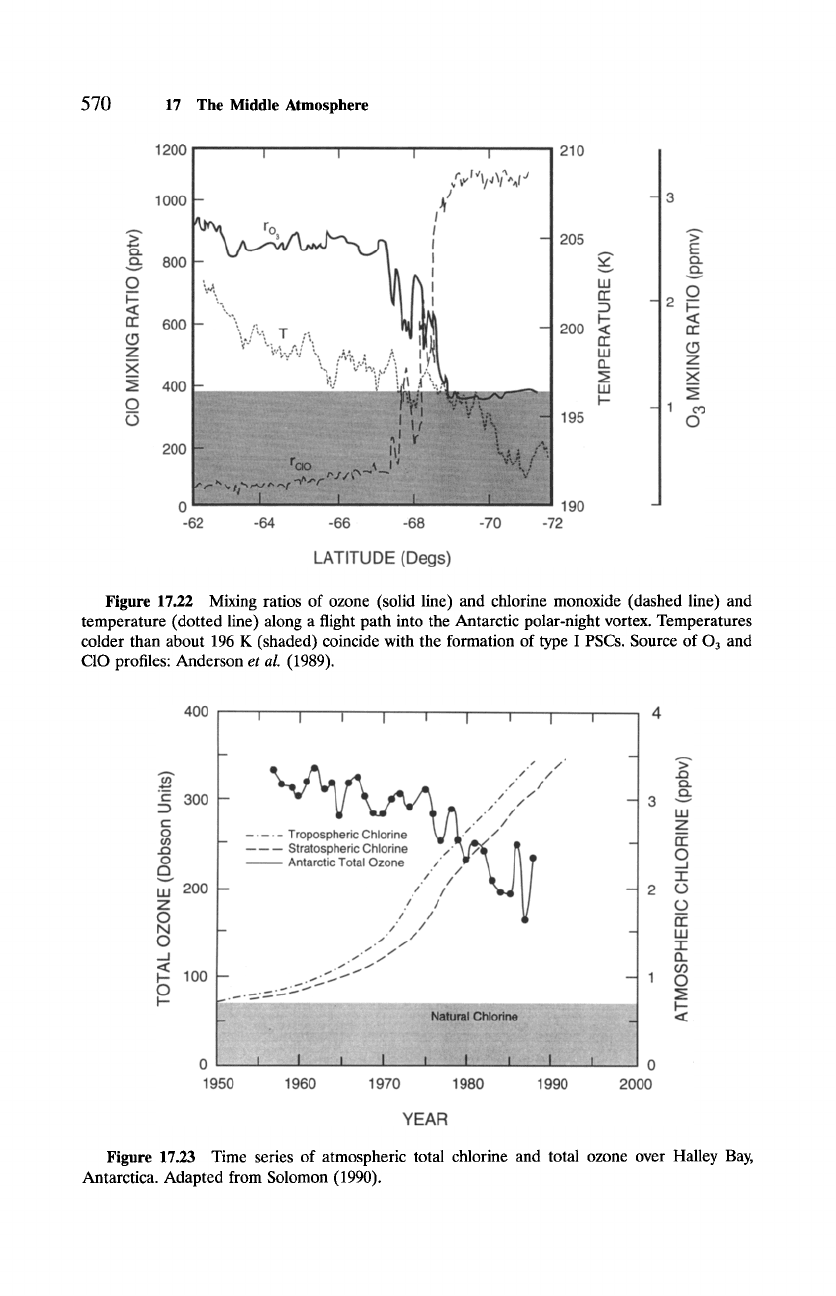
Figure 17.22 Mixing ratios of ozone (solid line) and chlorine monoxide (dashed line) and
temperature (dotted line) along a flight path into the Antarctic polar-night vortex. Temperatures
colder than about 196 K (shaded) coincide with the formation of type I PSCs. Source of O3 and
C10 profiles: Anderson
et al. (1989).
400 4
Or)
E 300
E
O
t~
.Q
O
lu 200
Z
O
N
O
_1
<
I-- 100
O
!-
I I I I ~ I t I i
-.-.-Tropospheric Chlorine ~/ .L -/_ ./
-
Stratospheric Chlorine
"/'~/~
Antarct'c Total Ozone
//"//I0- ~i~ l/ T
-
./// /11
~
-
./ ii
.I /
.I /
.I j
... . _. . ~'._~ :-""/" ~
>
t'3
t'~
t'~
-- 3
LU
Z
_
rr
O
._1
"1-
- 2 O
O
r
- uJ
"l-
fl..
or)
- 1 O
F--
<
0 0
1950 1960 1970 1980 1990 2000
YEAR
Figure 17.23 Time series of atmospheric total chlorine and total ozone over Halley Bay,
Antarctica. Adapted from Solomon (1990).

17.7
Heterogeneous Chemical Reactions
571
Antarctica emerges clearly after 1980, presumably when chlorine levels ex-
ceeded a threshold for reactions (17.20) to become an important sink of 03
(Solomon, 1990).
While converting inert forms of chlorine into reactive Clx, heterogeneous
reactions (17.19) have the opposite effect on reactive nitrogen. They convert
NOx into relatively inactive nitric acid. This bears importantly on ozone deple-
tion because NOx regulates the abundance of reactive chlorine. The principal
means by which Clx is converted back to inactive forms is via reaction with
nitrogen dioxide
C10 +
NO 2
4- M
--+ C1ONO 2
4- M.
(17.21)
The abundance of
NO 2
thus controls the duration over which reactive chlorine
is available to destroy ozone in (17.20). Since PSCs are composed of hydrated
forms of nitric acid (Sec. 9.3), their formation removes NOx from the gas
phase. Should PSC particles become large enough to undergo sedimentation,
NOx is removed entirely. The stratosphere is then denitrified, leaving reactive
chlorine available much longer to destroy ozone.
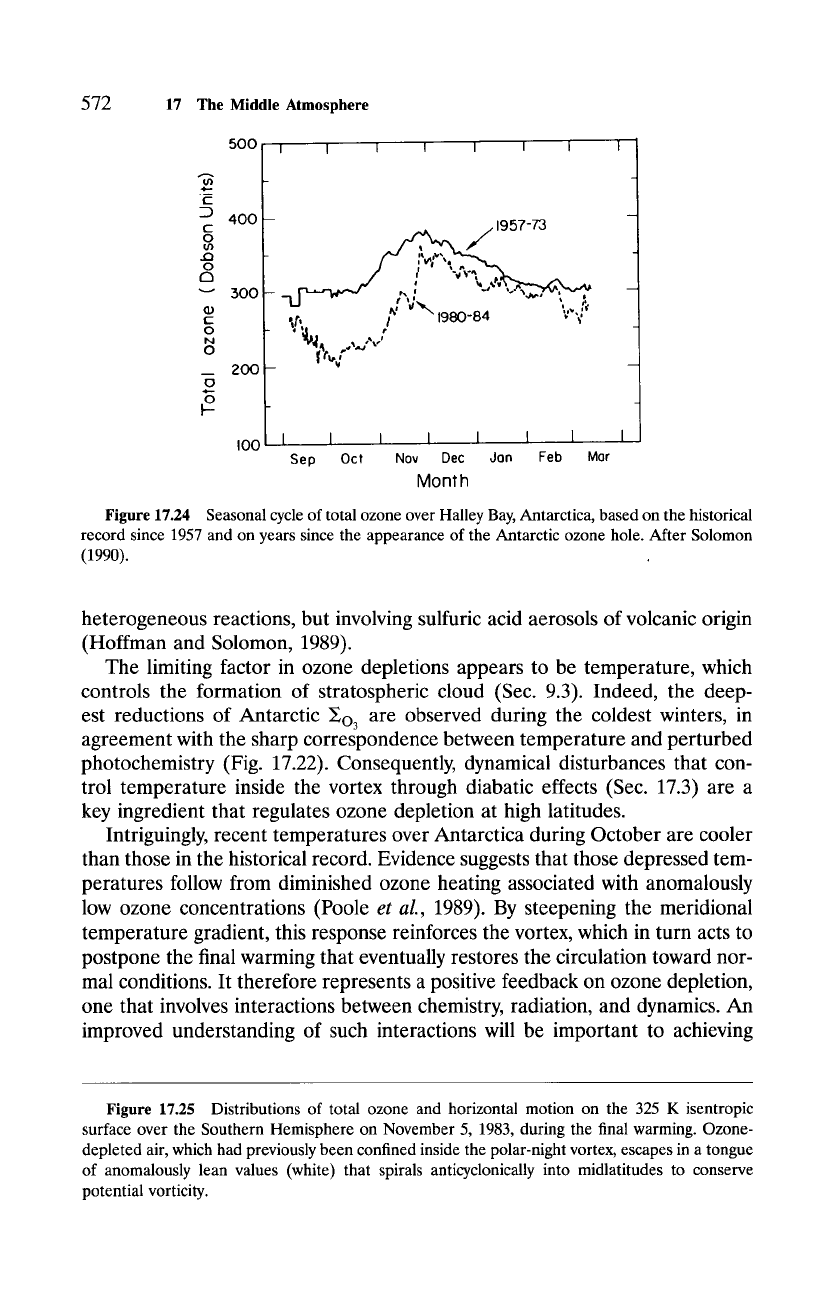
Figure 17.24 compares the seasonal cycle of ~o3 over Antarctica based
on the historical record against that based on years since the appearance of
the ozone hole. The two evolutions diverge near Austral spring, when solar
radiation triggers reactions (17.19.3) and (17.19.4), which release reactive chlo-
rine and set the stage for catalytic destruction of ozone in (17.20). Minimum
column abundances are observed in mid-October. By November, increasing
values restore ~o3 toward historical levels, when ozone-rich air is imported
from low latitudes during the final warming. But even then, values remain be-
low historical levels due to the dilution of subpolar air with ozone-depleted
air from inside the polar-night vortex. As illustrated by Fig. 17.25, the break-
down of the vortex during Austral spring involves a complex rearrangement
of air. Meridional displacements when the circumpolar flow weakens allow
ozone-depleted air that had been confined inside the vortex to escape from
the polar cap. Anomalies of mixing ratio created in this fashion can survive
for several weeks before they are eventually destroyed by deformation and
small-scale diffusion (see Hess and Holton, 1985).
Airborne observations have established that the same reactions operating
over the Antarctic occur in the Arctic stratosphere. But, owing to the warmer
temperature of the Arctic polar-night vortex, PSCs are a relatively infrequent
phenomenon. Moreover, free chlorine that is produced in isolated PSCs via
(17.19) is quickly acted on by dynamical effects that, by elevating temperature,
reverse the process through other chemical reactions (see, e.g., Garcia, 1994).
This limits the amount of reactive chlorine available when the sun rises over
the Arctic, which in turn limits catalytic destruction of ozone via (17.20).
Similar considerations apply to midlatitudes, where ozone depletions of 5
to 10% have been documented (WMO, 1991). Occurring at temperatures
too warm to support cloud formation, those depletions may also follow from
572
17 The Middle
Atmosphere
500 I
I i i I i I I
U3
.m
C
400
C
O
.Q
O
a
300
C
O
N
o
_ 200
O
O
F-
I00
1 I 1 1 1 I 1
Sep Oct Nov Dec Jan Feb Mor
Month
Figure 17.24 Seasonal cycle of total ozone over Halley Bay, Antarctica, based on the historical
record since 1957 and on years since the appearance of the Antarctic ozone hole. After Solomon
(1990).
heterogeneous reactions, but involving sulfuric acid aerosols of volcanic origin
(Hoffman and Solomon, 1989).
The limiting factor in ozone depletions appears to be temperature, which
controls the formation of stratospheric cloud (Sec. 9.3). Indeed, the deep-
est reductions of Antarctic Eo3 are observed during the coldest winters, in
agreement with the sharp correspondence between temperature and perturbed
photochemistry (Fig. 17.22). Consequently, dynamical disturbances that con-
trol temperature inside the vortex through diabatic effects (Sec. 17.3) are a
key ingredient that regulates ozone depletion at high latitudes.
Intriguingly, recent temperatures over Antarctica during October are cooler
than those in the historical record. Evidence suggests that those depressed tem-
peratures follow from diminished ozone heating associated with anomalously
low ozone concentrations (Poole
et al.,
1989). By steepening the meridional
temperature gradient, this response reinforces the vortex, which in turn acts to
postpone the final warming that eventually restores the circulation toward nor-
mal conditions. It therefore represents a positive feedback on ozone depletion,
one that involves interactions between chemistry, radiation, and dynamics. An
improved understanding of such interactions will be important to achieving
Figure 17.25 Distributions of total ozone and horizontal motion on the 325 K isentropic
surface over the Southern Hemisphere on November 5, 1983, during the final warming. Ozone-
depleted air, which had previously been confined inside the polar-night vortex, escapes in a tongue
of anomalously lean values (white) that spirals anticyclonically into midlatitudes to conserve
potential vorticity.

Problems
573
further progress on the ozone hole as well as on other complex problems
facing atmospheric science.
Suggested Reading
An Introduction to Dynamic Meteorology
(1992) by Holton provides a nice
introductory development of stratospheric waves and a quantitative description
of how they interact with the mean meridional circulation.
An advanced treatment of these topics is presented in
Middle Atmosphere
Dynamics
(1987) by Andrews
et aL
Holton (1984) discusses the historical view of the Brewer-Dobson circula-
tion in relation to transport of water vapor across the tropical tropopause.
WMO (1987) gives a more general development of stratosphere-troposphere
exchange in light of aircraft and satellite observations. Recent observations of
stratosphere-troposphere exchange and their interpretation are developed in
Holton
et al.
(1995).
Aeronomy of the Middle Atmosphere
(1986) by Brasseur and Solomon is a com-
prehensive treatment of gas phase photochemistry. Heterogeneous chemical
processes are reviewed in WMO (1991).
Solomon (1990) provides an excellent overview of the Antarctic ozone hole
and its relationship to polar stratospheric clouds.
Problems
17.1.
17.2.
17.3.
17.4.
Within the framework of Chapman chemistry, derive expressions for the
photochemical lifetimes 703 and
ZOx
for 03 and Ox, respectively, where
Zx 1 - -(1/X)dX/dt
is defined from reactions that destroy species X.
Given the rate coefficients in cgs units: k 2 - 6.0 x
10-34(300/T)[M],
k 3 =
8.0
•
lO-12e -2060/T,
where T is in Kelvin, and [M] is the number
density of air, J2 = 4.35
x 10 -15,
7.44
x 10 -11 ,
1.01 x 10 -9, and J3 = 4.47 x
10 -4, 7.80 x 10 -4, and 6.96 x 10 -3, at 100, 10, and 1 rob, respectively,
evaluate Zo3 and
ZOx
at (a) 100 mb, (b) 10 mb, and (c) 1 mb.
Ozone is useful as a tracer in the lower stratosphere. Yet, 03 has a
photochemical lifetime there of only hours (see Fig. 17.1). Resolve this
discrepancy.
(a) Construct an analytical approximation to the radiative-equilibrium
temperature in Fig. 17.10. (b) Construct an analytical approximation to
the zonal wind at 100 mb in Fig. 1.8. (c) Use the results of parts (a) and
(b) to plot the radiative-equilibrium wind in the middle atmosphere.
Use observed temperature (Fig. 1.7), radiative-equilibrium temperature
(Fig. 17.10), and the coefficient of Newtonian cooling (Fig. 8.29) to

574 17 The Middle Atmosphere
17.5.
17.6.
17.7.
17.8.
17.9.
17.10.
17.11.
estimate the radiative cooling rate (K day -I) inside the polar-night
vortex at (a) 100 mb, (b) 10 mb, and (c) 1 mb.
Describe how the polar-night vortex is maintained warmer than ra-
diative equilibrium by (a) thermal dissipation of planetary waves and
(b) mechanical dissipation of planetary waves.
Use the fact that upward momentum flux carried by planetary waves
is absorbed to describe how vertically propagating wave activity makes
the circulation of the middle atmosphere behave as a refrigerator.
If the middle atmosphere remains in thermodynamic equilibrium, net
heat transfer vanishes for individual air parcels, even though they may
temporarily sustain heat transfer during their motion about the pole
(see, e.g., Fig. 3.6). Likewise, if the middle atmosphere remains in
photochemical equilibrium, no ozone is produced. (a) Discuss net pro-
duction of ozone in relation to air being driven out of thermodynamic
equilibrium and photochemical equilibrium and relate those concepts
to the Brewer-Dobson circulation. (b) Extend the foregoing ideas to
explain the seasonal cycle of total ozone in Fig. 1.18.
Discuss how changes of radiative transfer introduced by depleted ozone
at high latitudes can reinforce the polar-night vortex to represent a
positive feedback.
According to the Brewer-Dobson circulation (Fig. 17.9), air enters the
stratosphere from the tropical troposphere. Estimate the water vapor
mixing ratio in ppmv (Problem 1.2) for the tropical lower stratosphere
if tropospheric air entering the stratosphere (a) evolves through a ther-
modynamic state corresponding to the mean temperature and height of
the tropical tropopause (Fig. 1.7), (b) were actually introduced through
isolated convective overshoots that have a mean height and tempera-
ture of 16.5 km and 185 K (see Fig. 1.25a), and (c) as in part (b), but if,
after entering the stratosphere, tropospheric air inside convective over-
shoots were to mix with subtropical stratospheric air that has a mixing
ratio of 5 ppmv.
The Brewer-Dobson circulation (Fig. 17.9) gives no indication of equa-
torward transport in the lower stratosphere. (a) How could subtropical
air in Problem 17.9, part (c), be supplied to the equator? (b) Why does
such transport not appear in the Brewer-Dobson circulation?
Methane oxidation is thought to be responsible for about 25% of strato-
spheric water vapor. Given the reactions
CH 4 -t- OH ~ H20 q- CH 3
(ka)
HzO + O ~ 2OH (kb),
with the rate coefficients in cgs units:
k a -
2.4
•
lO-12e -1710/T
and
kb --
2.2 • 10 -1~ calculate the photochemical-equilibrium mixing ratio

Problems
575
17.12.
17.13.
of water vapor produced by methane oxidation at 20 km. Compare this
value with observed mixing ratios at this altitude.
See Problem 4.8.
Consider Chapman chemistry, but augmented by the catalytic cycle
(17.13) involving CI~. Determine the photochemical lifetime of Ox at
100 mb for (a) rate coefficients for (17.13.1) and (17.13.2) in cgs units
of
ka
= 8.5
• 10 -12
and
k b
--
3.7
• 10 -11,
respectively, and a C10 mix-
ing ratio of 100 pptv, which are representative of gas-phase chemistry
under unperturbed conditions, and (b) rate coefficients for (17.13.1)
and (17.13.2) in cgs units of
k a
= 8.5 • 10 -l~ and
k b
=
3.7
• 10 -9,
respectively, and a C10 mixing ratio of 1000 pptv, which are represen-
tative of heterogeneous chemistry involving (17.20) under chemically
perturbed conditions. (c) Compare the photochemical lifetimes of Ox
in parts (a) and (b) with that under pure oxygen chemistry calculated
in Problem 17.1.
This Page Intentionally Left Blank
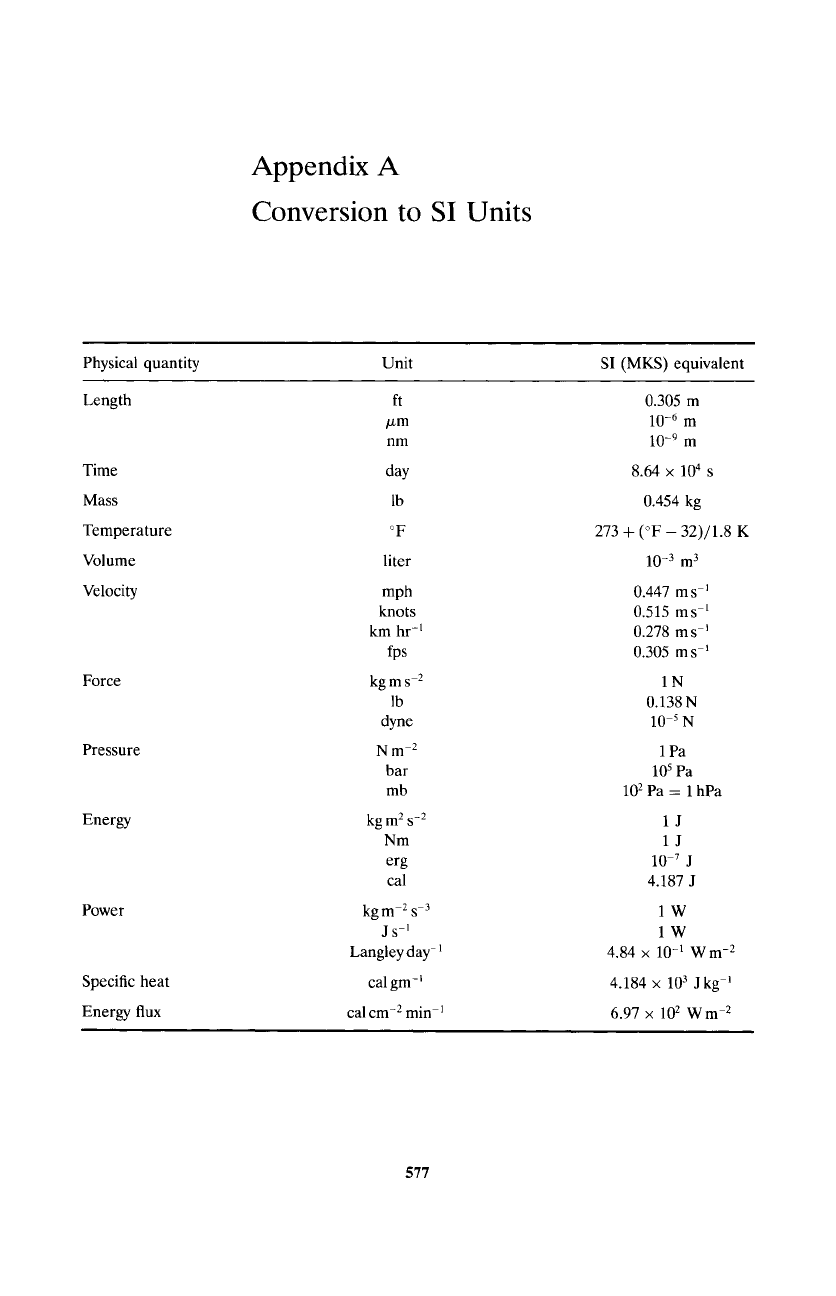
Appendix A
Conversion to SI Units
Physical quantity Unit
SI (MKS) equivalent
Length
Time
Mass
Temperature
Volume
Velocity
Force
Pressure
Energy
Power
Specific heat
Energy flux
ft
/xm
nm
day
lb
o F
liter
mph
knots
km hr -1
fps
kgms -2
lb
dyne
Nm-2
bar
mb
kg m 2 s -2
Nm
erg
cal
kg m -2 s -3
js-1
Langley day-
cal gm-
cal cm -2 min -1
0.305 m
10 -6 m
10 -9 m
8.64 • 104 s
0.454 kg
273 + (~ - 32)/1.8 K
10 -3 m 3
0.447 m s -1
0.515 ms -1
0.278 m s-1
0.305 m s-1
1N
0.138N
10 -5 N
1Pa
105
Pa
102 Pa = 1 hPa
1J
1J
10 .7 J
4.187 J
1W
lW
4.84 x 10 -1 Wm -2
4.184 x 103 J kg -1
6.97 x 102 W m -2
577
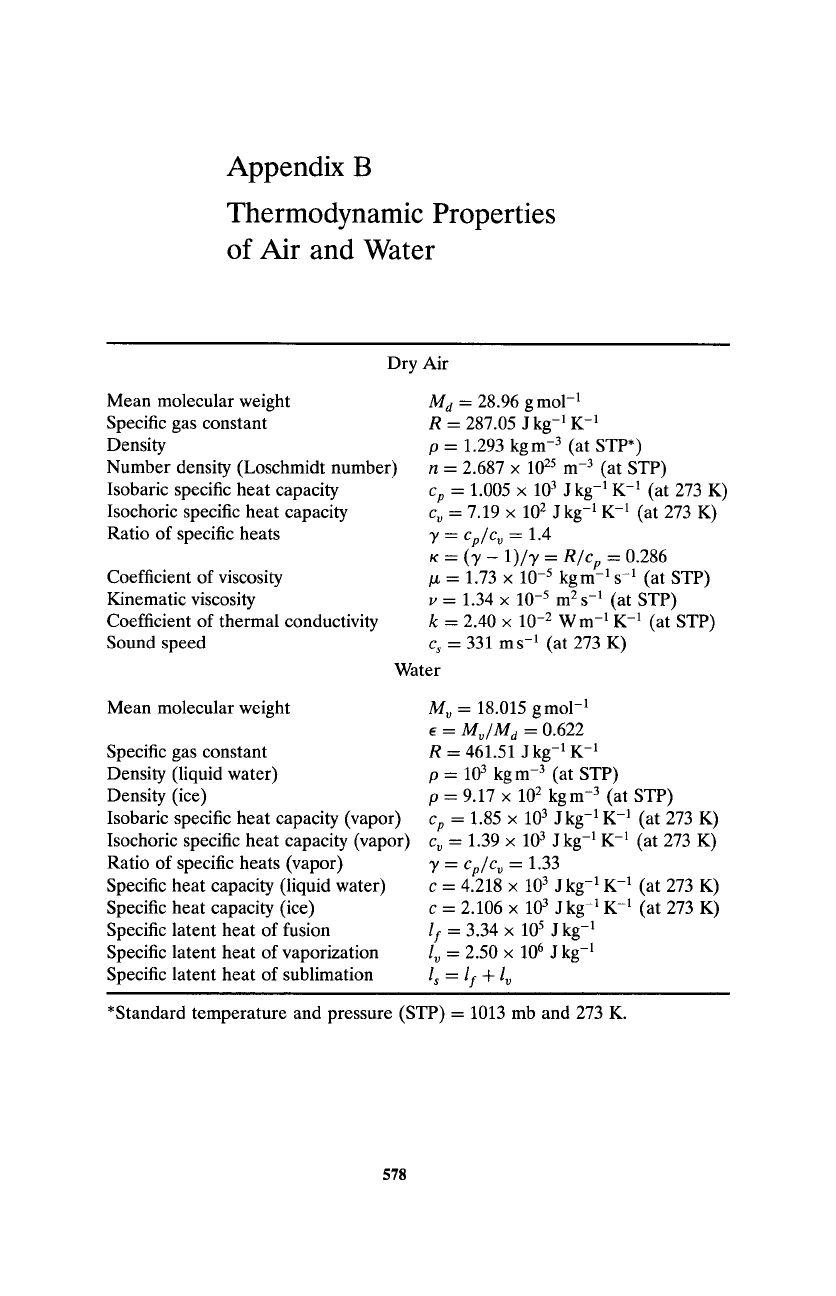
Appendix B
Thermodynamic Properties
of Air and Water
Dry Air
Mean molecular weight
Specific gas constant
Density
Number density (Loschmidt number)
Isobaric specific heat capacity
Isochoric specific heat capacity
Ratio of specific heats
Coefficient of viscosity
Kinematic viscosity
Coefficient of thermal conductivity
Sound speed
M d
= 28.96 gmo1-1
R = 287.05 J kg -1 K -1
p = 1.293 kg m -3 (at STP*)
n = 2.687 x 1025 m -3 (at STP)
Cp
= 1.005 x 103 J kg -1K -1 (at 273 K)
c o = 7.19 x 102 J kg -1K -1 (at 273 K)
y = Cp/Co
= 1.4
K = (y - 1)/y = R/cp
= 0.286
/z = 1.73 x 10 -5 kgm -a s -1 (at STP)
v = 1.34 x 10 -5 m 2 s -1 (at STP)
k = 2.40 x 10 -2 W m -1K -1 (at STP)
Cs
= 331 ms -1 (at 273 K)
Water
Mean molecular weight
Specific gas constant
Density (liquid water)
Density (ice)
Isobaric specific heat capacity (vapor)
Isochoric specific heat capacity (vapor)
Ratio of specific heats (vapor)
Specific heat capacity (liquid water)
Specific heat capacity (ice)
Specific latent heat of fusion
Specific latent heat of vaporization
Specific latent heat of sublimation
M o = 18.015 gmo1-1
9 = Mo/M a
= 0.622
R = 461.51 J kg-
a K- 1
p-- 103 kgm -3 (at STP)
p = 9.17 x 102 kgm -3 (at STP)
Cp
= 1.85 x 103 J kg -1K -1 (at 273 K)
c o = 1.39 x 103 J kg -a K -1 (at 273 K)
y = Cp/Co
= 1.33
c = 4.218 x 103 J kg -1K -1 (at 273 K)
c = 2.106 x 103 J kg -1K -1 (at 273 K)
If
= 3.34 x 105 J kg -1
l o = 2.50 x 106 J kg-a
l s = If + l o
*Standard temperature and pressure (STP) = 1013 mb and 273 K.
578

Appendix C
Physical Constants
Avogadro's number
Universal gas constant
Boltzmann constant
Planck constant
Stefan-Boltzmann constant
Speed of light
Solar constant
Radius of the earth
Standard gravity
Earth's angular velocity
NA = 6.022 X 10 23 mol- 1
R* = 8.314 J mo1-1 K -1
k = 1.381 x 10 -23 J K -1
h = 6.6261 • 10 -34 J s -1
tr - 5.67 x 10 -8 W m -2 K -4
c- 2.998 x 108 ms -1
F s = 1.372 x 10 3 Wm -2
a = 6.371 • 10 3 km
go = 9.806 m s -2
12 = 7.292 • 10 -5 s -1
579
