Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.

54 Lubricant Additives: Chemistry and Applications
and alkyl mercaptan. A white precipitate will also form, which has been determined to be a low-
sulfur- containing zinc pyrophosphate. The oil phase will contain varying amounts of S,S,S-tri -
alkyltetrathiophosphate, O,S,S-trialkyltrithiophosphate, and O,O,S-trialkyldithiophosphate depend-
ing on the alkyl chain and the extent of degradation. The decomposition products of ZDDPs made
from secondary alkyl alcohols, straight-chain primary alkyl alcohols, and branched primary alkyl
alcohols appear similar in content but differ in proportions. This implies a similar mechanism for
both primary and secondary ZDDP decomposition.
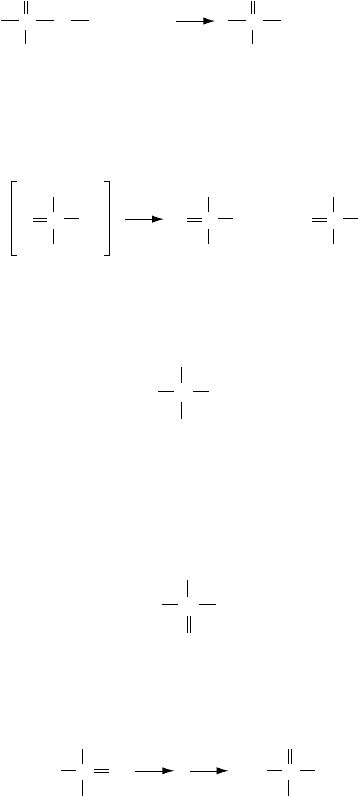
O-alkyl thiphosphate esters are powerful alkylating agents. The P–O–R group is susceptible
to nucleophilic attack, thus producing an alkylated nucleophile and thiophosphate anion. The
incoming nucleophile initiates the reaction by an attack on the alpha carbon. This shows a kinetic
dependence on alkyl structure
Nu
−
ROP
S
+ O
−
P
S
Nu−R+
(2.9)
Steric hindrance to the approach of the nucleophile will play a large rate-controlling factor here.
The only nucleophile initially present is the dithiophosphate itself. The decomposition is initiated
by one dithiophosphate anion attacking another, possibly on the same zinc atom:
P
S
S
−
2
OR
OR
P
S
S
−
O
−
OR
P
S
S
R
OR
OR
+
(2.10)
The resulting di-anion then attacks the triester, producing O,S-dialkyldithiophosphate anion
P
SSR
O
−
OR
(2.11)
resulting in the migration of an alkyl group from oxygen to sulfur. This anion then, in a route analo-
gous to the dialkyldithiophosphate anion, reacts with itself in a nucleophilic attack to effect another
alkyl transfer from oxygen to sulfur producing O,S,S-trialkyldithiophosphate
P
RS SR
OR
O
(2.12)
The net effect of the above reactions is a double alkyl migration from oxygen to sulfur
P
−
S
S
OR
OR
P
RS
SR
O
O
−
(2.13)
The major gases associated with ZDDP decomposition are dialkylsul de (RSR), alkyl mercaptan
(RSH), and ole n. The relative amounts of each of these gases depend on whether the alkyl group in
the ZDDP is primary, branched primary, or secondary [9]. In the presence of mercaptide anion (RS
–
)
CRC_59645_Ch002.indd 54CRC_59645_Ch002.indd 54 3/20/2009 5:33:55 PM3/20/2009 5:33:55 PM

Zinc Dithiophosphates 55
from the intermediate zinc mercaptide (Zn[RS
2
]), O,S,S-trialkyldithiophosphate will react with mer-
captide to produce alkyl mercaptan and results in the following structure:
P
RS
SR
O
−
O
(2.14)
The nucleophilic phosphoryl oxygen (P=O) will then attack another phosphorus atom to produce a
P–O–P bond as in the following reaction:
SR
−
SRP
++
OP P
O
O
P
−
(2.15)
A mercaptide anion subsequently cleaves the P–O–P bond at the original P–O site, giving rise to a
net exchange of one atom of oxygen for one atom of sulfur between the two phosphorus atoms:
−
SR SRP
++
+
P O
−
OPP
(2.16)
This gives rise to a net reaction for conversion of Structure 2.14 to S,S,S-trialkyltetrathiophosphate,
dialkylsul de and S-alkylthiophosphate di-anion as shown in the following reaction:
P
RS
SR
−
SR
3
O
O
−
P
RS
SR
S
SR
P
RS
O
−
2
O
O
−
++R
2
S
+
(2.17)
The dialkylsul de and S,S,S-trialkyltetrathiophosphate decomposition products are soluble in oil.
The S-alkylthiophosphate decomposition product can also react with itself by way of a phos-
phoryl nucleophilic attack and elimination of mercaptide anion as in Reaction 2.15. This process
will continue until a zinc pyro- and polypyrophosphate molecule with low sulfur content is formed.
The chain will continue to extend until the product precipitates out of solution.
The decomposition of primary alkyl ZDDPs can be accurately described as discussed earlier.
ZDDPs made from branched primary alcohols will decompose in a similar fashion, although at a
much slower rate. This can be explained by the fact that the alpha carbon of the branched primary
alkyl group, being more sterically hindered than the unbranched primary alkyl group, will be less
susceptible to nucleophilic attack, as described in Reaction 2.9. The increased steric hindrance from
beta carbon branching will also decrease the amount of successful mercaptide anion attack on the
branched alkyl P–O–R bond, resulting in less dialkylsul de formation and a higher yield of mercap-
tan, an ole n by-product (through a competing protonation or elimination reaction with mercaptide
anion). Lengthening the alkyl chain will have a much less pronounced effect on thermal stability
than branching at the beta carbon due to the greater steric hindrance derived from the latter.
The decomposition of secondary alkyl ZDDPs, although similar to primary decomposition,
shows that ole n formation is much more pronounced. The increase in elimination over nucleo-
philic substitution in secondary ZDDPs over primary ZDDPs is easily explained by the fact that
elimination is accelerated by increasing the alkyl substitution around the double bond formed.
Thus, secondary alkyl groups will favor a thermal decomposition into ole ns and phosphate
acids at the expense of the sulfur–oxygen interchange noted earlier. In a similar but much more
pronounced way, tertiary ZDDP decomposition will be dominated by facile production of ole n
through elimination. This occurs at even moderate temperatures, making their use in commercial
applications prohibitive.
CRC_59645_Ch002.indd 55CRC_59645_Ch002.indd 55 3/20/2009 5:33:55 PM3/20/2009 5:33:55 PM

56 Lubricant Additives: Chemistry and Applications
Aryl ZDDPs, due to the stability of the aromatic ring, are not susceptible to nucleophilic
attack. Thus, the initial thermal decomposition reaction described in Reaction 2.9 cannot occur.
Also the formation of ole n from an acid-catalyzed elimination reaction cannot occur. Aryl ZDDPs
are, therefore, very thermally stable.
A rating of various ZDDPs in terms of thermal stability would, therefore, be aryl > branched
primary alkyl > primary alkyl > secondary > tertiary. The varying amounts of decomposition
products that depend on the heat history and the alkyl or aryl chain involved will directly control the
amount of EP and wear protection the ZDDP will provide in a given circumstance [10].
Hydrolysis of ZDDP begins with cleavage of the carbon–oxygen bond of the thiophosphate
ester, with the hydroxide anion displacing the thiophosphate-anion-leaving group. The stability
of the intermediate alkyl cation determines the ease with which this cleavage takes place. The
secondary alkyl cation is more stable and more easily formed than the primary alkyl cation;
therefore, hydrolysis of secondary ZDDP occurs more easily than hydrolysis of primary ZDDP.
For the case of an aryl ZDDP, the carbon–oxygen bond cannot be broken, and the site of hydrolytic
attack is the phosphorus–oxygen bond with the displacement of phenoxide anion with hydroxide
anion. The order of hydrolytic stability is, therefore, primary > secondary > aryl.
2.5 OXIDATION INHIBITION
Base oils used in lubricants degrade by an autocatalytic reaction known as auto-oxidation. The
initial stages of oxidation are characterized by a slow, metal-catalyzed reaction with oxygen to form
an alkyl-free radical and a hydroperoxy-free radical as seen in the following reaction:
RH O R HOO
M
⫹⫹
2
+
→
**
(2.18)
This reaction is propagated by the reaction of the alkyl-free radical with oxygen to form an alkylp-
eroxy radical. This radical further reacts with the base oil hydrocarbon to form alkyl hydroperoxide
and another alkyl radical as seen in the following reaction:
R O ROO ROOH R
RH
**
⫹⫹
2
→→
*
(2.19)
This initial sequence is followed by chain branching and termination reactions forming high-
molecular-weight oxidation products [11].
The antioxidant functionality of ZDDP is ascribed to its af nity for peroxy radicals and
hydroperoxides in a complex pattern of interaction.
The initial oxidation step of ZDDP by hydroperoxide is the rapid reaction involving the oxidative
formation of the basic ZDDP salt as seen in the following reaction:
2
62
S
R′OHZn
4
OOOHR′Zn
(RO)
2
PS
4
+
++
S
(RO)
2
PS
S
(RO)
2
PS
(2.20)
In this reaction, 1 mol of alkyl hydroperoxide converts 4 mol of neutral ZDDP to 1 mol of basic ZDDP
and 2 mol of the dialkyldithiophosphoryl radical (which subsequently reacts to produce the disul-
de) [12]. The rate of hydroperoxide decomposition slows during an induction period during which
the basic zinc thermally breaks down into the neutral ZDDP and zinc oxide [6]. This is followed
by the neutral ZDDP further reacting with hydroperoxide to produce more dialkyldithiophosphoryl
disul de and more basic ZDDP. When the concentration of the basic ZDDP becomes low enough,
CRC_59645_Ch002.indd 56CRC_59645_Ch002.indd 56 3/20/2009 5:33:56 PM3/20/2009 5:33:56 PM
Zinc Dithiophosphates 57
a nal rapid neutral salt-induced decomposition of the hydroperoxide will occur in which the
dialkyldithiophosphoryl radical will not react with itself to form the disul de but will react with
hydroperoxide to form the dialkyldithiophosphoric acid as seen in the following reaction [13]:
S
R′OOH(RO)
2
PS
•
+
S
R′OO
•
(RO)
2
PSH +
(2.21)
The dialkyldithiophosphoric acid then rapidly reacts with alkyl hydroperoxide, producing oxidation
products that are inactive in oxidation chain reactions. The simplest reaction scheme for the reduction
of the hydroperoxide is seen in the following reaction:
2
S
R′OH H
2
OR′OOH
(RO)PSH
2
+++
S
(RO)
2
PS
(2.22)
Oxidation products include the disul de mentioned earlier, the analogous mono- and trisul des,
and compounds of the form (RO)
n
(RS)
3–n
P=S and (RO)
n
(RS)
3–n
P=O [3]. These products show
little activity as either oxidation inhibitors or antiwear agents.
The literature also reveals an ionic process that will produce more dialkyl-dithiophosphoric
acid as seen in the following reaction:
2
S
R′OO
•
R′OO
−
Zn
(RO)PS
++
+
S
(RO)
2
PS Zn
+
S
(RO)
2
PS
•
(2.23)
followed by Reaction 2.21 [14].
At low concentrations of ZDDP, hydrolysis of the ZDDP to the zinc basic double salt and
dialkyldithiophosphoric acid becomes viable. At temperatures >125°C, the dialkyldithiophos-
phoryl disul de decomposes into the dialkyldithiophosphoryl radicals, which further react with
hydroperoxide to produce more dialkyldithiophosphoric acid [6]. Thus, many pathways are avail-
able to form the active dialkyldithiophosphoric acid.
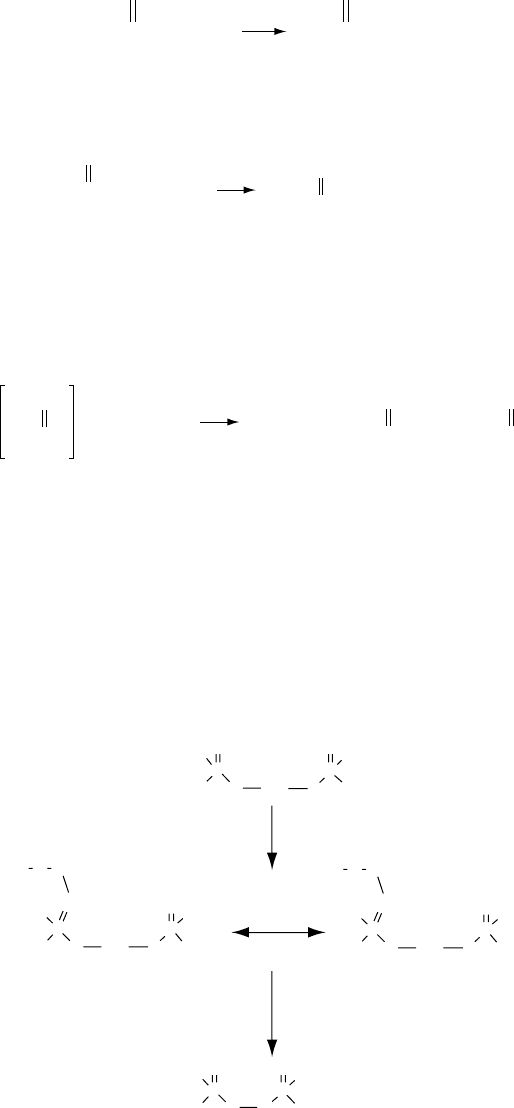
The neutral ZDDP also reacts with alkyl peroxy radicals. This is an electron-transfer mechanism
that involves the stabilization of a peroxy intermediate. An attack by a second peroxy radical leads
to the intramolecular dimerization of the resulting dithiophosphate radical forming the inactive
dialkyldithiophosphoryl disul de as seen in the following reaction:
RO
RO
R
RO
OR
OR
RO
OO
PP
RO
2
•
S
•
SS
S
Zn
OR
OR
Zn
PP
S
SS
S
RO
2
•
R
RO
OR
OR
RO
OO
P
•
P
S
•
SS
S
Zn
•
RO
OR
OR
SS
SS
PP
RO
++
2 RO
2
−
Zn
2+
(2.24)
CRC_59645_Ch002.indd 57CRC_59645_Ch002.indd 57 3/20/2009 5:33:57 PM3/20/2009 5:33:57 PM
58 Lubricant Additives: Chemistry and Applications
The zinc metal atom provides an easy route for heterolysis of the radical intermediate; thus, the
disul de, by itself, has little antioxidant functionality [15]. ZDDP acts as an oxidation inhibitor
not only by trapping the alkyl radicals, thus slowing the chain reaction mechanism, but also by
destroying alkyl hyperoxides and inhibiting the formulation of alkyl radicals. Empirical determi-
nation of the relative antioxidant capability of the three main classes of ZDDP shows secondary
ZDDP > primary > aryl ZDDP. The relative performance of each ZDDP type may correlate with
the stabilization of the dialkyl(aryl)dithiophosphoryl radical and its subsequent reactivity with
alkyl hydroperoxide to produce the catalyzing acid.
Commercial ZDPs are a mixture of both neutral and basic salts. It has recently been
determined that neutral and basic ZDDPs give essentially equivalent performance with respect to
antioxidant behavior. This can be explained by the equilibrium shown in Reaction 2.8. At elevated
temperatures, as would occur in an oxidation test, the basic ZDDP is converted into the neutral
ZDDP. As the temperature is lowered, the equilibrium shifts back toward the formation of the
basic ZDDP, indicating that the concentration of basic ZDDP as a function of temperature. The
solvent used and the presence of other additives also play a role in this equilibrium. Thus, the exact
composition of neutral versus basic salts at any time in an actual formulation is a complex function
of many variables.
2.6 ANTIWEAR AND EXTREME-PRESSURE FILM FORMATION
ZDDPs operate mainly as antiwear agents but exhibit mild EP characteristics. As an antiwear
agent, ZDDP operates under mixed lubrication conditions with a thin oil lm separating the metal
parts. Surface asperities, however, intermittently penetrate the liquid lm, giving rise to metal-on-
metal contact. The ZDDP reacts with these asperities to reduce the contact. Likewise, when the load
is high enough to collapse the oil lm, the ZDDP reacts with the entire metal surface to prevent
welding and to reduce wear. A great deal of study has been done to determine the nature of this
protective lm and the mechanism of deposition, where the thermal degradation products of the
ZDDP are the active antiwear agents.
The antiwear lm thickness and composition are directly related to temperature and the
extent of surface rubbing. Initially, ZDDP is reversibly absorbed onto the metal surface at low
temperatures. As the temperature increases, catalytic decomposition of ZDDP to dialkyldithio-
phosphoryl disul de occurs, with the disul de absorbed onto the metal surface. From here, the
thermal degradation products (as described in Section 2.3) are formed with increasing tempera-
ture and pressure until a lm is formed on the surface [16]. The thickness and composition of this
lm have been studied using many different analytical techniques, but no analysis gives a concise
description of the lm size and composition for the various kinds of metal-to-metal contact found
in industrial and automotive lubrication regimes. In general, the antiwear/EP ZDDP lm can be
said to be composed of various layers of ZDDP degradation products. Some of these degradation
products are reacted with the metal making up the lubricated surface. The composition of the lay-
ers is temperature-dependent.
The rst process that takes place is the reaction of sulfur (from the ZDDP thermal degrada-
tion products) with the exposed metal leading to the formation of a thin iron sul de layer [17].
Next, phosphate reacts to produce an amorphous layer of short-chain ortho- and metaphosphates
with minor sulfur incorporation. The phosphate chains become longer toward the surface, with
the minimum chain length approaching 20 phosphate units. Some studies have indicated that this
region is best described as a phosphate “glass” region in which zinc and iron cations act to stabilize
the glass structure. At the outermost region of the antiwear lm, the phosphate chains contain
more and more organic ligands, eventually giving way to a region composed of organic ZDDP
decomposition products and undegraded ZDDP itself. The thickness of the lm has been analyzed
to be as small as 20 nm using ultra thin lm interferometry and as large as 1 μm using electrical
capacitance [18–21].
CRC_59645_Ch002.indd 58CRC_59645_Ch002.indd 58 3/20/2009 5:33:58 PM3/20/2009 5:33:58 PM
Zinc Dithiophosphates 59
Recent work has concluded that, although the rate of lm formation is directly proportional
to temperature, a stronger correlation exists between lm formation and the extent of metal-to-
metal rubbing as quanti ed by the actual distance that the metal slides during a given test period.
The lm reaches a maximum thickness at which point a steady state between formation and
removal exists, the rate of formation being more temperature-dependent than the rate of removal.
It was also found that the ZDDP reaction lm has a “solid-like” nature (as opposed to be a highly
viscous liquid) due to the lack of reduction of lm thickness observed with time on a static test
ball [22].
Another mechanism of wear found to be inhibited by ZDDP is wear produced from the reaction
of alkyl hydroperoxides with metal surfaces. It was found that the wear rate of automobile engine
cam lobes is directly proportional to alkyl hydroperoxide concentration. The mechanism proposes
the direct attack of hydroperoxide (generally through fuel combustion and oil oxidation) on fresh
metal, causing the oxidation of an iron atom from a neutral charge state to Fe
+3
by reaction with 3
mol of alkyl hydroperoxide as described in the following reactions:
222
2
ROOH Fe RO OH Fe⫹⫹⫹
⫺⫹
→
∗
(2.25)
ROOH Fe RO OH Fe⫹⫹⫹
⫹⫺⫹23
→
∗
(2.26)
The ZDDP and its thermal degradation products neutralize the effect of the hydroperoxides by the
mechanism described in Reactions 2.20 through 2.23 in Section 2.5. It was also shown that peroxy
and alkoxy radicals were far less aggressive toward metal surfaces than hydroperoxides, indicating
that free-radical scavengers such as hindered phenols would be ineffective in controlling this kind
of engine wear. This may explain why the antiwear performance of ZDDP is directly related to its
antioxidation performance in the order of secondary ZDDP > primary ZDDP > aryl ZDDP rather
than correlating with the order of thermal stability (aryl > primary > secondary) [23].
A recent study has been conducted to investigate the difference in wear performance between
neutral and basic ZDDPs in the sequence VE engine test. The neutral ZDDP performed better in
value train wear protection than the basic ZDDP. The basic salt actually failed the sequence VE
engine test, indicating that using commercial ZDDPs with lower basic salt content may be preferred
when limited to 0.1% maximum phosphorus content (as mandated by the International Lubricant
Standardization and Approval Committee [ILSAC] GF-3 motor oil speci cation). It was suggested
that the increased wear protection by neutral ZDDP could be explained by the superior adsorption
of the oligomeric structure of the neutral salt, leading to the formation of longer polyphosphate
chains relative to the basic salt [5].
2.7 APPLICATIONS
ZDDPs are used in engine oils as antiwear and antioxidant agents. Primary and secondary ZDDPs
are both used in engine oil formulations, but it has been determined that secondary ZDDPs perform
better in cam lobe wear protection than primary ZDDPs. Secondary ZDDPs are generally used
when increased EP activity is required (i.e., during run-in to protect heavily loaded contacts such as
valve trains). ZDDPs are generally used in combination with detergents and dispersants (alkaline
earth sulfonate or phenate salts, polyalkenyl succine amides or Mannich-type dispersants), viscosity
index improvers, additional organic antioxidants (hindered phenols, alkyl diphenyl amines), and
pour point depressants. A typical lubricant additive package for engine oils can run in high at
25% in treatment level. The ILSAC has designated its GF-3 engine oil speci cation to include a
maximum limit of 0.1% phosphorus to minimize the engine oil’s negative impact on the emissions
catalyst. For the GF-4 speci cation, the limit in phosphorus was reduced even further. As a result
CRC_59645_Ch002.indd 59CRC_59645_Ch002.indd 59 3/20/2009 5:33:58 PM3/20/2009 5:33:58 PM
60 Lubricant Additives: Chemistry and Applications
of the minimum phosphorus requirement, the treatment level for ZDDP in organic oils is limited to
∼0.5 to 1.5%, depending on the alkyl chain length used.
The new challenge to motor oil formulators is in passing the required ILSAC tests while keeping
the ZDDP level low. Yamaguchi et al. have shown that the antioxidant effect of ZDDP is signi -
cantly enhanced in API group II base stocks with as much as 50% increase noted for a basic ZDDP.
An increase in antioxidancy was also noted when using ZDDPs in polyol ester [24]. Several studies
have also shown that ZDDP oxidation by-products are in effective antiwear agents. The use of these
base-stock effects to extend the oxidation life of the ZDDP may be a suitable method for the formu-
lator to reduce the level of ZDDP needed to accommodate the GF-3 limits.
The synergistic effect between organic molybdenum compounds and ZDDP in wear reduction
is currently being studied as a means of lowering phosphorus content in engine oils. In U.S. patent
5,736,491, molybdenum carboxylate is used with ZDDP to give a synergistic reduction in friction
coef cient by as much as 30%, thus allowing a reduction in the total phosphorus content and an
improvement in fuel economy [25]. The patent literature has sited other organic molybdenum com-
pounds such as molybdenum dithiocarbamates (MoDTC) and dialkyldithiophosphates (MoDTP) as
being useful, synergistic secondary antiwear agents [26].
ZDDPs are also used in hydraulic uids as antiwear agents and antioxidants. The treatment level
for ZDDP in hydraulic uids is lower than that used for engine oils, typically running between 0.2
and 0.7% by weight. They are used in combustion with detergents, dispersants, additional organic
antioxidants, viscosity index improvers, pour point depressants, corrosion inhibitors, defoarmers,
and demulsi ers for a total treatment level of between 0.5 and 1.25% [27]. Primary ZDDPs are
preferred over secondary ZDDPs due to their better thermal and hydrolytic stability. One problem
faced by hydraulic uid formulators is the need for a uid that will service both high-pressure
rotary vane pumps and axial piston pumps, preferably out of the same sump. High-pressure vane
pumps require a hydraulic uid with antiwear properties and oxidative stability commonly achieved
through the use of ZDDPs. High-pressure piston pumps need only rust and oxidation protection and
do not require ZDDPs. ZDDPs can cause catastrophic failure to axial piston systems by adversely
affecting the sliding steel–copper alloy interfaces. The patent literature has several examples of
formulators trying to overcome this problem with the use of additional wear-moderating chemis-
tries such as sulfurized ole ns, polyol esters or borates of them, fatty acid imidazolines, aliphatic
amines, and polyamines. Another problem faced by hydraulic uid formulators is the interaction
of ZDDPs with overbased alkaline earth detergent salts (as well as the interaction of carboxylic
acid and alkenyl succinic anhydride rust preventatives with these detergents) in the presence of
water to give lter-clogging by-products. Formulators have tried to overcome this problem of poor
“wet” lterability by using nonreactive rust inhibitors (i.e., alkenyl succinimides) and improving the
hydrolytic stability of ZDDP antiwear agent [28].
ZDDPs are used in EP applications such as gear oils, greases, and metalworking uids.
Secondary ZDDPs are preferred due to their thermal instability resulting in quick lm formation
under high loads. In automotive gear oils, ZDDPs are used at 1.5–4% in combination with EP agents
(such as sulfurized ole ns), corrosion inhibitors, foam inhibitors, demulsi ers, and detergents. Total
multifunctional additive package treatment levels for automotive gear lubricant additives are from
5 to 12% by weight. Industrial gear oil formulators have generally gone to ashless systems using
sulfur–phosphorus-based EP antiwear chemistries at total additive package treatment levels of
1.5–3%. In general, the recent focus in gear oil technology improvement has centered on increased
thermal stability and EP properties.
ZDDPs are used in greases in chemical systems that closely resemble gear oil formulations.
Many gear oil lubricant additives are used in EP greases. In general, the ZDDP treatment level
for greases is in the same range as that used for gear oils. ZDDP, usually secondary or a mixture
of secondary and primary, is used in combination with sulfurized ole ns, corrosion inhibitors,
ashless antioxidants, and additional friction modi ers. A recent advancement in grease technology
is the use of ZDDP/sulfurized ole n synergy to replace antimony and lead in high-EP grease
CRC_59645_Ch002.indd 60CRC_59645_Ch002.indd 60 3/20/2009 5:33:58 PM3/20/2009 5:33:58 PM
Zinc Dithiophosphates 61
formulations. This has generally been limited to the European market, having been pioneered in
Germany.
ZDDPs, in combination with sulfurized ole ns, are also used to replace chlorinated paraf ns
in medium- to heavy-duty metalworking uids. This is due to the possible carcinogenicity of the
low-molecular-weight analogs of chlorinated paraf n. European formulators, and to a certain extent
Japanese formulators, use ZDDPs in this way. The use of ZDDPs in metalworking uids in the
United States is limited due to environmental concerns. The U.S. Environmental Protection Agency
classi es them as marine pollutants.
In conclusion, after 50 years, ZDDPs still enjoy a wide variety of uses in the lubrication indus-
try, with production volumes remaining at high levels. The majority of ZDDP production is used
in automobile engine oil. The impact of the GF-2 and GF-3 phosphorus-level speci cation of 0.1%,
however, was reduction of ZDDP production in the past 10 years. The Ford Motor Company is
currently evaluating engine oils with 0–0.6% phosphorus levels in eet tests in preparation for the
looming GF-4 standard in 2004, which will require engine oils to have minimal impact on emis-
sion system deterioration. This could further negatively impact ZDDP production. The need to
understand clearly how ZDDPs function in terms of wear and oxidation protection is reinforced by
the need to develop satisfactory phosphorus-free alternatives to ZDDP. The development of such
chemistries, within the economic and functional limits that ZDDPs impose, will be a daunting task
for future researchers. Until that time, the elimination of ZDDPs from various industrial lubricants
will mandate either higher costs or less performance.
REFERENCES
1. Freuler, H.C. Modi ed lubricating oil. U.S. Patent 2,364,284 (December 5, 1944, Union OIL Co. of
California).
2. Adams, D.R. Manufacture of dihydrocarbyl dithiophosphats. U.S. Patent 5,672,294 (May 6, 1997, Exxon
Chemical Patents, Inc.).
3. Paddy, J.L. et al. Zinc dialkyldithiophosphate oxidation by cumene hydroperoxide: kinetic studies by
Raman and
31
P NMR spectroscopy. Trib Trans 33(1):15–20, 1990.
4. Yamaguchi, E.S. et al. Dynamic light scattering studies of neutral diisobutyl zinc dithiophosphate. Trib
Trans 40(2):330–337, 1997.
5. Yamaguchi, E.S. The relative wear performance of neutral and basic zinc dithiophosphates in engines.
Trib Trans 42(1):90–94, 1999.
6. Bridgewater, A.J., J.R. Dever, M.D. Sexton. Mechanisms of antioxidant action, part 2. Reactions of zinc
bis(O,O′-dialkyl(aryl)phosphorodithioates) and related compounds with hydroperoxides. J Chem Soc
Perkin II:1006–1016, 1980.
7. Buckley, T.F. Methods for preventing the precipitation of mixed zinc dialkyldithiophosphates which
contain high percentages of a lower alkyl group. U.S. Patent 4,577,037 (March 18, 1986, Chevron
Research Co.).
8. Yamaguchi, E.S. Oil soluble metal (lower) dialklyl dithiophosphate succinimide complex and
lubricating oil composition containing same. U.S. Patent 4,306,984 (December 22, 1981, Chevron
Research Co.).
9. Luther, H., E. Baumgarten, K. Ul-Islam. Investigations by gas chromatography into the thermal
decomposition of zinc dibutyldithiophosphates. Erdol und Kohle 26(9):501, 1973.
10. Coy, R.C., R.B. Jones. The chemistry of the thermal degradation of zinc dialkyldithiophosphate
additives. ASLE Trans 24(1):91–97, 1979.
11. Rasberger, M. Oxidative degradation and stabilization of mineral based lubricants, in R.M. Moritier and
S.T. Orszulik, eds., Chemistry and Technology of Lubricants, 2nd ed. London: Blackie Academic and
Professional, 1997, pp. 82–123.
12. Rossi, E., L. Imperoto. Chim Ind (Milan) 53:838–840, 1971.
13. Sexton, M.D., J Chem Soc Perkin Trans II:1771–1776, 1984.
14. Howard, S.A., S.B. Tong. Can J Chem 58:92–95, 1980.
15. Burn, A.J. The mechanism of the antioxidant action of zinc dialkyl dithiophosphates. Tetrahedron
22:2153–2161, 1966.
CRC_59645_Ch002.indd 61CRC_59645_Ch002.indd 61 3/20/2009 5:33:58 PM3/20/2009 5:33:58 PM
62 Lubricant Additives: Chemistry and Applications
16. Bovington, C.H., Darcre, B. The adsorption and reaction of decomposition products of zinc
dialkyldithiophosphate on steel. ASLE Trans 27:252–258, 1984.
17. Bell, J.C., K.M. Delargy. The composition and structure of model zinc dialkyldithiophosphate antiwear
lms, in M. Kozna, ed., Proceedings 6th
International Congress on Tribology Eurotrib ’93, Budapest,
2:328–332, 1993.
18. Willermet, P.A., R.O. Carter, E.N. Boulos, Lubricant-derived tribochemical lms—An infra-red
spectroscopic study. Trib Intl 25:371–380, 1992.
19. Fuller, M. et al. Chemical characterization of tribochemical and thermal lms generated from neutral
and basic ZDDPs using x-ray absorption spectroscopy. Trib Intl 30:305–315, 1997.
20. Allison-Greiner, A.F., J.A. Greenwood, A. Cameron. Thickness measurements and mechanical
properties of reaction lms formed by zinc dialkyldithiophosphate during running. Proceedings of
IMechE International Conference on Tribology—Friction, Lubrication and Wear 50 Years on, London,
IMechE, 1:565–569, 1987.
21. Tripaldi, G., A. Vettor, H.A. Spikes. Friction behavior of ZDDP lms in the mixed boundary/EHD
regime. SAE Tech. paper 962036, 1996.
22. Taylor, L., A. Dratva, H.A. Spikes. Friction and wear behavior of zinc dialkyldithiophosphate additive.
43(3):469–479, 2000.
23. Habeeb, J.J., W.H. Stover. The role of hydroperoxides in engine wear and the effect of zinc
dialkyldithiophosphates. ASLE Trans 30(4):419–426, 1987.
24. Yamaguchi, E.S. et al. The relative oxidation inhibition performance of some neutral and basic zinc
dithiophosphate salts. S.T.L.E. Preprint No. 99-AM-24, pp. 1–7, 1989.
25. Patel, J.A. Method of improving the fuel economy characteristics of a lubricant by friction reduction and
compositions useful therein. U.S. Patent 5,736,491 (April 7, 1998, Texaco, Inc.).
26. Naitoh, Y. Engine oil composition. U.S. Patent 6,063,741 (May 16, 2000, Japan Energy Corporation).
27. Brown, S.H. Hydraulic system using an improved antiwear hydraulic uid. U.S. Patent 5,849,675
(December 15, 1998. Chevron Chemical Co.).
28. Ryan, H.T. Hydraulic uids. U.S. Patent 5,767,045 (June 16, 1998, Ethyl Petroleum Additives, Ltd.).
CRC_59645_Ch002.indd 62CRC_59645_Ch002.indd 62 3/20/2009 5:33:58 PM3/20/2009 5:33:58 PM

63
3
Ashless Phosphorus–
Containing Lubricating
Oil Additives
W. David Phillips
CONTENTS
3.1 Introduction and Scope ...........................................................................................................64
3.2 Historical Background ............................................................................................................65
3.3 Manufacture of Phosphorus-Containing Lubricating Oil Additives ......................................68
3.3.1 Neutral Alkyl and Aryl Phosphate Esters ....................................................................68
3.3.1.1 Natural Phosphates..........................................................................................69
3.3.1.2 Synthetic Phosphates from Isopropylphenols ................................................. 70
3.3.1.3 Synthetic Phosphates from Tertiarybutylphenols ........................................... 70
3.3.2 Acid Phosphate Esters ..................................................................................................71
3.3.2.1 Alkyl and Aryl Acid Phosphates (Non-ethoxylated) ...................................... 71
3.3.2.2 Alkyl- and Alkylarylpolyethleneoxy Acid Phosphates ...................................72
3.3.3 Amine Salts of Acid Phosphates and of Polyethyleneoxy Acid Phosphates ................72
3.3.4 Neutral Phosphite Esters ...............................................................................................73
3.3.5 Alkyl and Aryl Acid Phosphites ................................................................................... 73
3.3.6 Dialkyl Alkyl Phosphonates ......................................................................................... 74
3.4 The Function of Lubricity Additives ....................................................................................... 74
3.4.1 The Basic Mechanism of Lubrication and Wear and the In uence of Additives ......... 75
3.5 Investigations into the Mechanism and Activity of Phosphorus-Containing Additives ......... 79
3.5.1 Early Investigations into Antiwear and Extreme Additives .........................................80
3.5.2 Neutral Alkyl and Aryl Phosphates ..............................................................................80
3.5.2.1 Historical Background ....................................................................................80
3.5.2.2 Recent Technical Developments .....................................................................86
3.5.2.3 Recent Commercial Developments .................................................................89
3.5.3 Alkyl and Aryl Acid Phosphates .................................................................................. 91
3.5.3.1 Non-ethoxylated .............................................................................................. 91
3.5.3.2 Alkyl and Alkarylpolyethleneoxy Acid Phosphates .......................................92
3.5.3.3 Amine Salts of Acid Phosphates .....................................................................95
3.5.4 Neutral Alkyl and Aryl Phosphites ..............................................................................98
3.5.4.1 Use as Antiwear/Extreme-Pressure Additives ................................................98
3.5.4.2 Use as Antioxidants for Lubricating Oils .......................................................99
3.5.5 Alkyl and Aryl Acid Phosphites ................................................................................. 100
3.5.5.1 Amine Salts of Acid Phosphites.................................................................... 102
3.5.6 Phosphonate and Phosphinate Esters.......................................................................... 103
3.5.7 A Summary of the Proposed Mechanism for Antiwear and Extreme-Pressure
Activity of Phosphorus-Based Additives ....................................................................104
3.6 Market Size and Commercial Availability............................................................................ 105
CRC_59645_Ch003.indd 63CRC_59645_Ch003.indd 63 3/19/2009 6:26:48 PM3/19/2009 6:26:48 PM
