Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.

594 Lubricant Additives: Chemistry and Applications
5. A calcium complex soap-thickened grease generally exhibits excellent oil separation, good
mechanical stability, and in general can be used to a maximum application temperature of
350°F (177°C). The thickener in this case provides a degree of AW and EP protection.
6. A lithium complex soap-thickened grease generally exhibits excellent oil separation, mod-
erate water resistance, and in general can be used to a maximum application temperature
of 350°F (177°C).
7. Clay-thickened grease has good to excellent water resistance, good pumpability, excellent
oil separation, and in general can be used at maximum application temperatures >350°F
(177°C).
8. Polyurea-thickened grease has excellent oxidative stability, excellent pumpability, and
excellent oil separation, but has poor worked stability and fair to modest antirust per-
formance. These greases can be used at a maximum application temperature of 350°F
(177°C). Polyurea greases soften easily but are reversible.
Chemical and physical processes caused by thermal and shear stresses degrade greases [29]. These
authors demonstrated that thermally aged lithium hydroxystearate greases were affected in terms of
oil lm thickness and oil release in a rolling contact under starved conditions.
23.6 OXIDATION INHIBITORS
The mechanism of hydrocarbon oxidation, because of its importance to lubricant chemistry and
performance, has been well studied and is reviewed in Chapter 1.
Hydrocarbons react with oxygen to initially produce peroxides and hydroperoxides, which fur-
ther react to give alcohols, aldehydes, ketones, and carboxylic acids. These oxidation reactions pro-
ceed through free radical chain processes. Grease, which is basically soap-thickened hydrocarbon,
is also susceptible to oxidation. In addition, the metals of the metal soaps can catalyze oxidation.
Examples of classes of antioxidants used in grease are
Hindered phenols, for example, 2,6-di-t-butyl phenol and 2,6-di-t-butyl-p-cresol
Aromatic amines, for example, diarylamines, di-octyldiphenylamine
Metal dialkyldithiophosphates, for example, zinc dithiophosphate
Metal dialkyldithiocarbamates, for example, zinc and molybdenum dithiocarbamates
Ashless dialkyldithiocarbamates
Sulfurized phenols, for example, phenolic thioesters, and phenolic thioethers
Phenothiazine
Disul des, for example, diaryldisul des
Trialkyl and triaryl phosphates and phosphites, for example, tris(di-t-butyl phenyl phosphite)
Alkylated phenol antioxidants are most effective at low temperatures. Secondary aromatic amines
such as phenyl alpha-naphthylamine (PANA), phenyl beta-naphthylamine (PBNA), di-octyldi-
phenylamine, and phenothiazines are most useful at high temperatures [5]. In practice, grease is
generally formulated to include a combination of alkylated or secondary amine- and phenol-type
antioxidants to provide performance over as wide a temperature range as possible.
In some cases, the combination of antioxidants (or other additives) provides an additive effect,
whereas in other cases, synergy is observed when both a hindered phenolic and an aryl amine anti-
oxidant are used together in the same formulation.
23.7 FRICTION AND WEAR
Friction is the force required to cause the motion of two surfaces or bodies in contact with each other.
Lubricants are used to reduce the frictional forces. High friction results in heat and because more
force or power is necessary to move the parts relative to one another, this friction reduces operating
•
•
•
•
•
•
•
•
•
CRC_59645_Ch023.indd 594CRC_59645_Ch023.indd 594 10/31/2008 3:18:49 PM10/31/2008 3:18:49 PM
Additives for Grease Applications 595
ef ciency. When the lubricant lm is insuf cient to protect the metal surfaces, there is wear on one or
both the components. Wear is material loss directly caused by the interaction of asperities on the two
surfaces while in relative motion to each other. As wear results in the loss of material and the scarring
changes the size and shape of the machined components, wear reduces the useful life of the compo-
nents. Extreme wear can result in failure of the equipment and in safety issues. There are three general
types of wear: abrasive, adhesive, and corrosive. These are generally addressed by formulating a grease
using additives designed to protect against these phenomena. When a lubricant is applied between
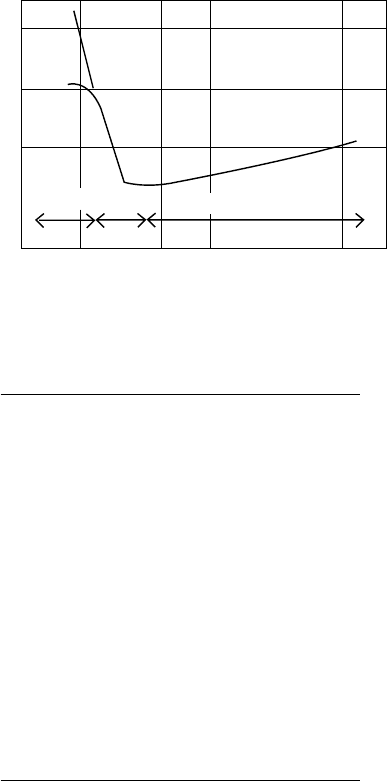
the rubbing surfaces, the friction and wear can be minimized. Three lubrication regimes are de ned
depending on the amount of lubricant lm separating the surfaces. These are
Boundary lubrication
Elastohydrodynamic lubrication
Hydrodynamic lubrication
These three lubrication regimes are indicated on a Stribeck curve shown in Figure 23.2 [30].
Approximate thickness of lms in each regime and how they are related to the size of asperities and
sliding wear debris in the boundary regime are shown in Table 23.5 [31].
•
•
•
TABLE 23.5
Dimensions of Films, Asperities, and
Debris Related to Boundary Lubrication
Approximate Size
Range (µm)
Monomolecular layer 0.002–0.2
Sliding wear debris 0.002–0.1
Boundary lm 0.002–3
Elastohydrodynamic lm 0.01–5
Asperity height 0.01–5
Rolling wear debris 0.07–10
Hydrodynamic lm 2–100
Asperity tip radius 10–1000
Concentrated contact width 30–500
FIGURE 23.2 Stribeck curve for a journal bearing.
Hydrodynamic
Mixed
Boundary
5
0.001
0.01
0.1
f
1
810
ηω/p
20
CRC_59645_Ch023.indd 595CRC_59645_Ch023.indd 595 10/31/2008 3:18:49 PM10/31/2008 3:18:49 PM
596 Lubricant Additives: Chemistry and Applications
Hydrodynamic lubrication is a regime where the moving surfaces are essentially separated from
each other. In this regime, the viscosity of the oil in combination with the motion of the mechanical
components can produce a uid pressure high enough to completely separate the two surfaces.
Elastohydrodynamic lubrication is a regime where the lm thickness is insuf cient to com-
pletely separate the surfaces. In this regime, the surface asperities make contact, which leads to
wear. The lubricant in the contact area is continually replenished at the front of the contact [32]. The
lm thickness in the elastohydrodynamic regime is larger than that in the boundary lubrication but
smaller than that in the hydrodynamic regime.
Boundary lubrication is a regime where lm thickness between the moving surfaces is only a
few molecules thick. In this regime, because of the closeness of the moving surfaces, friction and
wear are determined by properties of both the surfaces and the lubricant. Boundary lms form
because they reduce the surface energy and, therefore, are thermodynamically favored [33]. These
lms form by molecules that contain polar functional groups. Because of this, they orient onto
the surface by either chemical or physical adsorption. Even oxidation products derived from the
breakdown of the lubricating uid can adsorb onto metal parts and into contact areas that are being
lubricated. Boundary lubrication can range from mild to severe conditions.
Physical adsorption is a reversible process in which molecules adsorb and desorb from a sur-
face without chemical change. Additives that provide protection by physical adsorption are polar
structures. This is because at least two phenomena must occur: the molecule must have a prefer-
ential af nity for the surface and it should have a preferred orientation on the surface, so that a
more closely packed arrangement can be achieved. Alcohols, acids, and amines are examples of
long-chain molecules with functional groups at the end. Molecules that can pack tightly and orient
in a closely packed arrangement relative to the surface provide improved lm strength. Because the
forces involved in physical adsorption are relatively weak, these lms are effective at low to moder-
ate temperatures. New molecules from the bulk lubricant are constantly available to replace those
that physically desorb or are mechanically removed from the surface.
Chemical adsorption, however, is an irreversible process in which a lubricant uid molecule or
additive component reacts with the surface to form a low shear strength protective layer. As this new
low shear strength material is worn away, additional additive reacts to form a new protective layer.
Protection from chemical adsorption occurs at higher temperatures because chemical reactions are
required to generate the actual species that form the surface lms. EP additives can protect lubri-
cated surfaces at temperatures as high as 400°C.
Wear protection and friction reduction over a wide temperature range can be achieved by
combining additives that function by physical and chemical adsorption. Between the low-
temperature physically adsorbed layer and the high-temperature chemically adsorbed layer can be
a temperature range over which there is poorer protection. This has been experimentally demon-
strated where oleic acid was used as the normal wear additive and a chlorinated additive provided
EP protection at higher temperatures [34].
23.8 EXTREME-PRESSURE AND ANTIWEAR AGENTS
EP and AW additives are used to reduce friction and prevent wear under moderate to more severe
boundary lubrication conditions. Reactive compounds containing sulfur, phosphorus, or chlorine,
metals, or combinations are known to provide EP protection.
Under high loads, opposing metal surfaces contact each other, and as a result, high local
temperatures develop, enabling an EP agent to react with the metal surfaces, forming a surface
lm, preventing the welding of opposing asperities [35].
Some of the major group materials that have been used as EP and AW additives are as follows:
Sulfurized ole ns, fats, and esters
Chlorinated paraf ns
•
•
CRC_59645_Ch023.indd 596CRC_59645_Ch023.indd 596 10/31/2008 3:18:49 PM10/31/2008 3:18:49 PM
Additives for Grease Applications 597
Metal dialkyldithiophosphates, including antimony and zinc
Phosphate and thiophosphate esters, for example, tricresyl phosphate, di-n-octyl phosphite,
isodecyl diphenyl phosphite
Ammonium salts of phosphate esters
Borate esters
Metal dithiocarbamates, including antimony
Metal naphthenates, including bismuth and lead
Metal soaps, including lead
Sul des and disul des, for example, diaryldisul des
High-molecular-weight complex esters
23.8.1 SOLID ADDITIVES
Solid additives are organic or polymeric solid materials or inorganic compounds used to impart
EP and friction reduction properties to the grease and protection in case of lubricant loss. A more
detailed description of solid additives can be found in Chapter 6. Examples include the following:
Bismuth has recently been reported as being more environmentally-friendly than lead for
application as an EP additive [36,37]
Boron-containing additives—boric acid, borax, and metal borates
Boron nitride
Molybdenum disul de
Inorganic-sulfur-phosphorus additive—patented blend of phosphate and thiosulfate [38]
Fluorinated polymers, for example, per uorinated polyole ns
Graphite—in various forms. The merits of expanded graphite have been reported [39]
Calcium acetate, carbonate, and phosphate, cerium uoride
Zinc stearate, zinc oxide
Copper powder
Nickel powder
Phosphate glasses
EP properties and the mechanism of phosphate glasses in lubricating greases have been studied
where the authors compared phosphate glasses to molybdenum disul de, graphite, molybdenum
dithiocarbamate, polytri uoroethylene, and boron nitride [40]. Improvement in the load-carrying
capacity of greases using phosphate glass—a white, relatively inexpensive powder compared with
other solid additives—has been reported to provide very effective wear protection under severe
conditions [41,42]. Under light loads, the nely divided phosphate glass particles were found to
maintain their original round shape. The particles were performing as micro ball bearings under
these low-load conditions. At very high loads, the phosphate glass particles compressed and formed
a thick protective lm on the wear surface.
Greases nd a place in space applications, where these lubricants need to demonstrate long-term
use in situations involving vacuum. Additives used in these greases must have low volatility and excel-
lent lubricity. Greases for these applications have included per uoropolyalkylether (PFPAE) uids
for many years. Studies using deep-groove ball bearings lled with PFPAE-based grease have been
reported with long-run periods in vacuum of 10
–4
–10
–5
Pa at 2000 rpm [43].
23.9 RUST AND CORROSION INHIBITORS
Rust is a form of corrosion formed by electrochemical interaction between iron and atmospheric
oxygen and is accelerated in the presence of moisture due to the catalytic action of water [44]. Rusting
of iron and steel surfaces can reduce operating ef ciencies and cause part and equipment damage.
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
CRC_59645_Ch023.indd 597CRC_59645_Ch023.indd 597 10/31/2008 3:18:49 PM10/31/2008 3:18:49 PM
598 Lubricant Additives: Chemistry and Applications
The electrochemical oxidation of the surfaces of iron or steel can be prevented by the addi-
tion of speci c water-blocking additives to lubricating grease that inhibit the formation of rust (or
iron oxides). Rust inhibitors are typically highly polar surface-active oil-soluble compounds, which
attach to metal surfaces by physical adsorption.
Rust inhibitors incorporated into lubricating grease provide a protective lm against the effects
of moisture, water, and air. Corrosion inhibitors work by neutralizing corrosive acids formed by the
degradation of base uids and lubricant additives.
Examples of various chemical classes of rust and corrosion inhibitors used in grease are
Carboxylic acids, including fatty acids, for example, alkyl succinic acid half ester, and nonyl
phenoxy acetic acid
Salts of fatty acids and amines, for example, disodium sebacate
Succinates, for example, alkyl succinic acid half ester
Fatty amines and amides
Metal sulfonates, including ammonium, barium, and sodium
Metal naphthenates, including bismuth, lead, and zinc
Metal phenolates
Nitrogen-containing heterocyclic compounds, for example, substituted imidazolines
Amine phosphates
Salts of phosphates esters
23.9.1 METAL DEACTIVATORS
These materials reduce the catalytic effect of metal on the rate of oxidation. They act by forming an
inactive lm on metal surfaces by complexing with metallic ions. Several classes of materials have
been reported to be effective:
Organic complexes containing nitrogen or sulfur, amines, sul des, and phosphates
Derivates of 2,5-dimercapto-1,3,4-thiadiazole
Triazoles, benzotriazoles, and tolyltriazoles
Di-salicylidene-propanediamine
23.9.2 TACKINESS ADDITIVES
Grease may be formulated to withstand the heavy impact common in heavy-equipment applica-
tions. The adhesive and cohesive properties of grease can be improved to resist throw off from
bearings and ttings, while providing extra cushioning to reduce shock and noise through the use
of tackiness agents. The water resistance of such grease can also be signi cantly improved through
the use of tackiness additives.
High-molecular-weight polymers such as polyisobutylene (PIB), polybutene, ethylene-propylene
copolymer, ole n coplymer (OCP) and latex compounds are typical examples of tackiness additives.
Like all long-chain polymers, tackiness additives are susceptible to breakdown when exposed to high
rates of shear. A further discussion of these interesting materials is described in Chapter 13.
23.10 ENVIRONMENTALLY FRIENDLY GREASE
Fully formulated grease that combines needed high-performance features with environmental
safety and compliance has been developed not only for civilian industrial and automotive applica-
tions but also for the military [45].
The goal of environmentally friendly lubricants is to minimize or eliminate any potential harm
or damage to humans, wildlife, soil, or water. Depending on the nature of the application, the use
•
•
•
•
•
•
•
•
•
•
•
•
•
•
CRC_59645_Ch023.indd 598CRC_59645_Ch023.indd 598 10/31/2008 3:18:49 PM10/31/2008 3:18:49 PM
Additives for Grease Applications 599
of environmentally friendly (or “green”) lubricants will enable industry to reduce some of, and
perhaps even eliminate, the costs associated with the remediation and disposal of nonbiodegradable
and toxic lubricants.
One key raw material used in formulating biodegradable grease is vegetable oil. Vegetable oils
are obtained from renewable sources and are biodegradable and, as such, are more environmentally
friendly than conventional mineral oil–based lubricants [46].
A special class of vegetable oils, containing a high oleic content (≥75% oleic) and a low polyun-
saturated fatty acid content (linoleic or linolenic), displays good oxidative stability with acceptable
low-temperature properties. This makes them well suited for use in greases compared to conven-
tional vegetable oils [46].
In addition to the lubricating uids, the toxicity and biodegradability of additive components is
important. Over the years, many of the additives that were originally based on fractions cut from
petroleum or coal-derived liquids are now synthetic and, therefore, are of much higher purity.
Linear side chain hydrocarbon groups have in many instances replaced branched and aromatic
functional groups. This results in greater potential biodegradability. Toxicity of additives is related
to metals in many cases. Current trends are to replace metal-containing additives with ashless
varieties having similar or greater performance features. This will result in a lower pollutant load
on the environment. Even military formulations are moving toward more environmentally friendly
versions.
Environmentally friendly grease is used in applications such as agriculture, construction,
forestry, marine, mining, and railroad. Speci c applications include tramway tracks and railway
switches, wheel ange lubricant for railways, and farm tractors.
23.11 SUMMARY
In summary, grease nds applications where uid lubricants may drip or leak from the point of
application. Grease reduces the need to frequently lubricate a particular site, because the grease
structure serves as a lubricant reservoir. Grease is very effective where lubricant is needed on a ver-
tical machine component or at positions that are dif cult to reach. Grease acts as a physical barrier
that is effective in sealing out external contaminants and provides better protection when contami-
nated than does a liquid lubricant. Grease can also provide noise reduction in certain applications
and is effective in protecting equipment at high temperatures and pressures and under conditions
where there is shock loading in the lubricated components.
The formulation of grease can be considered as art or science depending on the researcher ques-
tioned, but certain guidelines are generally useful.
For low-temperature applications, grease can use low- and high-viscosity index (VI) lubricat-
ing uids with a relatively low thickener content. The uid lubricant should have a low pour point
and good pumpability and rust protection. Grease for high-temperature applications will typically
have a higher-viscosity lubricating uid and high-temperature complex thickener. This will result in
grease with a higher dropping point. This type of grease will also nd application where low am-
mability and, therefore, low volatility are design requirements.
Grease for applications where water contamination is an issue should be designed with a
water-resistant thickener and generally a high-viscosity lubricating uid. The grease should have
low water washout and low waster spray-off, which can be improved by incorporating a tacki-
ness additive. Any application in a wet environment requires that the grease has excellent rust
and corrosion protection. Careful formulation to balance the chemistries of the needed additives
is critical in achieving the described performance where surface protection is so important to
machine life. Where shock or heavy loads are applied, grease is formulated using high-viscosity
lubricating uids and should include higher concentrations of AW and EP additives. This combi-
nation will provide both thicker lubricant lm and appropriate additive chemistry to protect the
metal surfaces.
CRC_59645_Ch023.indd 599CRC_59645_Ch023.indd 599 10/31/2008 3:18:50 PM10/31/2008 3:18:50 PM

600 Lubricant Additives: Chemistry and Applications
Grease used in a centralized system needs to have a low to moderate thickener concentration
and a relatively low-viscosity lubricating uid, so that the grease exhibits a lower apparent viscosity
and can be readily pumped throughout the system.
23.12 EFFECTS OF INDIVIDUAL ADDITIVES AND GREASE SOAPS
This section includes a sample of application data in which various grease additives were employed to
modify the performance of various greases. The rst section describes the use of individual additives
with a particular type of grease. The second section demonstrates some synergistic effects of combining
two or more additives that individually may not provide all the performance bene ts needed.
An excellent general review of the fundamental characteristics of synthetic lubricating greases
has been published [47]. This review describes the very wide range of potential synthetic greases in
terms of properties and performance capabilities but also cautions that some of these higher-perfor-
mance capabilities are not always needed, and thus, the higher cost of synthetics may not always be
justi ed. Previously published chapters on greases can be found in earlier books [48,49].
These application data are meant to serve as a guideline, not as a recipe for greases. The actual
combination of all the components of grease, including the base oil, thickener, each additive, and the
order of addition, will affect the properties and performance of a nal grease formulation, and thus,
these issues are left for the formulator. A list of additive components is summarized in Table 23.6.
The data in Table 23.7 show the results of wear experiments on aluminum complex grease using
four organo-molybdenum-containing additives at the same concentration. Molyvan A is a powder,
and any heterogeneity in the lubricant might result in the observed higher wear scars in grease made
with this additive.
Comparison of aluminum complex and lithium complex grease with one level of Vanlube 829
showed improvement in both four-ball EP weld load and four-ball wear scar in the lithium complex
grease (Table 23.8). It should be noted that the weld load difference in these results is within experi-
mental error, but the combined improvement in weld load and wear scar is directionally desirable.
TABLE 23.6
Grease Additives—Chemical Components
Molyvan L: Molybdenum di(2-ethylhexyl) phosphorodithioate
Molyvan 822: Molybdenum dialkyldithiocarbamate in oil
Molyvan 855: Organo-molybdenum complex
Molyvan A: Molybdenum di-n-butyldithiocarbamate
Vanlube 829: 5,5-Dithio-bis(1,3,4-thiadiazole-2)3H-thione
Vanlube 73:Antimony tris(dialkyldithiocarbamate) in oil
Vanlube 7723: Methylene bis(dibutyldithiocarbamate)
Vanlube 622: Antimony o,o-dialkylphosphorodithioate in oil
Vanlube 8610: Antimony dithiocarbamate/sulfurized ole n blend
Vanlube NA: Alkylated diphenylamines
Desilube 88: Blend of phosphate and thiosulfate
Irgalube 63: Ashless dithiophosphate
Irgalube TPPT: Triphenyl phosphorothionate
Lubrizol 1395: Zinc dialkyldithiophosphate
Lubrizol 5235: Sulfur–phosphorus–zinc additive package
Lubrizol 5034A: Sulfur–phosphorus industrial gear oil additive package
Amine O: Substituted imidazoline
Sarkosyl O: N-oleyl sarcosine
Na-Sul ZS-HT: Zinc dinonylnaphthalene sulfonate/carboxylate complex
MoS
2
: Molybdenum disul de
CRC_59645_Ch023.indd 600CRC_59645_Ch023.indd 600 10/31/2008 3:18:50 PM10/31/2008 3:18:50 PM
Additives for Grease Applications 601
The data in Table 23.9 show the improved performance of an aluminum complex grease
containing Vanlube 622 over the grease containing a similar amount of Vanlube 73 or Vanlube
7723. There should be concern over the high sulfur content of Vanlube 622 and its effect on
copper corrosion for applications where the grease will be in contact with copper-containing
components.
Comparison of two antimony-containing EP/AW additives in aluminum complex grease showed
improved EP performance and lower wear scar diameter for the sulfurized antimony dithiocarba-
mate (DTC) (Vanlube 8610; Table 23.10). Comparison of two sulfur–phosphorus EP additives in
aluminum complex and lithium complex grease shows extremely high four-ball EP performance
using Desilube 88 compared with Irgalube 63 (Table 23.11).
TABLE 23.9
Effect of Antimony Compounds in Aluminum Complex Grease
Additive Wt%
Four-Ball EP
Weld Load (kg)
Four-Ball Wear Scar
Diameter (mm)
Aluminum complex base grease
(980 SUS paraf nic base oil blend)
—
126 0.68
Vanlube 73 3.0 315 0.84
Vanlube 7723 3.0 315 0.81
Vanlube 622 3.0 500 0.46
TABLE 23.7
Organo-Molybdenum Compounds in Aluminum Complex Grease
Additive Wt%
Four-Ball EP Weld
Load (kg)
Four-Ball Wear Scar
Diameter (mm)
Base grease (880 SUS)
paraf nic base oil blend)
— 126 0.68
Molyvan L 3.0 200 0.48
Molyvan 822 3.0 250 0.60
Molyvan 855 3.0 250 0.58
Molyvan A 3.0 250 0.76
TABLE 23.8
Thiadiazole Compound in Grease
Additive Wt%
Four-Ball EP
Weld Load (kg)
Four-Ball Wear Scar
Diameter (mm)
Aluminum complex base grease (900 SUS
paraf nic base oil blend)
— 140 0.59
Lithium complex base grease (600 SUS paraf nic
base oil)
— 180 0.61
Vanlube 829 in aluminum complex 3.0 620 0.66
Vanlube 829 in lithium complex 3.0 800 kg pass 0.50
CRC_59645_Ch023.indd 601CRC_59645_Ch023.indd 601 10/31/2008 3:18:50 PM10/31/2008 3:18:50 PM
602 Lubricant Additives: Chemistry and Applications
23.12.1 FOOD-GRADE GREASES
Food-grade greases are a special class of grease, which require nontoxic components approved for
this particular application. Additional discussion and details can be found in Chapter 22.
The data in Table 23.12 demonstrate the better balance of EP and wear protection by the addi-
tion of calcium carbonate when compared to Desilube 88 alone. Rust protection is demonstrated by
the synergistic effect of the two corrosion inhibitors using ASTM D1743. The individual additives
are not effective in providing suf cient EP and wear protection in this formulation.
23.12.2 SYNERGISTIC ADDITIVE EFFECTS
Frequently, combinations of two or more additives show enhanced performance over that of the
individual components. The occurrence and magnitude of synergistic behavior involving speci c
compounds very likely depend on the nature of the base grease, including the base oil and the
thickener.
The use of Vanlube 829 in combination with Irgalube 63 showed improvement in the four-ball
wear scar compared with Vanlube 829 in combination with Irgalube TPPT (Table 23.13). These
combinations were each better in EP performance than formulations containing only Irgalube
63 or Irgalube TIPPT in the absence of Vanlube 829. Desilube 88 and MoS
2
showed signi cant
improvement in EP and wear performance for aluminum complex grease when compared with
lithium complex grease (Table 23.14).
TABLE 23.11
Sulfur–Phosphorus Compounds in Grease
Additive Wt%
Four-Ball EP
Weld Load (kg)
Four-Ball Wear Scar
Diameter (mm)
Aluminum complex base grease
(900 SUS paraf nic base oil blend)
— 140 0.59
Lithium complex base grease
(600 SUS paraf nic base oil blend)
— 180 0.61
Desilube 88 in aluminum complex 3.0 500 0.76
Desilube 88 in lithium complex 3.0 620 0.88
Irgalube 63 in aluminum complex 3.0 250 0.60
Irgalube 63 in lithium complex 3.0 315 0.55
TABLE 23.10
Antimony DTC and Antimony DTC/Sulfurized Olefi n in
Aluminum Complex
Additive Wt%
Four-Ball EP
Weld Load (kg)
Four-Ball Wear Scar
Diameter (mm)
Aluminum complex base
grease (880 SUS paraf nic
base oil blend)
— 126 0.68
Vanlube 73 3.0 315 0.84
Vanlube 8610 3.0 400 0.74
CRC_59645_Ch023.indd 602CRC_59645_Ch023.indd 602 10/31/2008 3:18:50 PM10/31/2008 3:18:50 PM

Additives for Grease Applications 603
TABLE 23.12
Food-Grade Aluminum Complex Grease
Additive Wt%
Four-Ball EP
Weld Load (kg)
Four-Ball Wear Scar
Diameter (mm)
Rust Test ASTM
D1743
Aluminum complex base
grease (500 SUS technical
white mineral oil)
— 140 0.62 Fail
Calcium carbonate 4.0 315 0.49 —
Calcium carbonate 4.0 315 0.55 Pass
Amine O 0.5
Sarkosyl O 0.5
Amine O 0.5 160 0.80 Pass
Desilube 88 3.0 500 1.04 —
TABLE 23.13
Synergistic Compounds in Aluminum Complex Grease
Additive Wt%
Four-Ball EP Weld
Load (kg)
Four-Ball Wear Scar
Diameter (mm)
Aluminum complex base grease
(880 SUS paraf nic base oil blend)
— 126 0.68
Vanlube 829 1.0 400 0.88
Irgalube TPPT 3.0
Vanlube 829 1.0 400 0.60
Irgalube 63 3.0
Irgalube 63 3.0 250 0.50
Irgalube TPPT 3.0 200 0.55
TABLE 23.14
Addition of MoS
2
in Grease
Additive Wt%
Four-Ball EP Weld
Load (kg)
Four-Ball Wear Scar
Diameter (mm)
MoS
2
in lithium complex
(600 SUS paraf nic base oil)
3.0 500 0.72
MoS
2
in aluminum complex
(900 SUS paraf nic base oil blend)
3.0 400 0.90
MoS
2
1.3 500 1.0
Desilube 88 in lithium complex 3.0
MoS
2
1.3 800 0.80
Desilube 88 in aluminum complex 3.0
CRC_59645_Ch023.indd 603CRC_59645_Ch023.indd 603 10/31/2008 3:18:50 PM10/31/2008 3:18:50 PM
