Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.

564 Lubricant Additives: Chemistry and Applications
Representative proton NMR spectra for the diester oils are shown for DBS and DOS in
Figure 22.27. These are most similar to the oils that are widely used in disk drive uid dynamic
bearing spindle motors. The NMR measurements were done on a Bruker Aspect 3000 Nuclear
Magnetic Resonance Spectrometer attached to a 250 MHz superconducting magnet with an
automatic sample changer. The samples for proton NMR were 10% w/w oil in chloroform-d1. The
solution spectra were measured in 5 mm NMR tubes from Kontes Scienti c Glassware (Grade 6,
5 pack, #897235). The proton NMR measurements were done using a spectral width of 5 kHz, 45°
pulse width of 3.0 µs, 1 s relaxation delay, and 512 scans.
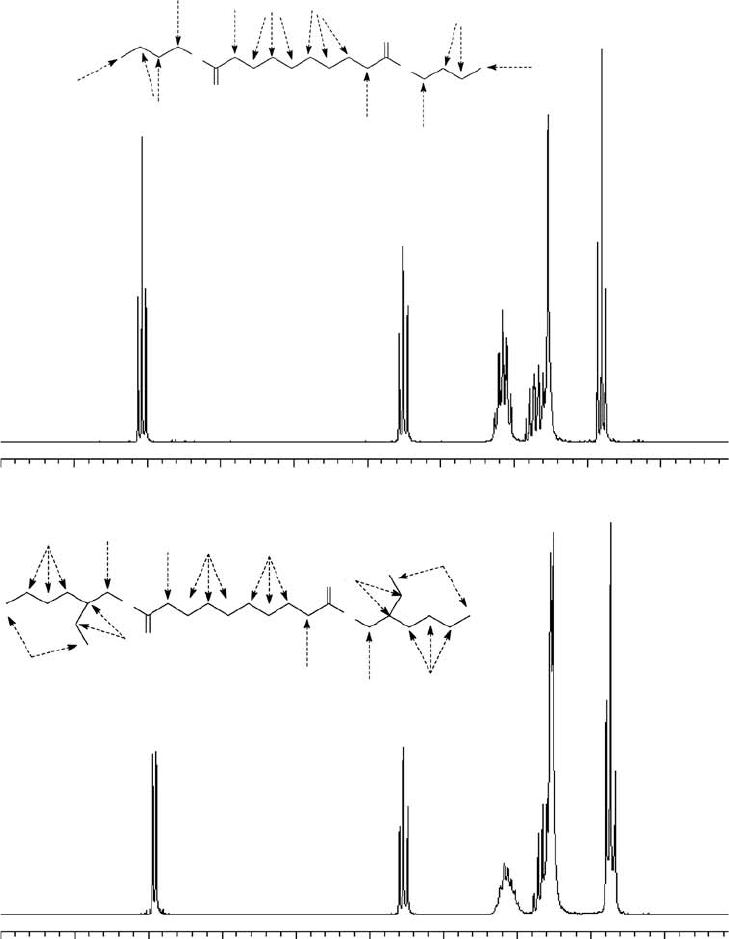
The proton corresponding to each peak in the NMR spectra in Figure 22.27 [1] is indicated by a
letter, and an arrow shows the location of the corresponding proton on the molecular structure. Peak
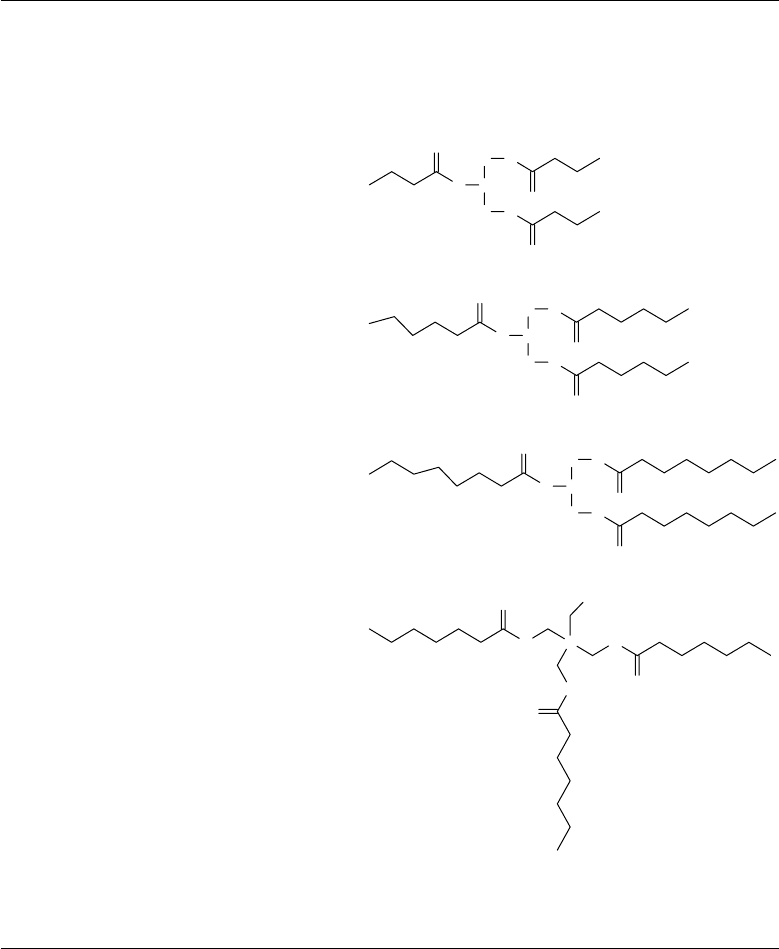
TABLE 22.17
Molecular Structures of the Triester Oils Investigated for Fluid Dynamic Bearing Motors
Acronym
Molecular
Weight (g/mol) Structure
TRIB 302
H
2
C
CH
H
2
C
O
O
O
O
O
O
TRIH 387
H
2
C
CH
H
2
C
O
O
O
O
O
O
TRIO 471
H
2
C
CH
H
2
C
O
O
O
O
O
O
TMP 471
CO
O
O
O
O
O
CH
3
Source: Adapted from Rudnick, L.R. (ed.), Synthetics, Mineral Oils, and Bio-Based Lubricants Chemistry and Technol-
ogy, Chapter 38, CRC Press, Taylor & Francis Group, Boca Raton, FL, 2006.
CRC_59645_Ch022.indd 564CRC_59645_Ch022.indd 564 3/20/2009 5:44:36 PM3/20/2009 5:44:36 PM
Lubricants for the Disk Drive Industry 565
assignments corresponding to these structures are listed in Table 22.19 [1]. The peak area is proportional
to the number of protons at each chemical shift. The peak areas were calculated from the NMR spectra
with commercial software (NUTS NMR Data Processing Software, Version 4.27, Acorn NMR, Inc.,
Fremont, California, United States). The reference peak area (indicated by “a” in the rst column of the
peak assignment tables) was assigned to the expected area. The rest of the integrated areas are relative
to the reference peak area. Both the integrated peak areas and the peak areas expected from the abun-
dance of each type of proton in the model chemical structure agree within 10%. Small changes in the
chemical shifts are due to differences in the chemical environment of the protons.
22.3.3.1 Viscosity and Vapor Pressure
Oil viscosity and vapor pressure are thermodynamically interrelated by the dispersion and dipolar
forces between the molecules. All of the intermolecular potential energy must be surmounted to evap-
orate a molecule from the liquid, and part of the intermolecular potential energy must be surmounted
for ow. To rst order, the dispersion force is proportional to the number and type of atoms, thus to
molecular weight. For the relatively low-molecular-weight oils useful in uid bearings, the ow is
mostly by the whole molecule, rather than segments of the molecule. Thus, increasing the molecular
weight, or polarity, to reduce the vapor pressure increases the viscosity. The relationship between oil
vapor pressure and viscosity is thoroughly investigated with Eyring’s chemical reaction rate theory of
evaporation and ow (Karis and Nagaraj [40]). The essence of their ndings is summarized later.
TABLE 22.18
Molecular Structures of the Nonpolar Oils Investigated for Fluid Dynamic Bearing Motors
Acronym
Molecular
Weight
(g/mol) Structure
2,2-DPP 196
1,3-DPP 196
PAO 240
PRS 269
SQL 423
Source: Adapted from Rudnick, L.R. (ed.), Synthetics, Mineral Oils, and Bio-Based Lubricants Chemistry and Technology,
Chapter 38, CRC Press, Taylor & Francis Group, Boca Raton, FL, 2006.
CRC_59645_Ch022.indd 565CRC_59645_Ch022.indd 565 3/20/2009 5:44:36 PM3/20/2009 5:44:36 PM
566 Lubricant Additives: Chemistry and Applications
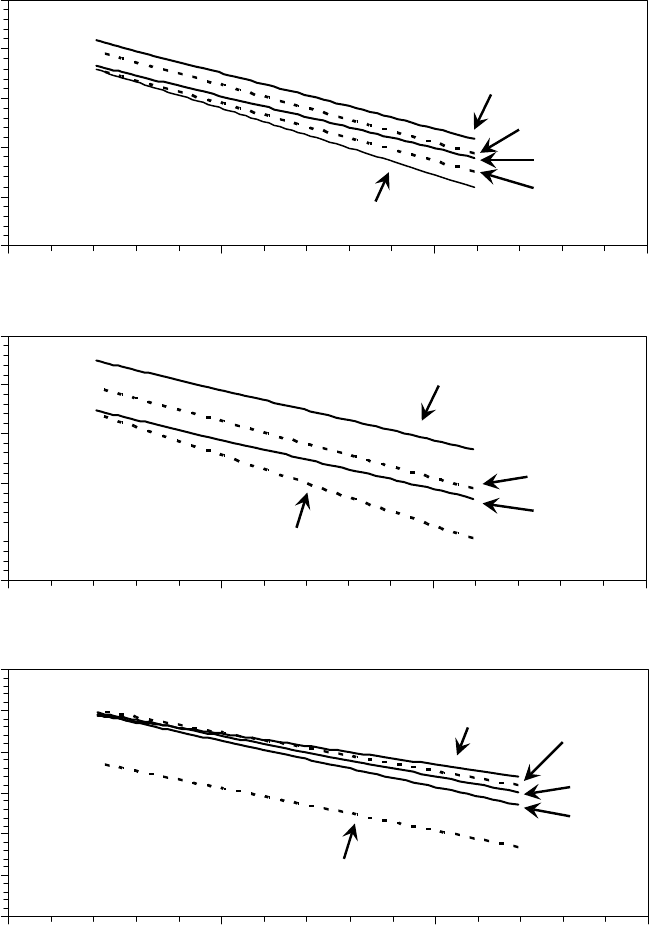
According to the Eyring Equation 22.6, a plot of ln(η) versus 1/T is a straight line with slope,
∆E
vis
/R and intercept ln(Nh
p
/V
l
) − ∆S
vis
/R. The viscosity of several low-molecular-weight ester and
hydrocarbon oils is shown plotted as ln(η) versus 1/T i n F i g u r e 2 2 . 2 8 [1]. T h e r e i s s o m e s ys t e m a t i c d e v i -
ation from linearity, but overall the general trend is t by the Eyring equation between −20 and 100°C.
4.5 4.0
3.5
3.0 2.5 2.0 1.5 1.0 0.5
4.5 4.0
3.5
3.0 2.5 2.0 1.5 1.0 0.5 ppm
ppm
O
O
O
O
(a)
(b)
(c)
(d)
(e)
O
O
O
O
(A)
(B)
(e)
(e)
(b)
(b)
(b)
(b)
(b)
(a)
(a)
(a)
(a)
(a)
(c)
(c)
(c)
(c)
(d)
(d)
(d)
(d)
(d)
(d)
(d)
(c)
(e)
(e)
(e)
FIGURE 22.27 Proton NMR spectrum of diester uid dynamic bearing oil (A) DBS and (B) DOS. The peak
assignments are indicated on the molecular structures. (Adapted from Rudnick, L.R. (ed.), Synthetics, Mineral
Oils, and Bio-Based Lubricants Chemistry and Technology, Chapter 38, CRC Press, Taylor & Francis Group,
Boca Raton, FL, 2006.)
CRC_59645_Ch022.indd 566CRC_59645_Ch022.indd 566 3/20/2009 5:44:38 PM3/20/2009 5:44:38 PM

Lubricants for the Disk Drive Industry 567
The ow activation entropy ∆S
vis
= ∆S
vis
trans
+ ∆S
rot
vis
is the sum of the translational and rotational
contributions. The slope and intercept of the ln(η) versus 1/T plot is employed to calculate ∆S
vis
and
∆E
vis
for each of the oils. These thermodynamic properties for ow are listed in Table 22.20 [1].
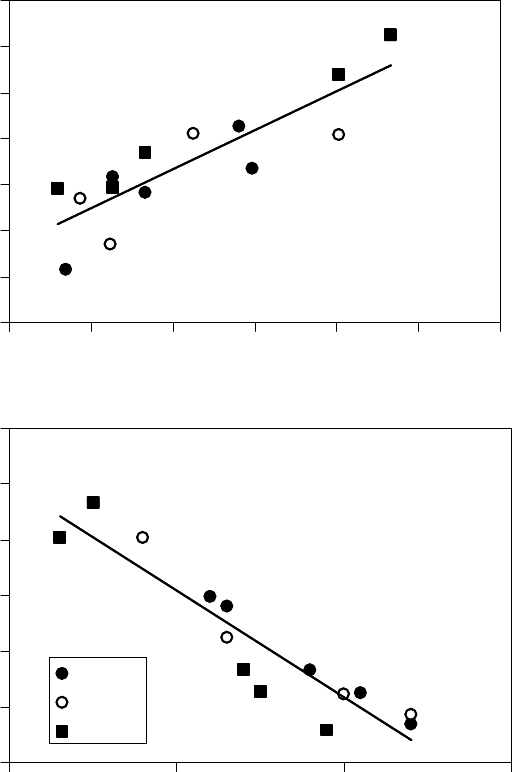
A similar analysis was performed for the vapor pressure of these oils. According to the Claperyron
Equation 22.15, a plot of ln(P
0
) versus 1/T is a straight line with slope −∆E
vap
/R and intercept
ln(P) − 1 + ∆S
vap
/R. The vapor pressure of several low-molecular-weight ester and hydrocarbon
oil is shown plotted as ln(P
0
) versus 1/T in Figure 22.29 [1]. The vaporization entropy ∆S
vap
=
∆S
trans
vap
+ ∆S
rot
vap
is approximately the sum of the translational and rotational contributions. The inter-
cept of the ln(P
0
) versus 1/T plot is then employed to calculate ∆S
vap
and ∆E
vap
for each of the oils.
The translational component of the vaporization is approximately equal to the vapor-phase entropy
given by Equa tion 22.16, and the rotational component is similarly calculated in the vapor phase
with Equa tion 22.17 [40]. The vaporization entropy and the calculated vapor-phase translational and
rotational entropy are employed to estimate the liquid-phase entropy as S
liq
≈ S
vap
− ∆S
vap
. These
thermodynamic ow properties for vaporization are listed in Table 22.20.
Further insight into the relationship between the evaporation and ow properties of the poten-
tial uid bearing motor oils is obtained by combining the results together within the framework
of thermodynamics and the reaction rate model for evaporation and ow [65,66]. The ratio of the
vaporization energy to the ow activation energy n = ∆E
vap
/∆E
vis
measures the partial decoupling
of intermolecular forces between molecules during ow relative to the complete decoupling that
takes place upon vaporization [67]. Another thermodynamic quantity that plays a key role in the
ow process is the ow activation rotational entropy. The ow activation rotational entropy, ∆S
rot
vis
,
was calculated from the combined vapor pressure and viscosity versus temperature, given in Karis
and Nagaraj [40]. If there is a way to lower the viscosity without increasing the vapor pressure, it
seems that it can be done only by increasing the ow activation entropy, ∆S
vis
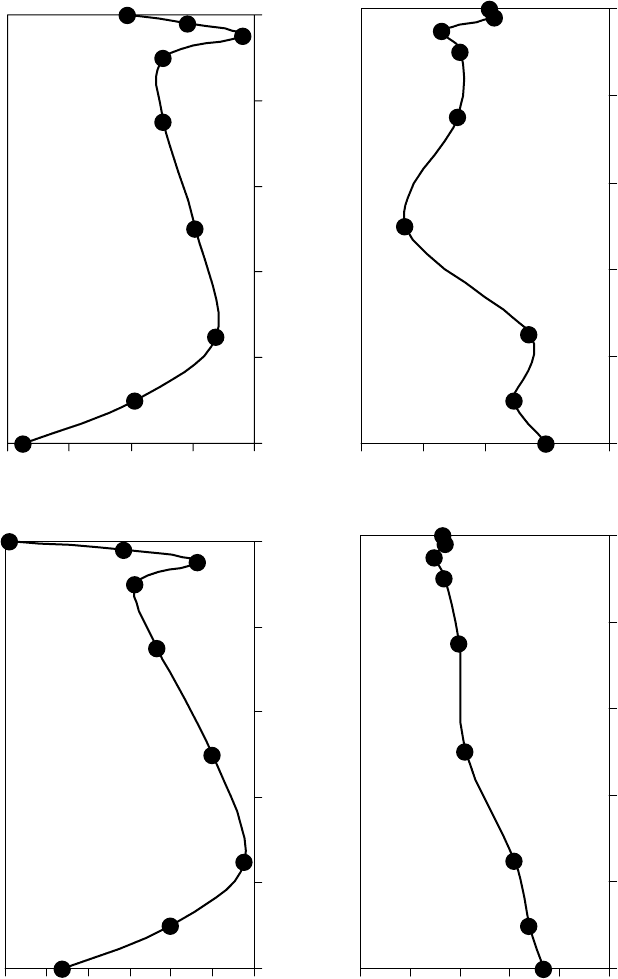
. For the oils shown
here, ∆S
vis
is proportional to ∆S
rot
vis
, as shown in Figure 22.30a [1]. However, ∆S
rot
vis
decreases with n,
as shown in Figure 22.30b. Thus, the ow activation rotational entropy decreases with the amount
of decoupling or asymmetry between vaporization and ow. For example, spherical molecules are
TABLE 22.19
Proton NMR Chemical Shifts and Peak Assignments
for the Spectra in Figure 22.27
Proton
Chemical Shift
(ppm) Measured Expected
Peak Area for DBS
(a)
a
4.01 4 4
(b) 2.23 4.2 4
(c) 1.57 8.4 8
(d) 1.36 12.4 12
(e) 0.91 6.1 6
Peak Area for DOS
(a)
a
3.97 4 4
(b) 2.27 4.1 4
(c) 1.59 6.6 6
(d) 1.28 24.6 24
(e) 0.86 12.1 12
a
Reference peak used as the basis for the other peak areas.
Source: Adapted from Rudnick, L.R. (ed.), Synthetics, Mineral Oils, and
Bio-Based Lubricants Chemistry and Technology, Chapter 38,
CRC Press, Taylor & Francis Group, Boca Raton, FL, 2006.
CRC_59645_Ch022.indd 567CRC_59645_Ch022.indd 567 3/20/2009 5:44:38 PM3/20/2009 5:44:38 PM
568 Lubricant Additives: Chemistry and Applications
more freely rotating in the ow-activated state, whereas rotation of rod-like molecules becomes less
likely in the ow-activated state, as they surmount the energy barrier between adjacent molecules
in the surrounding liquid.
Although the preceding discussion shows how the thermodynamic properties of vaporization
and ow govern the oil vapor pressure and viscosity, little can be said about speci cally what
molecular structure and compositions can be synthesized to provide an improved uid bearing
−7
−6
−5
−4
−3
−2
−1
0.0025 0.0030 0.0035 0.004
0
1/Temperature (K
−1
)
ln (Viscosity) (Pa s)
DBS
DOA
DOZ
DOS
DIA
Diesters
(a)
−7
−6
−5
−4
−3
−2
−1
0.0025 0.003 0.0035 0.004
1/Temperature (K
−1
)
ln (Viscosity) (Pa s)
TRIB
TRIH
TRIO
TMP
Triesters
(b)
−8
−6
−4
−2
0
0.0025 0.003 0.0035 0.004
Temperature (°C)
ln (Viscosity) (Pa s)
2,2-DPP
1,3-DPP
PAO
PRS
SQL
Nonpolar
(c)
FIGURE 22.28 Natural logarithm of viscosity as a function of inverse temperature of (a) diesters, (b) tri-
esters, and (c) hydrocarbon oils. The line is a t to the Eyring equation. (Adapted from Rudnick, L.R. (ed.),
Synthetics, Mineral Oils, and Bio-Based Lubricants Chemistry and Technology, Chapter 38, CRC Press,
Taylor & Francis Group, Boca Raton, FL, 2006.)
CRC_59645_Ch022.indd 568CRC_59645_Ch022.indd 568 3/20/2009 5:44:38 PM3/20/2009 5:44:38 PM

Lubricants for the Disk Drive Industry 569
motor. Recent developments on molecular dynamic simulation promise to bridge this gap between
molecular structure and thermodynamics. For example, Bair et al. [68] have demonstrated good
agreement between high shear viscosity measurements and predictions from a molecular dynam-
ics simulation. Yet, the model requires input of a particular molecular structure, in their case,
squalane, and numerically intensive computations that are required to predict the viscosity. Even
more complexity is involved if one tries to similarly predict the viscosity of a more complex polar
molecule such as diesters.
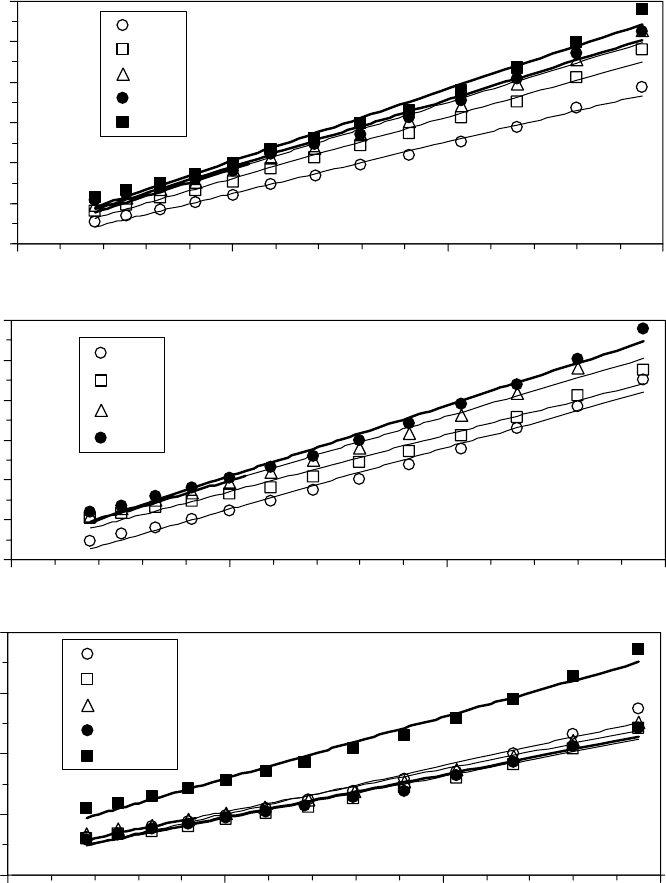
Oil blends, or viscosity-reducing additives, have also been considered. Blending oils change the
pressure–viscosity coef cient [55] and provide an intermediate viscosity [69,70]. The vaporization
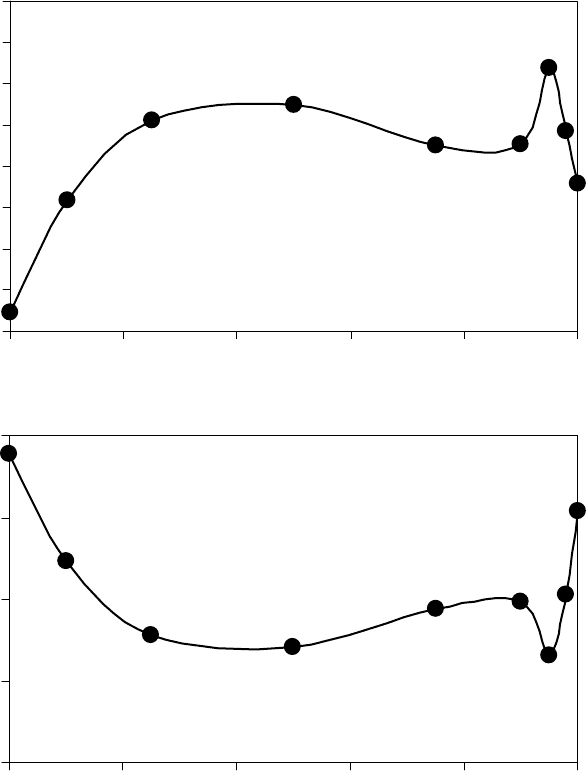
energy and entropy and ow activation energy and entropy are shown for a blend of polar diester
oil in nonpolar poly alpha ole n (PAO) oil in Figure 22.31 [1]. In this case, the vaporization energy
of the blend was less than that of the pure components. The entropy of the blend was higher than
that of the pure components. The reduction in the vaporization energy outweighed the effect of the
increase in liquid entropy, and the vapor pressure of the blend was higher than that of the pure com-
ponents. There is, however, an interesting dip in the properties near 95% DOS, which was reproduc-
ible. The ow activation energy and entropy of the blends are shown in Figures 22.31c and 22.31d.
The ow activation energy is approximately given by a linear combination of the weight fractions
of blend components. While the blend viscosity is actually less than the linear combination due
to an increase in the ow activation entropy near 50%. Taking the analysis one step further, the
ow activation rotational entropy has a sharp maximum near 95% DOS in Figure 22.32a. The ratio
n = ∆E
vap
/∆E
vis
has a sharp minimum near 95% DOS (Figure 22.32b). This unusual behavior near
95% DOS suggests that synergistic effects are possible in oil blends. These probably arise from clus-
tering of the nonpolar oil with the aliphatic chains of the diester. Perhaps, this type of effect could be
exploited to develop viscosity-reducing additives that have a lower vapor pressure than the base oil.
TABLE 22.20
Evaporation and Flow Thermodynamic Properties Calculated from Vapor Pressure and
Viscosity Data for Model Fluid Dynamic Bearing Oils
Evaporation Flow
Oil
∆E
vap
(kJ/mol)
∆S
vap
(J/mol K)
S
liq
(J/mol K)
∆E
vis
(kJ/mol)
∆S
vis
(J/mol K)
∆S
vis
rot
(J/mol K) n
DBS 90.7 160 114 20.6 −4.2 −3.1 4.4
DOA 94.4 160 117 24.7 4.1 6.61 3.8
DOZ 84.5 125 154 26.7 6.8 19.8 3.2
DOS 108.6 175 105 26.7 5.8 2.6 4.1
DIA 94.1 143 137 28.9 11.2 18.1 3.3
TRIB 81.8 154 163 25.1 10.5 12.5 3.3
TRIH 93.3 156 167 23.2 −1.5 2.4 4.0
TRIO 116.3 185 143 26.2 3.5 −1.4 4.4
TMP 81.0 110 218 28.9 10.4 30.3 2.8
2,2-DPP 59.1 120 144 25.5 17.0 30.4 2.3
1,3-DPP 74.2 159 105 22.3 8.4 6.6 3.4
PAO 89.6 188 80 22.7 4.5 −4.2 3.9
PRS 78.5 166 104 22.8 4.6 2.6 3.5
SQL 82.8 124 156 32.9 21.2 36.6 2.5
Note: Evaporation properties are referenced to 100°C and atmospheric pressure (100 kPa). Flow properties are referenced
to 40°C.
Source: Adapted from Rudnick, L.R. (ed.), Synthetics, Mineral Oils, and Bio-Based Lubricants Chemistry and Technology,
Chapter 38, CRC Press, Taylor & Francis Group, Boca Raton, FL, 2006.
CRC_59645_Ch022.indd 569CRC_59645_Ch022.indd 569 3/20/2009 5:44:39 PM3/20/2009 5:44:39 PM
570 Lubricant Additives: Chemistry and Applications
However, for a blend to be useful, it would also need to be capable of forming an azeotrope, and
then it could only be used in motors at the azeotropic composition. Otherwise, the evaporation of
the oil component with the highest vapor pressure would gradually change the oil blend viscosity
and vapor pressure with time.
−15
−10
−5
0
5
10
0.0020 0.0025 0.0030 0.0035
0.0020 0.0025 0.0030 0.0035
1/Temperature (K
−1
)
1/Temperature (K
−1
)
0.0020 0.0025 0.0030 0.0035
1/Temperature (K
−1
)
ln (P
0
) (Pa)
−15
−10
−5
0
5
10
ln (P
0
) (Pa)
−15
−10
0
−5
5
10
15
ln (P
0
) (Pa)
Diesters
(a)
(b)
(c)
DBS
DOA
DOZ
DIA
DOS
Triesters
TRIB
TRIH
TMP
TRIO
Nonpolar
2,2-DPP
1,3-DPP
PRS
PAO
SQL
FIGURE 22.29 Natural logarithm of vapor pressure as a function of inverse temperature of (a) diesters,
(b) triesters, and (c) hydrocarbon oils. The line is a t to the Clapeyron equation. Ambient pressure P =
100 kPa. (Adapted from Rudnick, L.R. (ed.), Synthetics, Mineral Oils, and Bio-Based Lubricants Chemistry
and Technology, Chapter 38, CRC Press, Taylor & Francis Group, Boca Raton, FL, 2006.)
CRC_59645_Ch022.indd 570CRC_59645_Ch022.indd 570 3/20/2009 5:44:39 PM3/20/2009 5:44:39 PM
Lubricants for the Disk Drive Industry 571
22.3.4 OIL FORMULATION CHEMISTRY
Thermal stability is imparted to grease and oil through formulation with additives. Over the past
two decades, we employed the principles of oil oxidation chemistry in developing formulations for
ball bearing grease [71] and uid dynamic bearing [72] spindle motors at the leading edge of the
industry in our laboratory. The general outline of the chemical mechanisms for oxidation and stabi-
lization, potential additives, and accelerated test results is published in Ref. 50. Here, we summarize
the low-temperature elementary reactions for each type of oxidation pathway including inhibition
by antioxidants. The rate equations are solved numerically in nondimensional form, and the model
results are compared with thermal oxidation life test data to illustrate the synergistic effects of pri-
mary antioxidant PriAOX and secondary antioxidant SecAOX and metal catalysis.
Diesters
Triesters
Nonpolar
−10
−5
0
5
10
15
20
25
50
40
30
20
10
0
−10
−10
01020304050
(a)
(
b
)
∆S
vis
(J/mol K)
∆S
vis
(J/mol K)
n
rot
∆S
vis
(J/mol K)
rot
234
5
FIGURE 22.30 Flow activation entropy versus ow activation rotational entropy (a) and the ow activation
rotational entropy versus the ratio of the vaporization energy to the ow activation energy (b) for the oils listed in
Tables 22.16 through 22.18. (Adapted from Rudnick, L.R. (ed.), Synthetics, Mineral Oils, and Bio-Based Lubri-
cants Chemistry and Technology, Chapter 38, CRC Press, Taylor & Francis Group, Boca Raton, FL, 2006.)
Diesters
Triesters
Nonpolar
−10
−5
0
5
10
15
20
25
50
40
30
20
10
0
−10
−10
01020304050
(a)
(
b
)
∆S
vis
(J/mol K)
∆S
vis
(J/mol K)
n
rot
∆S
vis
(J/mol K)
rot
234
5
CRC_59645_Ch022.indd 571CRC_59645_Ch022.indd 571 3/20/2009 5:44:40 PM3/20/2009 5:44:40 PM
572 Lubricant Additives: Chemistry and Applications
FIGURE 22.31 Vaporization activation energy and entropy change (a, b) and ow activation and entropy change (c, d) for blends of DOS in PAO. (Adapted from
Rudnick, L.R. (ed.), Synthetics, Mineral Oils, and Bio-Based Lubricants Chemistry and Technology, Chapter 38, CRC Press, Taylor & Francis Group, Boca Raton, FL,
2006.)
65
70
75
80
85
90
95
wt% DOS in PAO
∆E
vap
(kJ/mol)
∆E
vis
(kJ/mol)
∆S
vis
(J/mol K)
(a)
(d)(c)
20
22
24
26
28
30
2
4
6
8
10
0 20406080100
wt% DOS in PAO
0 20 40 60 80 100
wt% DOS in PAO
0 20 40 60 80 100
∆S
vap
(J/mol K)
(b)
100
120
140
160
180
wt% DOS in PAO
0 20406080100
CRC_59645_Ch022.indd 572CRC_59645_Ch022.indd 572 3/20/2009 5:44:40 PM3/20/2009 5:44:40 PM
Lubricants for the Disk Drive Industry 573
The following sets of elementary reactions, derived from schemes in Refs 50 and 73, are employed
to model the data. Intrachain proton abstraction and self-termination are not included. Some of the
non-rate-controlling reactants and products are not shown. The initiation reactions and the propo-
gation reactions are shown in Figures 22.33a and 22.33b, respectively. The dot denotes a carbon
radical. Only interchain proton abstraction (no cleavage reaction) is considered because this is the
dominant reaction pathway that we observed with ester oils. Proton abstraction from RH by thermal
or mechanical excitation is the rate-limiting step in the initiation mechanism. Decomposition of the
hydroperoxide ROOH is the rate-limiting step in the propagation mechanism.
PriAOX protonates radicals, as shown in Figure 22.34a. Examples of PriAOX are butylated
hydroxy toluene (BHT), dioctyldiphenyl amine (DDA), and phenyl naphthylamine (PNA).
SecAOX decomposes hydroperoxide, as shown in Figure 22.34b. Examples of SecAOX are zinc
−10
−5
0
5
10
15
20
25
30
0 20 40 60 80 100
wt% DOS in PAO
0 20 40 60 80 100
wt% DOS in PAO
(a)
(
b
)
2.0
2.5
3.0
3.5
4.0
∆S
vis
(J/mol K)
rot
n
FIGURE 22.32 The rotational component of the ow activation entropy (a) and the ratio of the vaporization
energy to the ow activation energy, n, (b) for blends of DOS in PAO.
CRC_59645_Ch022.indd 573CRC_59645_Ch022.indd 573 3/20/2009 5:44:40 PM3/20/2009 5:44:40 PM
