Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.

534 Lubricant Additives: Chemistry and Applications
22.2.1.3 Thin Film Viscosity
The aforementioned results have shown that in bulk PFPE disk lubricants, viscosity increases
exponentially as the measurement temperature approaches the glass transition temperature. This is
because chain motions are progressively frozen out as the thermal energy becomes less than their
activation energy. The lubricant viscosity also increases as the lubricant lm thickness decreases,
which helps to prevent the lubricant from owing completely off of the magnetic recording disks in
the air shear [32]. Viscosity enhancement of thin lms arises from a different mechanism than that
found with decreasing temperature.
Dispersive interaction has a dramatic effect on the viscosity of the molecular layers closest
to the surface, which can be explained in terms of the rate theory for viscous ow. Within the
Demnum S100
(a)
Krytox 143AD
Demnum SA2
Krytox COOH
(b)
Demnum S100
Krytox 143AD
Demnum SA2
Krytox COOH
1×10
−16
1×10
4
1×10
2
1×10
0
1×10
−2
1×10
−4
1×10
−14
1×10
−12
1×10
−10
1×10
−8
1×10
−6
1×10
−4
1×10
−2
1×10
0
1×10
−16
1×10
4
1×10
2
1×10
0
1×10
−2
1×10
−4
1×10
−14
1×10
−12
1×10
−10
1×10
−8
1×10
−6
1×10
−4
1×10
−2
1×10
0
G′′ (Pa)G′ (Pa)
ωa
T
g
(rad/s)
ωa
T
g
(rad/s)
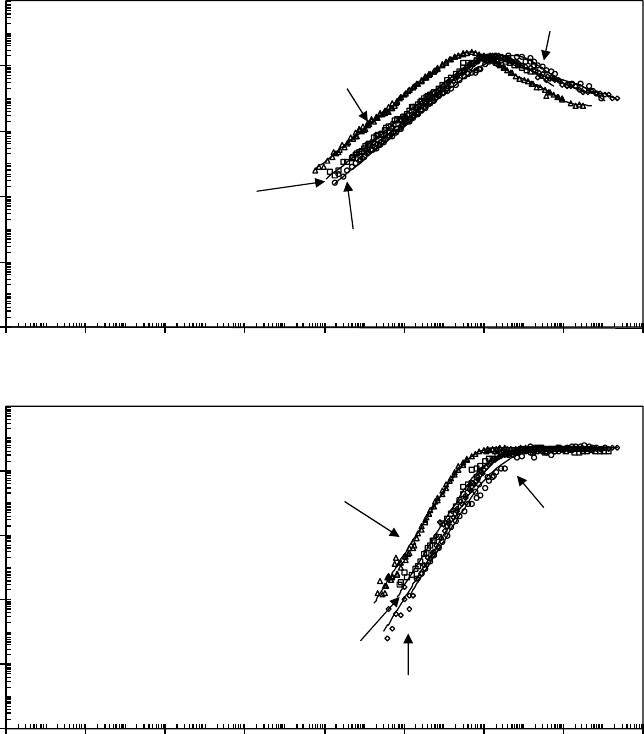
FIGURE 22.6 Shear loss (a) and storage (b) modulus master curves for the Demnum and Krytox series. The
smooth curves are from the discrete relaxation time series t to the frequency–temperature shifted data. Ref-
erence temperature is T
g
. (Adapted from Rudnick, L.R. (ed.), Synthetics, Mineral Oils, and Bio-Based Lubri-
cants Chemistry and Technology, Chapter 38, CRC Press, Taylor & Francis Group, Boca Raton, FL, 2006.)
CRC_59645_Ch022.indd 534CRC_59645_Ch022.indd 534 3/20/2009 5:44:25 PM3/20/2009 5:44:25 PM
Lubricants for the Disk Drive Industry 535
rate theory, a ow event comprises the transition of a ow unit from its normal or quiescent state,
through a ow-activated state, to a region of lower free energy in an external stress eld. For small
molecules, the ow unit is the whole molecule, whereas for longer chains, the ow unit is a segment
of the whole molecule. By analogy with chemical reaction rate theory, there is a ow activation
enthalpy, ∆H
vis
, and entropy, ∆S
vis
, for transition into the ow-activated state.
A ow unit is approximated by a particle in a box, with the energy being partitioned among
rotational and translational degrees of freedom, which govern the transition probability. On this
basis, the viscosity η = (Nh
p
/V
l
) exp (∆G
vis
/RT), where N is Avogadro’s number, h
p
the Planck’s
constant, V
l
the molar volume, R the universal gas constant, T the temperature, and ∆G
vis
=
∆H
vis
− T∆S
vis
the ow activation Gibbs free energy. The ow activation enthalpy ∆H
vis
= ∆E
vis
+ ∆(pV)
vis
, where ∆E
vis
is the ow activation energy and ∆(pV)
vis
the pressure–volume work. At
constant pressure, ∆(pV) = p∆V
vis
. For PFPE Z, the ow activation volume ∆V
vis
≈ 0.1 nm
3
[17],
Z03
Zdiac
Zdeal
Ztetraol 2000
Ztx
Zdol 2500
Relaxation time (s)
0.8 1.2 1.4 1.6 1.8
m/n
Relaxation time (s)
1×10
−4
1×10
−5
1×10
−6
1×10
−4
1×10
−5
1×10
−6
1
(a)
(
b
)
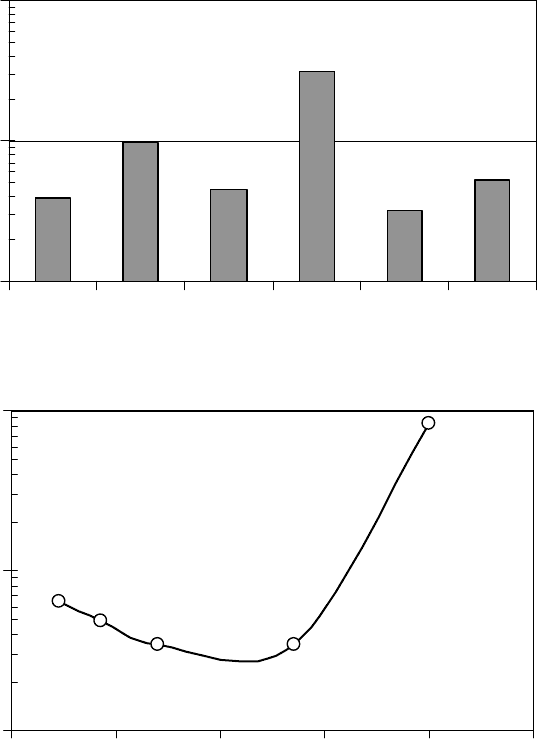
FIGURE 22.7 Longest relaxation time at 50°C for the Z series of lubricants (a) and Zdo14K series with a
range of monomer chain composition (b) calculated from the dynamic rheological measurements with time–
temperature superposition. (Adapted from Rudnick, L.R. (ed.), Synthetics, Mineral Oils, and Bio-Based Lubri-
cants Chemistry and Technology, Chapter 38, CRC Press, Taylor & Francis Group, Boca Raton, FL, 2006.)
CRC_59645_Ch022.indd 535CRC_59645_Ch022.indd 535 3/20/2009 5:44:25 PM3/20/2009 5:44:25 PM
536 Lubricant Additives: Chemistry and Applications
which is equivalent to a spherical region ≈0.6 nm in diameter. At ambient pressure (100 kPa),
∆(pV)
vis
≈ 6.2 J/mol, so that near ambient conditions, ∆H
vis
≈ ∆E
vis
. Therefore, the viscosity is given by
⫽
⫺
Nh
V
ETS
RT
p
l
()
exp
∆∆
vis vis
(22.6)
1×10
8
1×10
6
1×10
4
1×10
2
1×10
0
1×10
−2
1×10
−4
1×10
8
1×10
6
1×10
4
1×10
2
1×10
0
1×10
−2
1×10
−5
1×10
−1
1×10
3
1×10
7
1×10
11
1×10
15
1×10
−5
1×10
−1
1×10
3
1×10
7
1×10
11
1×10
15
ε′′ε′
ωa
T
o
(rad/s)
ωa
T
o
(rad/s)
Ztetraol 1000
Ztetraol 2000
Zdol 4000
(a)
(b)
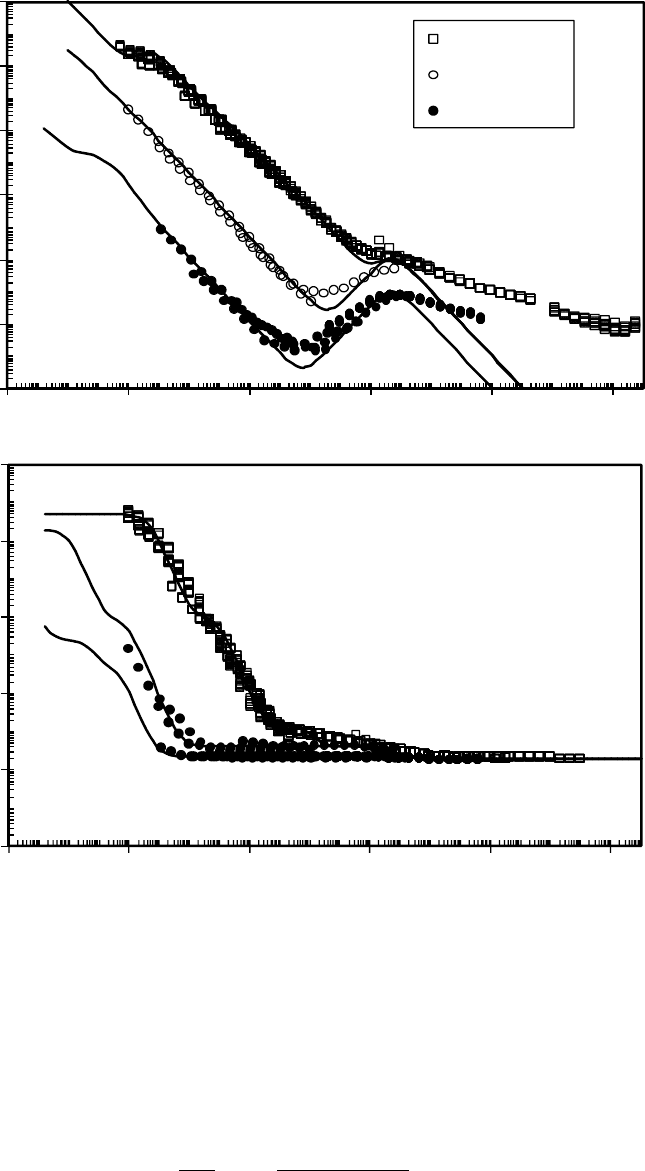
FIGURE 22.8 Dielectric loss factor (a) and relative permittivity (b) master curves for the Ztetraol 2000,
Ztetraol 1000, and Zdol 4000. The smooth curves are from the discrete relaxation time series t to the
frequency–temperature shifted data. Reference temperature is T
0
= 50ºC. (Adapted from Rudnick, L.R. (ed.),
Synthetics, Mineral Oils, and Bio-Based Lubricants Chemistry and Technology, Chapter 38, CRC Press,
Taylor & Francis Group, Boca Raton, FL, 2006.)
CRC_59645_Ch022.indd 536CRC_59645_Ch022.indd 536 3/20/2009 5:44:25 PM3/20/2009 5:44:25 PM

Lubricants for the Disk Drive Industry 537
A regression t to the bulk viscosity as a function of temperature [32] provided ∆E
vis
= 34.7 kJ/mol
and ∆S
vis
= 9.87 J/mol K. The ow activation energy is close to that reported for bulk Zdol with a
molecular weight of 3100 Da in Refs 33 and 34. A positive value for the ow activation entropy of
bulk Zdol means that the entropy of the ow unit increases on going into the ow- activated state.
Changes in the lubricant ow activation energy and entropy near the solid surface cause changes
in the viscosity with decreasing lm thickness. The ow activation energy near a solid surface is
estimated from the thin lm vaporization energy as follows. In an ideal gas, the chemical potential
µ (or partial molar Gibbs free energy) is given by
d ⫽ RT Pd ln
(22.7)
where P is the partial pressure of the lubricant in the vapor phase.
The chemical potential energy per unit volume in the lubricant lm is µ/V
l
= Π. The ratio of the
lm surface vapor pressure to the vapor pressure of the bulk lubricant, P
o
(h)/P
o
(∞), is derived by
integrating Equation 22.7
() ( ) ln
()
()
hRT
Ph
P
⫺⫽⬁
⬁
o
o
(22.8)
The reference state is taken to be the chemical potential and vapor pressure of the bulk lubri-
cant, u(∞) = 0, and P
o
(∞) is the vapor pressure of the bulk liquid.
In general, as the surface energy is de ned as the free energy per unit area, the total disjoining
pressure (Π) for these uids can be derived from the experimental surface energy (contact angle)
data by
⫽⫺ ⫹
∂
∂
()
h
dp
(22.9)
TABLE 22.7
The Coeffi cients of the Debye Equation from the Dielectric Master Curves at
Reference Temperature T
0
= 50°C
Lubricant
Parameter Zdol 4000 Ztetraol 2000 Ztetraol 1000
ε′(0) 3.3 × 10
3
1.1 × 10
4
1.1 × 10
7
σ(S/m) 1 × 10
−11
4 × 10
−8
6 × 10
−7
ε
s,1
3 × 10
3
1 × 10
4
1 × 10
7
τ
1
(s) 500 50 8
ε
s,2
3 × 10
2
1 × 10
3
1 × 10
6
τ
2
(s) 50 5 0.8
ε
s,3
30 100 100,000
τ
3
(s) 5 0.5 0.06
ε
s,4
24 5
τ
4
(s) 1 × 10
−8
1 × 10
−8
1 × 10
−8
Note: The high frequency ε
∞
≈ 1.7 from the index of refraction.
Source: Adapted from Rudnick, L.R. (ed.), Synthetics, Mineral Oils, and Bio-Based Lubricants Chemistry and
Technology, Chapter 38, CRC Press, Taylor & Francis Group, Boca Raton, FL, 2006.
CRC_59645_Ch022.indd 537CRC_59645_Ch022.indd 537 3/20/2009 5:44:27 PM3/20/2009 5:44:27 PM
538 Lubricant Additives: Chemistry and Applications
Here γ
d
and γ
p
are the dispersive and polar components of the surface energy, respectively, and
h the lm thickness.
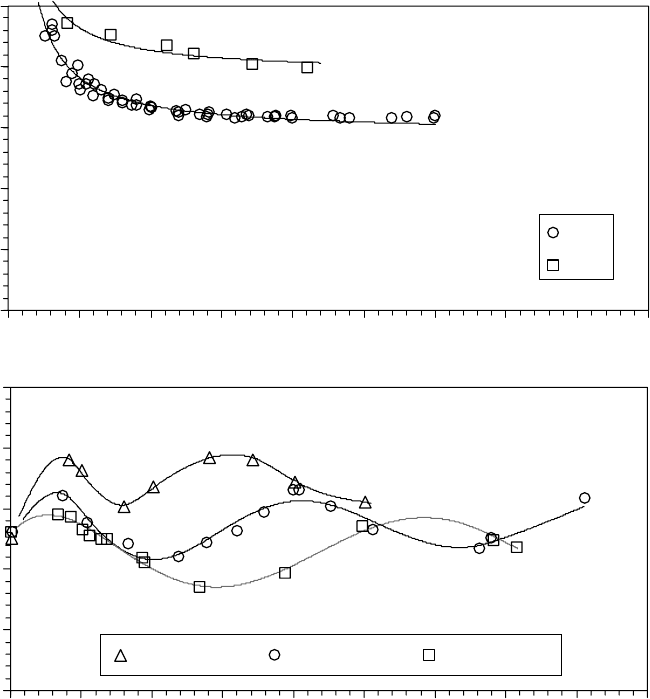
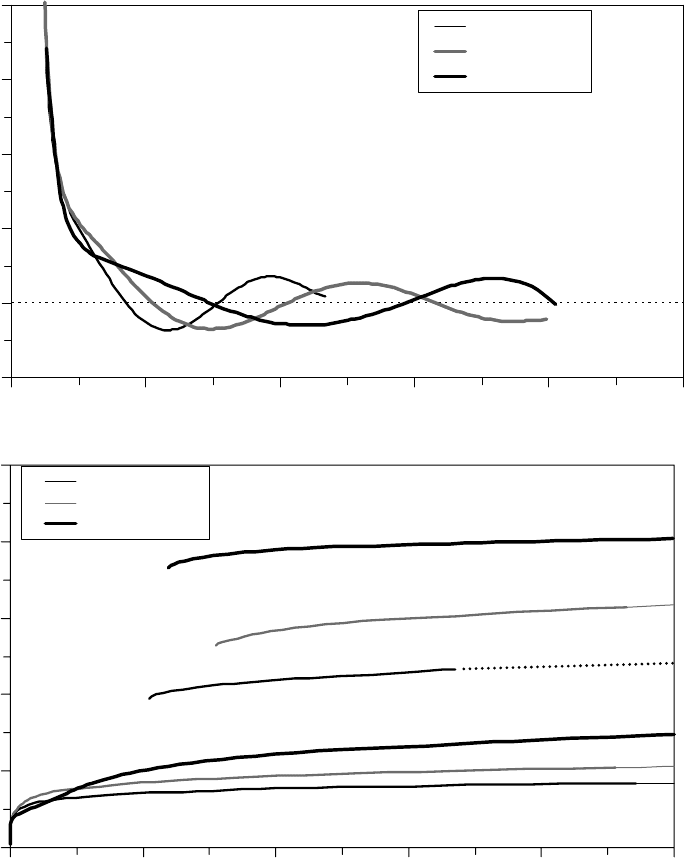
The regression t to the surface energy data, shown as smooth curves in Figures 22.9a and 22.9b [1],
was numerically differentiated to obtain the disjoining pressure [35,36]. The total disjoining pres-
sure, including the individual contributions from the dispersive and polar components, is shown
in Figure 22.10a [1]. Notice that γ
d
decreases monotonically with h, which is consistent with
Equation 22.10. Below lm thicknesses of ∼0.5 nm, Π at each molecular weight is dominated by
γ
d
, which increases rapidly with decreasing lm thickness and is largely independent of molecular
weight. The γ
p
, however, oscillates with lm thickness and becomes larger in magnitude than γ
d
as
h increases. Oscillations in γ
p
provide an additional contribution to Π for PFPE Zdol that produces
alternating regions of stable and unstable lm thickness [37]. The sum of the two contributions
0
5
10
15
20
25
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5
Zdol thickness (nm)
Polar (mN/m)
1100 Da
1600 Da
3100 Da
(b)
0
5
10
15
20
25
0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5
Thickness (nm)
Dispersive (mN/m)
Zdol
Z
(a)
FIGURE 22.9 The components of the surface energy measured on CHx-overcoated thin lm magnetic
recording media with fractionated Zdol of narrow polydispersity index. (a) The dispersive component of the
surface energy for PFPE Z and Zdol. (b) The polar component of the surface energy for PFPE Zdol. (Adapted
from Rudnick, L.R. (ed.), Synthetics, Mineral Oils, and Bio-Based Lubricants Chemistry and Technology,
Chapter 38, CRC Press, Taylor & Francis Group, Boca Raton, FL, 2006.)
CRC_59645_Ch022.indd 538CRC_59645_Ch022.indd 538 3/20/2009 5:44:27 PM3/20/2009 5:44:27 PM
Lubricants for the Disk Drive Industry 539
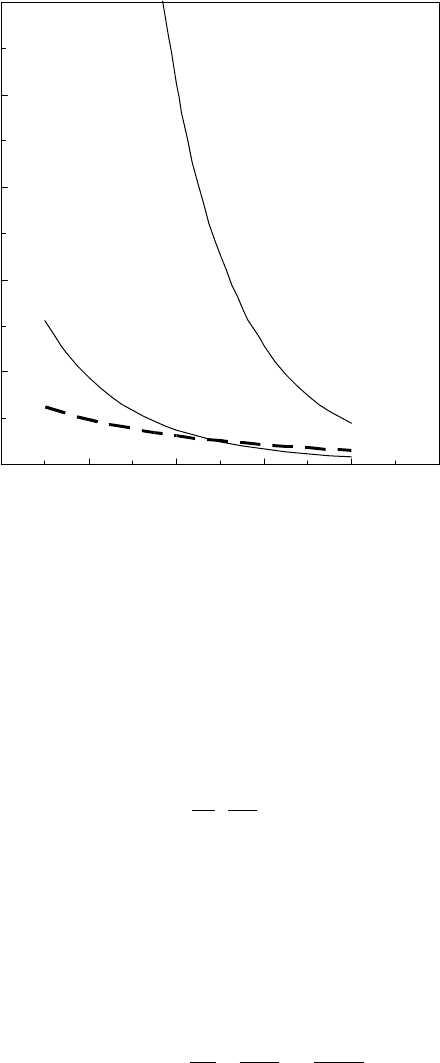
gives rise to oscillations in the total disjoining pressure. It is remarkable that the disjoining pressure
from the surface energies as a function of lm thickness, and Equation 22.8 relating the disjoin-
ing pressure to the degree of saturation predict the adsorption isotherms for low-molecular-weight
Zdols, according to P/P
0
= exp(−ΠV
l
/RT), which are shown in Figure 22.10b. There are two ther-
modynamically stable regions of lm thickness for degrees of saturation corresponding to regions
where Π > 0 and ∂Π/∂h < 0. For thicknesses in between these regions, condensing Zdol molecules
will either reevaporate or form islands at the next higher stable lm thickness.
0
1
2
3
4
5
0 0.2 0.4 0.6 0.8 1
P/P
0
Zdol thickness (nm)
1100 Da
1600 Da
3100 Da
(b)
−20
0
20
40
60
80
5
Zdol thickness (nm)
Π (Mpa)
1100 Da
1600 Da
3100 Da
(a)
01234
FIGURE 22.10 (a) The disjoining pressure from the fractionated Zdol surface energies in Figure 22.9a and
(b) the corresponding Zdol adsorption isotherms at 60°C. (Adapted from Rudnick, L.R. (ed.), Synthetics, Min-
eral Oils, and Bio-Based Lubricants Chemistry and Technology, Chapter 38, CRC Press, Taylor & Francis
Group, Boca Raton, FL, 2006.)
CRC_59645_Ch022.indd 539CRC_59645_Ch022.indd 539 3/20/2009 5:44:27 PM3/20/2009 5:44:27 PM

540 Lubricant Additives: Chemistry and Applications
For the purpose of explaining the viscosity increase of thin lms, surface chemical potential is
approximated by the unretarded atom-slab dispersive interaction energy
⫽⫺
VA
h
l
6
3
(22.10)
The dispersive interaction coef cient A is also referred to as the Hamaker constant and A = 10
−19
J
for Zdol.
As mentioned, the vaporization energy is the energy required to remove a molecule from the liquid
without leaving behind a hole, which is the energy needed to form a hole of the size of a molecule
in the liquid. The free volume needed for a ow unit to transition into the ow-activated state is less
than the size of the entire molecule. It is found that the ratio n ≡ ∆E
vap,∞
/∆E
vis,∞
> 3, where ∆E
vap,∞
and ∆E
vis,∞
are the vaporization and ow activation energies of the bulk liquid, respectively. Thus,
the ow activation energy near the surface is given approximately by
EE
n
vis vis,
⫽⫺
⬁
(22.11)
For linear chains longer than 5 or 10 carbon atoms, n increases due to the onset of segmental ow.
In practice, n is experimentally determined from the measured values of the vaporization and ow
activation energy. For PFPE Zdol 4000, ∆E
vap,∞
= 166 kJ/mol, giving n ≈ 4.8. This is consistent
with segmental ow.
To calculate the thin lm viscosity with Equation 22.6, the ow activation entropy near the
surface is also needed. Experimental ow activation entropy is calculated from the spin-off data
[32] with Equations 22.6 and 22.11 as follows. The experimental η versus h is determined from
dh/dt during air shear-induced ow on a rotating disk. Equation 22.6 is then solved for ∆S
vis
versus
h using Equation 22.11 for ∆E
vis
.
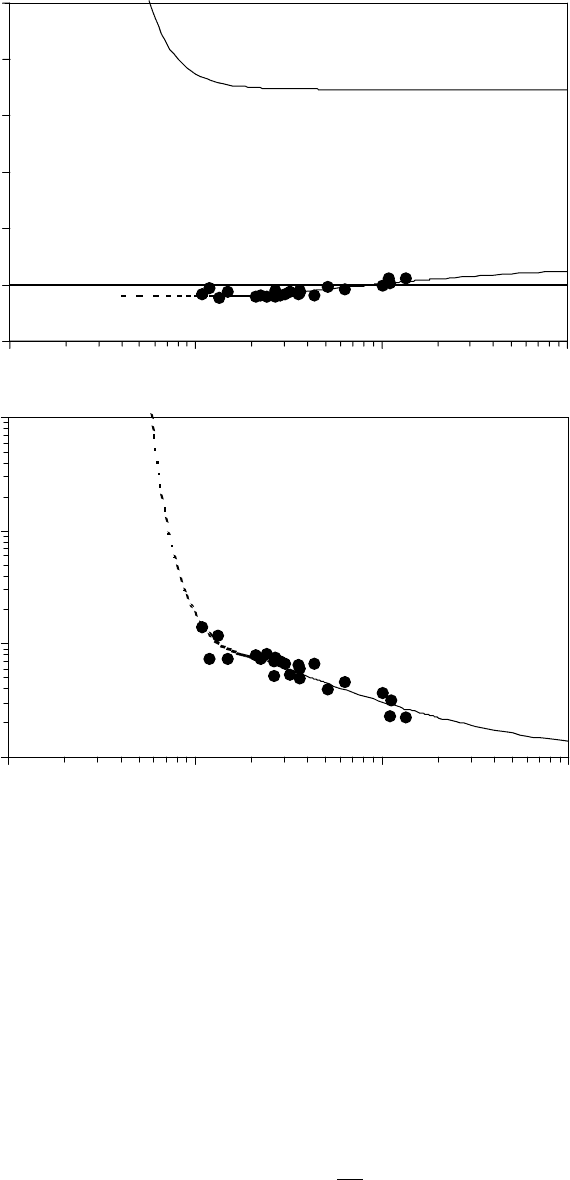
The ow activation energy and entropy are shown in Figure 22.11a [1]. The ow activation energy
suddenly increases below ∼0.8 nm due to the strong lm thickness dependence of the dispersion force.
The retarding effect of this increase on ow is compounded by the apparent effect of con nement on
restricting the degrees of freedom in the ow transition state, as seen by the negative entropic contri-
bution in Figure 22.11a. Below 2.3 nm, T∆S
vis
≈ −1.9 kJ/mol, which corresponds to the critical con-
gurational entropy change for ow (−R ln 2 ≈ −5.76 J/mol °K). The combined effects give rise to the
observed increase in viscosity with lm thickness shown in Figure 22.11b and enables extrapolation of
the viscosity to even thinner lms where the spin-off is so slow that it takes years to measure.
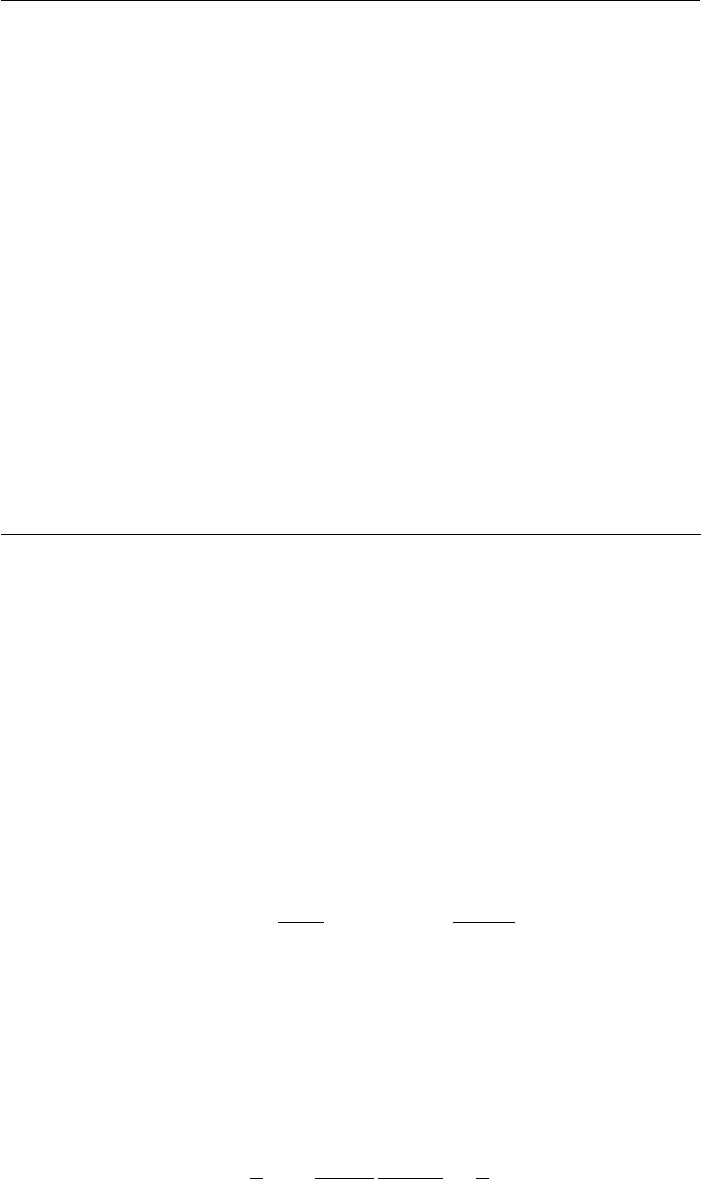
The viscosity increases by a large amount with lm thickness, which is much greater than the
increase with temperatures that might normally be encountered in the disk drive. The bulk viscosity
for several PFPE lubricants is shown in Figure 22.12 [1]. As the increase in viscosity with thickness
below ∼0.8 nm is much more than the increase with temperature between 0 and 60°C, the operating
temperature of disk drives should have no signi cant effect on lubricant spin-off from the disk by
air shear. That is, excluding air shear force due to the head suspension assembly and the air bearing
[38] and dispersion force between the lubricant and a low- ying slider [39].
22.2.1.4 Vapor Pressure
The vapor pressure of PFPE lubricants must be low to prevent evaporation from the disk. A method
to measure the vapor pressure was developed as follows. A model was derived to calculate the vapor
pressure from the measured Zdol molecular weight distribution and evaporation rate. Molecular
weight distributions were measured by GPC, as described in Karis et al. [28]. The vapor pressure
of discrete molecular masses was calculated from the evaporation rate measured by isothermal
thermogravimetric analysis (TGA) with a stagnant lm diffusion model as described by Karis and
CRC_59645_Ch022.indd 540CRC_59645_Ch022.indd 540 3/20/2009 5:44:27 PM3/20/2009 5:44:27 PM
Lubricants for the Disk Drive Industry 541
Nagaraj [40]. Polymers such as Zdol differ from the low-molecular-weight synthetic hydrocarbon
oils in that polymers comprise various different molecular weights. Further considerations must be
taken into account in modeling the evaporation of polymers, as described later.
A numerical model was developed to simulate the evaporation of a polymer from an initial
molecular weight distribution measured by GPC. The evaporation simulation is written in terms of
mass ux and the discrete form of the molecular weight distribution w
i
(t) as
wt wt t
A
m
t
ii i
() () ()
⫹
⫽⫺flux
0
(22.12)
−10
0
10
20
30
40
50
0.1 1.0 10.0 100.0
Zdol thickness (nm)
kJ/mol
(a)
1
10
100
1000
0.1 1.0 10.0 100.
0
Zdol thickness (nm)(b)
T∆S
vis
∆E
vis
η/η
∞
FIGURE 22.11 The vertical axis is de ned by the annotations on the curves in (a) Flow activation energy and
the entropic component of the ow activation free energy and (b) dispersion-enhanced viscosity as a function
of lm thickness. The lled symbols are from the spin-off measurements, and the dashed region of the curve
was calculated with constant ow activation entropy below 2.3 nm. Fractionated Zdol molecular weight 4500
Da, temperature 50°C. (Adapted from Rudnick, L.R. (ed.), Synthetics, Mineral Oils, and Bio-Based Lubricants
Chemistry and Technology, Chapter 38, CRC Press, Taylor & Francis Group, Boca Raton, FL, 2006.)
CRC_59645_Ch022.indd 541CRC_59645_Ch022.indd 541 3/20/2009 5:44:28 PM3/20/2009 5:44:28 PM
542 Lubricant Additives: Chemistry and Applications
where
A = surface area of the evaporating lubricant
m
0
= initial mass of lubricant
∆t = time step in the simulation
The mass ux of the ith molecular weight fraction M
i
is given by stagnant lm diffusion:
flux
i
ii
i
t
DM
RT
P()⫽
(22.13)
Here D
i
is the vapor-phase diffusion coef cient and δ the diffusion length (calculated or measured
with a liquid of known vapor pressure).
The mass ux divided by the mass density yields the rate of lm thickness change. The solution
vapor pressure for the ith molecular fraction was approximated assuming an ideal solution according
to Raoult’s law P
i
= x
i
P
i
0
, where x
i
is the mole fraction of the ith molecular fraction.
The Hirschfelder approximation [41] is used for the vapor-phase diffusion coef cient
D
MM
T
P
i
ii
⫽⫻ ⫹
⫺
1 858 10
11
4
12
32
.
/
/
gas
2
(22.14)
where
M
gas
= molecular weight of the ambient atmosphere (air or nitrogen to suppress oxidation)
P = ambient pressure
σ
i
= (σ
i
lube
+ σ
i
gas
)/2 = collision diameter
0.0
0.1
0.2
0.3
0.4
0.5
0 20 40 60 80 100
T (°C)
η
∞
(Pa s)
Z03
Zdol 4000
Ztetraol 2000
FIGURE 22.12 Bulk viscosity and ow activation energy of several PFPE lubricants as a function of tem-
perature. (Adapted from Rudnick, L.R. (ed.), Synthetics, Mineral Oils, and Bio-Based Lubricants Chemistry
and Technology, Chapter 38, CRC Press, Taylor & Francis Group, Boca Raton, FL, 2006.)
CRC_59645_Ch022.indd 542CRC_59645_Ch022.indd 542 3/20/2009 5:44:28 PM3/20/2009 5:44:28 PM
Lubricants for the Disk Drive Industry 543
For nitrogen, σ
i
gas
= 0.315 nm. The vapor-phase molecular diameter of the ith molecular fraction
employed in estimating the binary mass diffusion coef cient is approximately given by σ
i
lube
=
2 × R
g,i
≈ 0.05 ×
√
___
M
i
, where the molecular weight M
i
is in daltons and the radius of gyration R
g,i
is
in nanometers. This expression was derived from the radius of gyration for Zdol measured in Freon
[42,43]. The molecular diameters from this approximation for a range of ideal monodisperse Zdol
molecular weights are listed in Table 22.8 [1].
By analogy to hydrocarbon oils, the collision integral Ω for collision between molecules in the
gas phase is a function of their binary Lennard–Jones interaction potential. The collision integral
between Zdol molecules and nitrogen molecules was taken to be the same as that for collision
between hydrocarbon molecules and nitrogen, Ω = 1.2.
The Clapeyron equation is employed to calculate the pure component vapor pressure:
PP
S
R
E
RT
i
ii
0
1⫽⫺
⫺
exp exp exp
vap vap
{}
(22.15)
where the vaporization entropy ∆S
i
vap
= S
i
vap
– S
liq
is the difference between the entropy in the
vapor state and that in the liquid state. The Zdol liquid entropy is assumed to be independent
of molecular weight. It was determined along with the activation energy by comparison of the
simulated evaporation with isothermal TGA evaporation weight loss data. The liquid entropy for
Zdol, S
liq
= 107 J/mol K, is within the range obtained for the synthetic hydrocarbon oils. The vapor-
phase translational entropy for oils is approximated with the Sackur–Tetrode equation:
SR
h
RT
NP
M
p
vap,trans
i
i
⫽⫹ ⫹
5
2
2
3
2
32 52
4
ln ln
//
() ( )
()
3
(22.16)
TABLE 22.8
The Gas-Phase Molecular Diameter for Ideal Monodisperse Zdol Fractions
Calculated from the Radius of Gyration in Theta Solvent d = 0.05 √M
M (Da) d (nm)
Degree of
Polymerization
Contour
Length (nm)
Equilibrium
Thickness (nm)
500 1.12 3.54 2.71 0.66
750 1.37 6.29 4.06 0.74
1000 1.58 9.03 5.41 0.82
1350 1.84 12.88 7.29 0.93
1500 1.94 14.53 8.10 0.98
2000 2.24 20.02 10.79 1.14
3000 2.74 31.01 16.18 1.46
4000 3.16 42.00 21.56 1.79
4300 3.28 45.30 23.18 1.88
5400 3.67 57.38 29.10 2.24
Note: Also included are the degree of polymerization, the contour length measured along the chain from one
end to the other, and the equilibrium thickness. The equilibrium thickness is the maximum stable lm
thickness, or dewettting thickness, determined from the rst zero crossing of the disjoining pressure
with increasing lm thickness.
Source: Adapted from Rudnick, L.R. (ed.), Synthetics, Mineral Oils, and Bio-Based Lubricants Chemistry
and Technology, Chapter 38, CRC Press, Taylor & Francis Group, Boca Raton, FL, 2006.
CRC_59645_Ch022.indd 543CRC_59645_Ch022.indd 543 3/20/2009 5:44:28 PM3/20/2009 5:44:28 PM
