Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.


74 Lubricant Additives: Chemistry and Applications
Little mention is made in the literature of the use of aryl acid phosphites, and there is no known
oil industry using ethoxylated neutral or acid phosphites. Phosphites are, however, generally unsuit-
able for applications where water contamination is likely in view of their hydrolytic instability, and
ethoxylation, certainly in respect of water-soluble products, would not offer any obvious advantage.
3.3.6 DIALKYL ALKYL PHOSPHONATES
Although these products are isomeric with the dialkyl phosphites (Figure 3.2), they are a distinct
class of materials with different properties. They are claimed as friction modi ers as well as AW/EP
additives and are prepared by the Arbusov rearrangement in which a trialkyl phosphite is heated
with an alkyl halide, for example, an iodide (reaction 3.19):
P(OR) R I R PO(OR) RI
32
dialkyl alkyl phosphonate
⫹⫹
′
→
′
(3.19)
Commercially available materials range from the dimethyl methyl derivative to products based on
dodecyl phosphite, although the higher-molecular-weight products are likely to be of greatest inter-
est for oil applications. Polyethyleneoxy phosphonates, produced by the reaction of diphosphites
with epoxides, have been claimed as friction modi ers [61], whereas diaryl hydrogen phosphonates,
such as diphenyl phosphonate, are produced by hydrolysis of the corresponding phosphite with
water.
3.4 THE FUNCTION OF LUBRICITY ADDITIVES
The earliest additives used for improving lubrication performance were known as oiliness additives
and lm strength additives. While these descriptions are no longer used, others are now employed.
The current terminology together with typical examples of the chemistries employed is shown in
Table 3.2.
TABLE 3.2
Different types of additives used to improve lubrication performance
Additive Description Performance Mechanism Typical Chemistries
Friction modi er Reduces friction under
near-boundary
lubrication conditions
Physical adsorption of polar
materials on metal surfaces
Long-chain fatty acids and
esters, sulfurized fatty acids,
molybdenum compounds,
long-chain phosphites, and
phosphonates
Antiwear additive (usually
with mild EP properties)
Reduces wear at low to
medium loads
Reacts chemically with the
metal surface to from a layer
(normally a metal soap) that
reduces frictional wear at
low-medium temperature
and loads
Neutral organic phosphates
and phosphites, zinc
di-alkyldithiophosphates
Extreme-pressure additive,
also known as:
-fi lm strength additive,
-load-carrying additive
-antiscuffi ng additive
Increases the load at
which scuffi ng,
scoring, or seizure
occurs
Reacts chemically with the
metal surface to form a
layer, e.g. as a metal halide
or sulfi de which reduces
frictional wear at high
temperatures/loads
Sulfurized or chlorinated
hydrocarbons, acidic
phosphorus-containing
materials, and mixtures
thereof; some metal soaps,
e.g. of lead, antimony, and
molybdenum
CRC_59645_Ch003.indd 74CRC_59645_Ch003.indd 74 3/19/2009 6:26:52 PM3/19/2009 6:26:52 PM
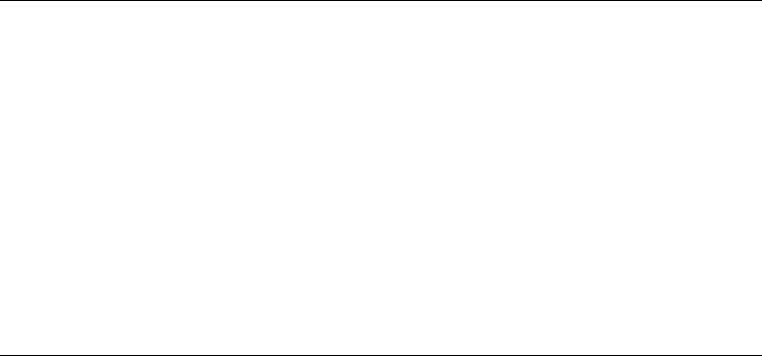
Ashless Phosphorus-Containing Lubricating Oil Additives 75
Table 3.3 [62] offers a generalized classi cation of the different chemical types of additives used
to improve lubrication performance, but, depending on the structure of the additive, some variation
in the performance can be expected. In reality, the distinction between AW and EP additives is not
clear-cut. AW additives may have mild EP properties, whereas EP additives can have moderate
AW performance, and both produce coatings on the metal surface. In fact, EP additives have been
described as additives that reduce or prevent severe wear [63]. However, as seen from the Table 3.3,
EP additives are unlikely to function satisfactorily as friction modi ers, and vice versa.
3.4.1 THE BASIC MECHANISM OF LUBRICATION AND WEAR AND THE INFLUENCE OF ADDITIVES
An understanding of the basic mechanism of lubrication is useful to appreciate the way in which
additives behave and their relative performance. The following is therefore a somewhat simpli ed
explanation of a complex process.
Lubrication can be described as the ability of oil (or another liquid) to minimize the wear and
scuf ng of surfaces in relative motion. It is a function of the properties of the lubricant (e.g., vis-
cosity), the applied load, the relative movement of the surfaces (e.g., sliding speeds), temperature,
surface roughness, and the nature of the surface lm (hardness and reactivity, etc.).
All surfaces are rough. Even those that appear smooth to the naked eye, when examined micro-
scopically, consist of a series of peaks and troughs. The simplest situation arises when the lubricat-
ing lm is thick enough to completely separate the two surfaces so that metal-to-metal contact does
not occur (Figure 3.5). Such a situation could arise at low loads or with highly viscous liquids, and
the lubricating characteristics depend on the properties of the lubricant as the load is fully supported
by the lubricant. This condition is known as hydrodynamic or full- lm lubrication.
As the load increases, the lubricating lm becomes thinner and eventually reaches a condition
where the thickness is similar to the combined height of the asperities on the mating surfaces. At this
stage, metal contact commences, and as the asperities collide, they are thought to weld momentarily
(causing friction) before shearing with loss of metal (wear) (Figure 3.5). The wear particles then
abrade the surface and adversely affect friction, with the resulting damage depending on the hard-
ness of the particle and the surface it contacts. This condition is known as mixed- lm lubrication as
it is a mixture of full- lm lubrication and boundary lubrication with the trend toward the latter with
increasing load.
As the lm thins still further, the load is increasingly supported by the metal surface and fric-
tion rises rapidly. When eventually a lm that is only a few molecules thick separates the surfaces,
TABLE 3.3
A General Classifi cation of Chemicals as Friction Modifi ers, AW, and EP Additives
Additive Friction Modifi er AW Additive EP Additive
Natural oils and fats 1 4 5
Long-chain fatty acids, amines, and alcohols 1 4 5
Organo-molybdenum compounds 1 2 4
Synthetic esters 2 3 4
Organo-sulfur compounds 2 2 3
ZDDP 3 1 3
Phosphorus compounds 3 1 3
Sulfur compounds 4 3 1
Chlorine compounds 5 4 1
Note: The lower the number, the better the rating.
CRC_59645_Ch003.indd 75CRC_59645_Ch003.indd 75 3/19/2009 6:26:53 PM3/19/2009 6:26:53 PM
76 Lubricant Additives: Chemistry and Applications
the roughness, composition, and melting point of the surfaces strongly in uence the resulting fric-
tion. At this stage, viscosity plays little or no part in the frictional behavior. This stage is known
as boundary lubrication and is characterized by high frictional values that now change little with
further increases in load or sliding speed. The wear process that takes place under boundary condi-
tions is perhaps the most complicated of those involved in lubrication in that it involves four differ-
ent types of wear: corrosive, fatigue, ploughing, and adhesive.
Corrosive wear occurs when the metal surfaces react with their environment to form a bound-
ary lm, whereas fatigue wear is the process of the fracture of asperities from repeated high stress.
Micropitting is an example of this form of wear, which is the subject of considerable investigation
today. Micropitting is the result of plastic deformation of the surface that eventually causes the frac-
ture of the asperity, leaving a small pit in the surface. Ploughing wear arises when a sharp particle
is forced along the surface, leaving a groove behind, whereas adhesive wear is the tendency of very
clean surfaces to adhere to each other. However, this action requires the generation of fresh surfaces
during the wear process, perhaps by plastic deformation. It is now thought that this mechanism is
much less prevalent than was earlier believed [64].
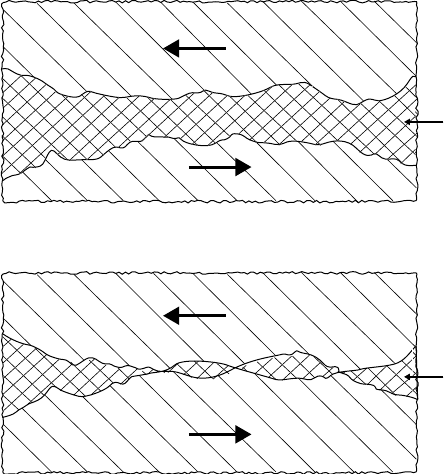
The relationship between friction, viscosity, load, and sliding speeds can be represented graphically
for a bearing by what is known as a Stribeck curve. This is shown in Figure 3.6 [65], where the frictional
coef cient is plotted against the dimensionless expression ZN/P, where Z represents the uid viscosity,
N the sliding speed, and P the load. Friction is reduced as the value of ZN/P is lowered until a minimum
is reached. For a bearing, this minimum value is ∼0.002 for an ideal hydrodynamic condition. At this
point, metal contact begins, and friction rises and continues to do so with increasing contact. In the
mixed friction zone, the friction value lies in the region of 0.02–0.10. Eventually, when the lm is very
thin, friction becomes independent of viscosity, speed, and load and can reach a value of 0.25.
By experiment it was established that
• Continually increasing the load reduced the ZN/P value, assuming that speed and the
viscosity remained constant. The same results can be obtained by reducing either the speed
or viscosity, or both, provided the unit load remains constant or is increased.
Metal
Metal
Metal
Metal
Oil
Oil
Full-film (hydrodynamic)
lubrication
Mixed-film lubrication
FIGURE 3.5 A diagrammatic representation of full- lm (hydrodynamic) and mixed- lm lubrication.
CRC_59645_Ch003.indd 76CRC_59645_Ch003.indd 76 3/19/2009 6:26:53 PM3/19/2009 6:26:53 PM
Ashless Phosphorus-Containing Lubricating Oil Additives 77
• Friction varied directly with viscosity; it was proportional to velocity at lower speeds but
varied inversely with velocity at higher speeds [65].
As the surfaces move closer together, the lubricant is squeezed out from between them. Some addi-
tives, when adsorbed onto the surface, display a molecular orientation perpendicular to the surface
that reduces the level of contact and hence lowers the friction. Such products are known as friction
modi ers. Those additives effective in reducing wear and (usually) friction in the mixed friction
zone are called antiwear additives, whereas products effective in reducing wear (and increasing
seizure loads) in the boundary lubrication process are known as extreme-pressure additives. How-
ever, due to the importance of temperature in the lubrication process, it has been pointed out in the
past that the latter should, perhaps, be better described as extreme-temperature additives.
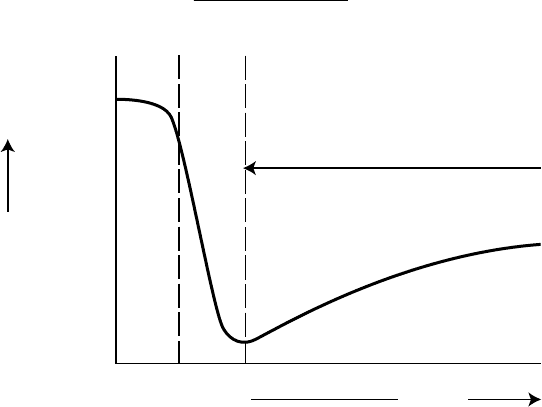
The temperature at which an additive reacts physically or chemically with the metal or metal
oxide surface signi cantly affects its activity. Each AW/EP additive type has a range of temperature
over which it is active (Figure 3.7) [66]. The lowest temperature in the range would normally be
the temperature at which physical adsorption takes place. This can occur at ambient or at higher
temperatures depending on the polarity of the additive and the impact on surface energy. The
greater the reduction in surface energy, the stronger will be the absorption of the surface lm and
the greater will be the likelihood that the additive remains in place for a chemical reaction with the
surface. Additives that are only weakly bound to the surface may desorb as the temperature rises
and cease to function further in the wear-reducing process.
As the temperature increases so does the surface reactivity. Fatty acids and esters react at fairly
low temperatures to produce metal soaps followed by chlorine-containing compounds (to form
chlorides), phosphorus (as phosphates, polyphosphates, and/or phosphides), and, nally, sulfur,
which reacts at very high temperatures to form metal sul des [66].
Chlorine-based additives can be lm-forming even at ambient temperatures, but as the tem-
perature rises they become aggressive and, with the release of HCl, can cause signi cant corrosion.
Although the FeCl
2
lm has a fairly well-de ned melting point at 670°C, the optimum operating
temperature is much lower. Klamann [67] indicates that the ef ciency of metal chlorides starts to
Friction
Viscosity × speed
Boundary
friction
0.15−0.25
0.001−0.002
Minimum fluid
friction
Boundary lubrication
Mixed
Hydrodynamic lubrication
(Fluid film)
Regions of lubrication
load
(ZN/P )
FIGURE 3.6 Relationship between coef cient of friction and ZN/P.
CRC_59645_Ch003.indd 77CRC_59645_Ch003.indd 77 3/19/2009 6:26:53 PM3/19/2009 6:26:53 PM
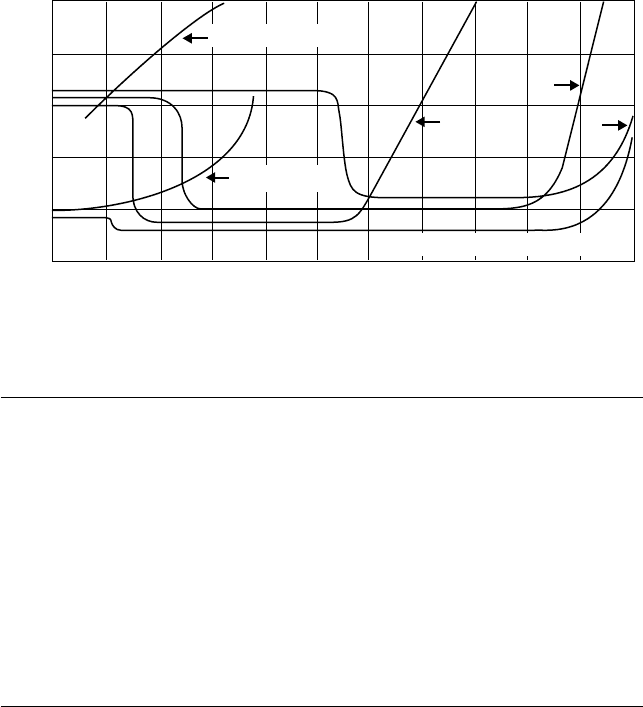
78 Lubricant Additives: Chemistry and Applications
drop above 300°C and that the friction coef cient at 400°C is already a multiple of the optimum
value. However, the dry friction coef cient of the chloride lm is substantially lower than that for
iron sul de (Table 3.4) [68]. The relatively low friction associated with this lm is probably one
reason why chlorinated products are so effective as EP additives. Phosphorus, by comparison, does
not react until at higher temperatures and then at slower rates. However, the upper temperature limit
of ~550°C in an air environment is thought to be a result of the oxidation of the carbon in the lm
rather than the degradation of a metal soap (Forster, N.H., Private Communication, July 2007).
The soaps, phosphates/phosphides, chlorides, and sul des formed on the metal surface
were originally considered to produce a lower melting and less-shear stable lm than that of the
metal/metal oxide. This lm would cause a smoothing of the metal surface that was then able to
support a higher unit loading. This is now thought to be an oversimpli ed explanation as research
has found the EP lms to be considerably different to those postulated and without the expected
lower shear stability [69]. What it certainly does not consider are additional “subprocesses” of
removal of the lm by mechanical wear and its possible regeneration in situ by further action of the
AW/EP additive (Figure 3.8).
Since surface temperature is largely dependent on load, additives that might be effective at
high loads may be completely ineffective at low loads (and vice versa). Under such circumstances,
therefore, signi cant wear could occur before the load-carrying properties of the EP additive come
into play. To minimize this effect, additives are often used in combination, resulting in extending
the temperature (and load) range over which they are active.
Mineral base oil
Fatty acids
Cl
S
Cl + P + S + fatty acids
P
0 100 200 300 400 500 600 700 800 90 1000 1100
Temperature (°C)
Friction coefficient
0
0.1
0.2
0.3
0.4
0.5
FIGURE 3.7 Effect of temperature on EP additive activity. (From Mandakovic, R., J. Syn. Lubr., 16(1),
13–26, 1999. With permission.)
TABLE 3.4
Corrosion Films Formed on Sliding Iron Surfaces
Lubricant Type Nature
Friction Coeffi cient
(Dry)
Melting Point
(°C)
Dry or hydrocarbon Fe 1.0 1535
FeO 0.3 1420
Fe
3
O
4
0.5 1538
Fe
2
O
3
0.6 1565
Chlorine FeCl
2
0.1 670
Sulfur FeS 0.5 1193
Source: Fundamentals of Wear, Lubrication, 12(6), 61–72, 1957. Permission from Chevron.
CRC_59645_Ch003.indd 78CRC_59645_Ch003.indd 78 3/19/2009 6:26:53 PM3/19/2009 6:26:53 PM
Ashless Phosphorus-Containing Lubricating Oil Additives 79
Although single AW/EP additives can be used to meet application and speci cation requirements,
combinations of additives can produce both synergistic and antagonistic effects. The use of mixtures
of phosphorus- and chlorine- or sulfur-containing compounds, to extend the temperature range over
which a lubricating lm is available, has already been mentioned. Another example of synergism was
reported by Beeck et al. [5], who described the effect of combinations of TCP and long-chain fatty
acids. It was suggested that the use of such mixtures in some way improved the packing of the lm on
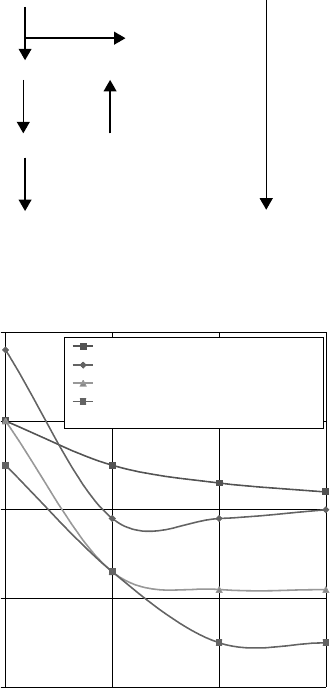
the surface and therefore helped to reduce metal contact. Figure 3.9 [68] in fact shows that combina-
tions of phosphate and fatty acid can result in lower wear rates than either component. Such synergy
is useful in that it reduces additive costs and the possibility that the additives might have an adverse
effect on product stability, etc. An example of additive antagonism is given in Section 3.12.
3.5 INVESTIGATIONS INTO THE MECHANISM AND ACTIVITY
OF PHOSPHORUS-CONTAINING ADDITIVES
Many papers have been written about the way in which TCP and other phosphorus-containing
compounds work as AW/EP additives. As might be expected, researchers have had differences
Physical adsorption
Desorption
Regeneration
of film
Increase in
temperature
Chemical reaction
Mechanical wear
Destruction of film
FIGURE 3.8 Basic processes involved in the mechanism of action of lubricity additives.
−11
−10
−9
−8
−7
10 100 1000 10000
Additive (none)
0.4% Organic phosphate
0.4% Fatty acid
0.4% Organic phosphate and
0.4% fatty acid
FIGURE 3.9 Effect of fatty acid and phosphate ester on wear rate. (Fundamentals of Wear, Lubrication,
12(6), 61–72, 1957. Permission from Chevron.)
CRC_59645_Ch003.indd 79CRC_59645_Ch003.indd 79 3/19/2009 6:26:54 PM3/19/2009 6:26:54 PM
80 Lubricant Additives: Chemistry and Applications
of opinion. These have probably arisen as a result of the different test conditions found in the
wide variety of test equipment developed for measuring wear. For example, different test specimen
geometries, surface nish, sliding speeds, and the use of additives with different levels of purity
have meant that the data have not been strictly comparable.
After a brief review of the early development of AW/EP additives, a number of papers exploring
the mechanism of action of different phosphorus-based additives are summarized in this section.
It is not inclusive, and the results of many other workers could have been mentioned. An additional
selection of papers on the topic is therefore given in Appendix B. Some papers evaluate several
classes of product (e.g., phosphates, phosphites, phosphonates, etc.); these may be located in sec-
tions other than that on neutral phosphates if information on these other structures is limited.
3.5.1 EARLY INVESTIGATIONS INTO ANTIWEAR AND EXTREME ADDITIVES
Some of the earliest experiments into the effects of different lubricants on friction were carried out
by Hardy in 1919 [70], who noted the superior performance of castor oil and oleic acid. He found
that good lubricating properties were closely related to the ability of substances to lower surface
energy. A series of papers from Hardy and Doubleday followed in 1922–1923 examining the activ-
ity of lubricants under boundary conditions.
In 1920, Wells and Southcombe [71] discovered that the addition of a small amount of a long-chain
fatty acid signi cantly reduced the static coef cient of friction of mineral oil. Bragg postulated in
1925 [72] that long-chain molecules with a polar terminal group were attached to the surface by
adsorption of the polar group and that the long hydrocarbon chains were orientated perpendicular
to the surface. He also suggested that the formation of lms on both the moving surfaces assisted
lubrication by sliding over one another, with their long chains being “ attened” as the distance
between the surfaces was reduced. However, in 1936, Clark and Sterrett [73] showed that the lubri-
cating lm could be up to 200 molecules in thickness but that only the rst layer would have the
strength to withstand the shearing stresses produced under sliding conditions. They also found
that certain ring structures (e.g., trichlorophenol) that were active as “ lm strength” additives also
showed molecular orientation, in this case, parallel to the metal surface, and attributed the good
load-carrying performance to the ability of the layers to slide over one another. Orientation was not
the only factor involved, as compounds with a similar orientation could show a wide difference in
performance.
The mechanism and in uence of additives on boundary lubrication were rst investigated and
reported by Beeck et al. [4]. They found that friction was reasonably constant with sliding velocity
up to a critical velocity, beyond which there was a signi cant reduction. Additives were found to
reduce the friction at low speeds relative to the base oil alone and also had a signi cant, but variable,
effect on the reduction at different critical velocities. Low critical velocities were found for com-
pounds that were strongly adsorbed and that showed orientation of the surface lm. It was recog-
nized that the adsorbed layer is thinner than the roughness of even the best machined surfaces and
that high temperatures (or loads) at points of contact would cause decomposition of the molecules
with the formation of a high-melting corrosion product and an increase in friction. If the surfaces
were highly polished, then sliding could take place without destruction of the surface lm. It was
concluded that most of the friction-reducing compounds, principally, the long-chain fatty acids,
were not able to produce a highly polished surface and therefore were not effective AW additives.
3.5.2 NEUTRAL ALKYL AND ARYL PHOSPHATES
3.5.2.1 Historical Background
One additive examined by Beeck et al. [5] that was able to reduce both friction and wear was TCP,
a product that was, at that time, beginning to nd widespread commercial use as an AW additive.
The authors proposed that TCP acted by a corrosive action, preferentially reacting with the high
CRC_59645_Ch003.indd 80CRC_59645_Ch003.indd 80 3/19/2009 6:26:54 PM3/19/2009 6:26:54 PM
Ashless Phosphorus-Containing Lubricating Oil Additives 81
spot on the surface, where the surface temperatures are highest (from metal contact). It was thought
that in the reaction, the phosphate ester formed a lower melting phosphide (or possibly an iron/iron
phosphide eutectic) that owed over the surface and caused a smoothing or chemical polishing
effect. They also observed that there appeared to be an optimum level of addition of the TCP (1.5%),
a conclusion later con rmed by other workers in the eld.
Beeck et al. claimed in these papers that their research had produced a better understanding of
the AW mechanism and enabled more precise distinctions to be drawn between the different types
of additives; more speci cally, that
A wear prevention agent reduces pressure and temperature through better distribution of the load over
the apparent surface. If the resulting minimum pressure is still too high for the maintenance of a stable
lm, metal to metal contact will take place in spite of the high polish. Since in this case the surface of
actual contact is relatively very large, seizure and breakdown will follow very rapidly …
The intervention of the war years encouraged German researchers to prepare and evaluate a num-
ber of phosphorus compounds as EP/AW additives, principally phosphinic acid derivatives and
also acid phosphates [6,7], while other workers [74] continued to investigate the behavior of TCP.
The performance of the latter in white oil was examined, and it was suggested that the additive
reacted with steel to form a thin, solid, nonconducting lm that prevented seizure by shearing in
preference to metal-to-metal contacts. The improved behavior of blends of TCP with fatty acids
was explained as being due to the improved adsorption of the fatty acid onto the surface of the
chemically formed lm.
In 1950, an extensive evaluation of different neutral alkyl and aryl phosphates and phosphites,
in some cases containing chlorine and sulfur, was undertaken [75]. The results of this investigation
showed that the action of sulfur and chlorine on the surface is to form a sul de and a chloride lm,
respectively. In the presence of phosphorus, mixed lms of phosphide/sul de or phosphide/chloride
were formed. The presence of phosphide was established chemically by the liberation of phosphine
in the presence of hydrochloric acid.
Although the concept of phosphide lm formation was challenged at this time [76,77], it
remained as the generally held theory until the mid-1960s when several papers appeared with con-
tradictory data. Godfrey [78] pointed out that the experiments that had indicated the presence of
phosphide had all been static, high-temperature investigations, and none had identi ed phosphide
on a sliding surface lubricated with TCP. He experimented with the lubrication of steel-on-steel sur-
faces by TCP followed by an examination of the metal surface. This revealed the presence of white
crystalline material, which was shown by electron diffraction measurements to be predominantly a
mixture of ferric phosphate, FePO
4
, and its dihydrate, FePO
4
.2H
2
O. Phosphides, if present, were in
extremely small quantities. Furthermore, a paste made from the dihydrate showed similar frictional
characteristics to TCP, whereas a paste from iron phosphide showed no signi cant reduction in fric-
tion. Tests also suggested the importance of air to the performance of TCP as tests carried out under
nitrogen revealed substantially increased wear. Pure TCP was evaluated and, unlike commercial
material, showed no signi cant friction-reducing properties.
The presence and role of impurities in the activity of commercial TCP was the subject of inves-
tigations using radioactive P
32
[79]. Results suggested that the phosphorus-containing polar impu-
rities—not the neutral TCP—were adsorbed onto the metal surface. The P
32
found in the wear
scar appeared to the chemically bound—not physically adsorbed, but the latter process seemed
to be the way that the phosphorus was initially made available on the surface. The authors indi-
cated that the impurities resembled acid phosphates (rather than phosphoric acid, which Godfrey
had assumed) and carried out wear tests comparing the neutral ester with both an acid phos-
phate (dilauryl acid phosphate) and hydrolyzed TCP. They found that lower concentrations of these
compounds generally gave equivalent performance to the neutral ester. Of interest was the obser-
vation that, although TCP showed no wear minimum in the reported tests (cf. the results given by
CRC_59645_Ch003.indd 81CRC_59645_Ch003.indd 81 3/19/2009 6:26:54 PM3/19/2009 6:26:54 PM

82 Lubricant Additives: Chemistry and Applications
Beeck et al. [5]), the data on acid phosphate, acid phosphite, and phosphoric acid did display such
minima.
The work using radioactive P
32
also allowed a study of the competition between TCP and dif-
ferent types of additives for the metal surface. This was determined by measuring the residual
surface radioactivity after wear tests. Table 3.5 shows the effect of various types of additives on the
adsorption of P
32
from TCP. The lower the number of counts, the greater the interaction between
the additive and TCP.
Radiochemical analysis was also the technique used to investigate the deposition of phosphorus
on steel surfaces in engine tests [80]. In this study, the effect of different types of aryl phosphates
(TPP, TCP, and TXP) on case-hardened tappets was examined. The results suggested that the ef -
cacy of these additives is correlated directly with their hydrolytic stability, that is, their ability to
produce acid phosphates as degradation products. This was con rmed by tests on a series of other
phosphates (largely alkyldiaryl phosphates), which showed good correlation between antiscuf ng
performance and hydrolytic stability (Table 3.6). Examination of the tappet surface revealed the
presence of aryl acid phosphates on the surface and the absence of phosphides. Adsorption studies
of the neutral aryl and acid phosphates on steel surfaces indicated that, although the lm of neutral
ester could be more easily removed, the adsorption of the acid phosphate was irreversible, suggest-
ing salt formation. These studies led the authors to conclude that the mechanism involved initial
adsorption of the phosphate on the metal surface followed by hydrolytic decomposition to give an
acid phosphate. This reacted with the surface to give iron organophosphates, which then decom-
posed further to give iron phosphates.
The importance of impurities in determining the level of activity of TCP was con rmed in yet
another paper [81]. The composition of impurities in commercial grades of TCP was determined
using thin-layer chromatography and analysis by neutron activation. Acidic impurities, probably the
monocresyl and dicresyl acid phosphate (and also small amounts [2 × 10
−4
%] of phosphoric acid),
were found at 0.1–0.2%, that is, at levels that had previously been shown to produce a signi cant
reduction in wear when added to mineral oil. Other impurities ranged from 0.2 to 0.8%. This latter
category was assumed to contain chlorophosphates based on the amount of chloride ion produced.
TABLE 3.5
Effects of Various Additives on the Adsorption of P
32
Additive Concentration (wt%) Activity (Counts/min)
0.5% TCP alone 280
+2% Barium sulfonate A 0
+2% Barium sulfonate B 80
+0.1% Rust inhibitor 16
+0.5% Diisopropyl acid phosphite 25
+0.1% Dilauryl acid phosphate 24
+5.5% Acryloid dispersant 82
+7.9% Polymeric thickener 78
+0.7% Sulfur–chlorine EP additive 120
+0.5% Thiophosphate 150
+0.5% 2,2′-Methylene-bis(2-methyl,
4-tertiarybutyl phenol)
250
+0.5% Sulfurized terpene 290
Source: Klaus, E.E., Bieber, H.E., ASLE Preprint 64-LC-2, 1964.
With permission.
CRC_59645_Ch003.indd 82CRC_59645_Ch003.indd 82 3/19/2009 6:26:54 PM3/19/2009 6:26:54 PM

Ashless Phosphorus-Containing Lubricating Oil Additives 83
The authors commented that the TCP used for the investigation was the best grade available, but
even this material contained up to 25% polar impurities. It was thought typical of the TCP used in
the wear studies to date and reported in the literature.
Wear tests on TCP, acid phosphates, and phosphites in a super-re ned mineral oil and a syn-
thetic ester (di-3-methylbutyl adipate) indicated that relatively small amounts (0.01%) of additive
can produce a signi cant wear reduction in mineral oils and that the acidic materials were more
active. However, in the polar base stock, where there is competition for the surface, the amount of
TCP required to provide a similar reduction in wear is substantially greater. The effectiveness of
the alkyl acid phosphates is not signi cantly reduced in the synthetic ester, suggesting that their
polarity (and hence adsorption) is greater than that of the neutral phosphate, the synthetic ester,
and its impurities (Table 3.7). The authors concluded that the activity of TCP was due to the acidic
impurities and that the neutral ester acted as a reservoir for the formation of these impurities during
the life of the lubricant.
Until about 1969, the theory regarding the production of a phosphate lm on the steel surface
seemed to be widely accepted. Reports then appeared suggested that the situation was more com-
plicated. One paper [82] examined and compared the corrosivity toward steel, the load- carrying
capacity, and the AW performance of several phosphorus compounds. Using the hot-wire technique
at 500°C [83] followed by an x-ray analysis of the surface lms that were produced, the reactivity (or
corrosivity) was studied. Perhaps, not surprisingly, the neutral phosphate and phosphite evaluated
showed relatively little reactivity with the steel, whereas the acid phosphate and phosphite produced
substantially more corrosion. The anomaly was the behavior of a neutral alkyl trithiophosphite,
which showed a very high reactivity but low load-carrying ability, suggesting a different mode of
breakdown. Analysis of the lms formed con rmed the major presence of basic iron phosphate (or
principally iron sul de in the case of the thiophosphite), but small amounts of iron phosphide were
TABLE 3.6
Correlation between the Antiscuffi ng Performance and Ease of Hydrolysis
(Acid Formation) of Organic Phosphates
Additive (0.08% wt Added P)
Relative Ease
of Hydrolysis
a
Time to Scuffi ng (min)
b
at a
Spring Load
305 lb 340 lb
Benzyldiphenyl phosphate 100 >30 9
Allyldiphenyl phosphate 100 >30 Not tested
Ethyldiphenyl phosphate 80 28 Not tested
Octyldiphenyl phosphate 50 15 6
Triphenyl phosphate 50 15 5
Tritolyl phosphate 30 8 Not tested
2-Ethylhexyldiphenyl phosphate 5 2–3 Not tested
None — 2–3 Not tested
Note: Camshaft, Ford Consul (cams phosphated); Tappet, Ford Consul (non-phosphated); Camshaft
speed, 1500 rpm (equivalent engine speed 3000 rpm); Base oil, SAE 10W/30 oil without EP
additive.
a
Because of the wide range of hydrolytic stability of these compounds, it was not possible to compare
the stabilities of all these compounds in the same acid medium. Consequently, an arbitrary scale was
drawn up with benzyldiphenyl phosphate assigned a value of 100.
b
Mean of several tests.
Source: Barcroft, F.T., Daniel, S.G., ASME J. Basic Eng., 64-Lub-22, 1964. With permission.
CRC_59645_Ch003.indd 83CRC_59645_Ch003.indd 83 3/19/2009 6:26:55 PM3/19/2009 6:26:55 PM
