Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.

104 Lubricant Additives: Chemistry and Applications
been used in water-based formulations with good pump wear characteristics [155,156]. One of the
most recent applications has been in refrigeration compressor oils (e.g., for automobiles) that are
compatible with the more ecologically acceptable refrigerants. The reason for their selection in this
application has probably been their good hydrolytic stability in view of the need for a long uid life
[157,158]. Other automotive industry applications for these products include use as friction modi-
ers, for example, in automatic transmission uids [159], or possibly as detergents in engine oils
[160–162] to keep insoluble combustion and oil oxidation products dispersed in the oil.
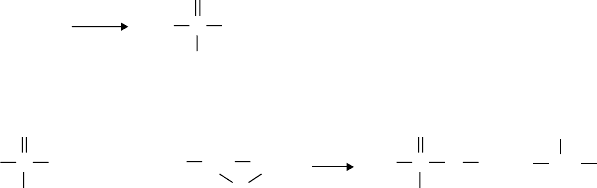
An alternative method for incorporating phosphorus into dispersant is exempli ed in Ref. 160.
This method involves reacting P
2
S
5
with a sulfurized hydrocarbon, such as sulfurized polyisobutyl-
ene, at high temperatures to form a thiophosphorus acid (see reaction 3.23, Figure 3.22). This inter-
mediate is then reacted with propylene oxide to form the hydroxypropyl esters of the phosphorus
acid (see reaction 3.24, Figure 3.22).
Aminoethane phosphonate copolymers have also been claimed to provide dispersancy, corrosion
protection, and pour point depression [163]. Among other applications mentioned in the literature
for these products or their salts in lubricating oils are the extrusion, cold rolling, and cold forging of
aluminum [164], offering improved rust inhibition [165] and antioxidant performance [166].
3.5.7 A SUMMARY OF THE PROPOSED MECHANISM FOR ANTIWEAR AND
E
XTREME-PRESSURE ACTIVITY OF PHOSPHORUS-BASED ADDITIVES
In attempting to produce an explanation for the activity of phosphorus-containing additives, it is
not easy, as explained earlier, to compare the results of the preceding investigations because condi-
tions vary from one investigation to another. No report evaluates all the different types of additives
with the same (high) level of purity under identical test conditions. However, it is possible to draw
together some of the more consistent “threads” running through the many papers. One parameter
highlighted in past reports (and con rmed by recent observations) is that the presence of oxygen
on the metal surface appears to be important for the activity of neutral aryl phosphates. This could
perhaps be one of the major reasons why TCP is sometimes found to be inactive. The composition
of the lm formed on the surface is not yet completely de ned, but current work points toward the
formation of a self-regenerating polyphosphate layer in which amorphous carbon may be providing
the lubrication bene ts. The mechanism of formation of the polyphosphate layer and the role, for
example, of moisture is not yet clear but appears to be a stepwise process as follows:
• The adsorption of the material onto the surface (occurring through the
–
P=O and
–
P
–
OH
bonds in the molecule).
• Either the hydrolysis of a –P–OR bond to form
–
P
–
OH (probably arising from water on the
surface but may also occur in solution) with the formation of acid phosphates/phosphites
PIB + P
2
S
5
P
OHPIB
S
OH
P
OH
O
PIB
S
OH
P
OPIB
S
OH
H
2
O
+
2
(CH
3
CH CH
2
)
(OCH
2
CH
CH
3
OH)
2
where PIB = polyisobutylene
(3.23)
(3.24)
FIGURE 3.22 An example of the preparation of a phosphorus-based detergent. (From Colyer, C.C., Gergel,
W.C., Chemistry and Technology of Lubricants, VCH Publishers, New York, 1992. With permission.)
CRC_59645_Ch003.indd 104CRC_59645_Ch003.indd 104 3/19/2009 6:27:00 PM3/19/2009 6:27:00 PM
Ashless Phosphorus-Containing Lubricating Oil Additives 105
or, in the case of neutral phosphates, the cleavage of the C–O bond to release an aryl radical
and a residual
–
P
–
O
•
radical.
• Either reaction of the
–
P
–
OH or
–
P
–
(OH)
2
with the metal surface to form an iron salt,
possibly followed by further hydrolysis to release the remaining hydrocarbon moieties and
reaction of the new
–
P
–
OH groups with the surface to form polyphosphate, or the reaction
of the residual
–
P
–
O
•
radical with the iron surface to form a succession of Fe–O–P– bonds
leading to the formation of polyphosphate.
• Products that contain
–
P
–
C bonds (e.g., the phosphonates and particularly the phosphi-
nates) are less likely to operate by a mechanism involving hydrolysis, and the stability of
the P
–
C bond might be expected to prevent or delay the formation of the phosphorus-rich
surface layer with an adverse effect on EP properties. However, the same stability could
result in better friction-modi cation properties. The fact that phosphinates and phospho-
nates are active as AW/EP additives suggests that the
–
P=O bond is also involved in the
surface adsorption process, but that either the nature of the surface lm may be different or
a polyphosphate lm is produced as a result of the scission of a
–
P
–
C bond.
• The formation of amine salts results in an increase in activity, possibly as a result of the
stability of the ion and improved adsorption on the metal surface.
The mechanism of formation of phosphide, which is reported in many instances, has not yet been
clari ed but might possibly involve the amorphous carbon that then acts as a reducing agent on the
phosphate/phosphite layer as it forms on the surface.
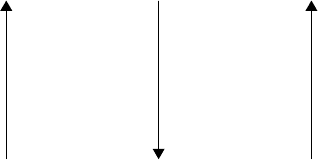
These conclusions lead, as a broad generalization, to the order in activity and impact on surface
chemistry/stability as shown in Figure 3.23.
The preceding comments are, however, a simpli cation of the situation. Depending on the
length of the alkyl or alkaryl chain, if the iron salts that are formed are soluble in the oil they may
desorb from the surface, leading to poor AW/EP performance. Interaction with other surface-active
materials will inevitably in uence the performance of AW/EP additives, whereas depletion in use
due to oxidation, etc., will also affect performance.
3.6 MARKET SIZE AND COMMERCIAL AVAILABILITY
Information on the market size for ashless phosphorus-containing AW/EP additives is limited. An
approximate total market of ∼10,000 tpa is broken down, as given in Table 3.16. The data exclude
the use of phosphites as antioxidants in oil applications, which is separately estimated to be between
100 and 200 tpa.
The wide use of phosphorus-containing AW/EP additives is due, in addition to their good lubric-
ity performance, to the following features of value to formulators:
• Ashless
• Low odor, color, and volatility
• Low acidity/noncorrosive (applies to the neutral esters only)
Amine phosphites
Amine phosphates
Acid phosphites
Acid phosphates
Neutral phosphites
Neutral phosphates
Neutral phosphonates
Improvement in
EP properties
Improvement in
AW properties
Impact on
stability, etc.
FIGURE 3.23 An approximate ranking of the effect of structure on the AW, EP, and stability properties of
the base stock.
CRC_59645_Ch003.indd 105CRC_59645_Ch003.indd 105 3/19/2009 6:27:01 PM3/19/2009 6:27:01 PM

106 Lubricant Additives: Chemistry and Applications
• Low toxicity
• Biodegradable (many but not all products)
• Compatible with most other types of additives (particularly the neutral esters)
• Soluble in a wide range of base stocks, both mineral oil and synthetic, and able to assist the
solvency of other additives
Although the physical properties of phosphorus-containing additives are not critical, the values for
the most widely used types of phosphate ester AW additives are given in Table 3.17, with TiBP as
an example of an alkyl phosphate, TCP as a natural phosphate, and ISO 32 grades of both types of
synthetic ester.
The major suppliers of phosphorus-containing lubricant additives are listed in Table 3.18, and
their current oil industry applications are summarized in Table 3.19. Undoubtedly, the most impor-
tant applications for the neutral aryl phosphates are hydraulic, turbine, and general circulatory oils,
whereas almost the entire market for the ethoxylated alkyl and aryl acid phosphates is to be found
in metalworking. The acid phosphates, acid phosphites, and amine salts of these acidic materials are
used in a mixture of metalworking, gear oils, hydraulic oils, etc., as indicated in Table 3.19.
The selection of an AW/EP additive depends on the speci c requirements for the application;
for example, whether both AW and EP performance is needed and what levels are required. When
this has been ascertained, secondary considerations may be the level of stability required (oxida-
tive or hydrolytic), potential interaction with other components of the formulation, and the effect on
surface-active properties such as foaming. Table 3.20 attempts to identify the additives that should
TABLE 3.16
A Breakdown of the Market for Ashless Phosphorus-
Containing AW/EP Additives
Product Market Size (t)
Alkyl phosphates 100
Aryl phosphates 6000
Acid phosphates, ethoxylated alkyl and aryl phosphates,
and amine salts of acid phosphates
3000
Phosphites, acid phosphites, dialkyl alkyl phosphonates,
and amine salts of acidic products
1000
TABLE 3.17
Typical Physical Properties of the Most Widely Used Grades of Phosphorus-Based
AW Additives
Property Unit TiBP TCP IPP/32 TBPP/32
Viscosity at 40°C cSt 2.9 25.0 32.3 33
Viscosity at 0°C cSt 10.0 1000 990 1500
Speci c gravity 20/20°C 0.965 1.140 1.153 1.170
Pour point °C <–90 –28 –27 –26
Acid number mg KOH/g 0.06 0.05 0.05 0.06
Water content % 0.01 0.06 0.05 0.05
Phosphorus level % 11.7 8.3 8.0 8.1
Flash point °C 155 240 245 255
CRC_59645_Ch003.indd 106CRC_59645_Ch003.indd 106 3/19/2009 6:27:01 PM3/19/2009 6:27:01 PM

Ashless Phosphorus-Containing Lubricating Oil Additives 107
TABLE 3.18
Principal Suppliers of Ashless Phosphorus-Containing Lubricating Oil Additives
Producer
Neutral
Phosphates Alkyl/Aryl Acid Phosphates
Amine
Phosphates
Neutral
Phosphites
Acid
Phosphites
Alkyl
PhosphonatesAlkyl Aryl Nonethoxylated Ethoxylated Alkyl Aryl
Asahi Denka ××
Chemtura × × × × ×
Ciba Spec. Chem. × × ×
Croda × × ×
Daihachi × ×
Dover Chemicals ××
Johoku Chemicals × × × × ×
Krishna ××
Lanxess × ×
Libra Chemicals × × ×
Rhein Chemie × × ×
Rhodia × × × × × × × × ×
Sumitomo ××
Supresta × ×
United Phosphorus × × ×
Vanderbilt ×
CRC_59645_Ch003.indd 107CRC_59645_Ch003.indd 107 3/19/2009 6:27:01 PM3/19/2009 6:27:01 PM

108 Lubricant Additives: Chemistry and Applications
be given prime consideration when taking these secondary requirements into account. Products that
demonstrate better AW than EP activity, and vice versa, are shown. However, the boundary between
AW and EP performance is not clear-cut and much depends on the application requirements.
3.7 TOXICITY AND ECOTOXICITY
It was mentioned earlier in this chapter that concern had been expressed in the past regarding
the toxicity of phosphorus-containing products, particularly TCP. Today, with increasing focus on the
environmental behavior of chemicals, their ecotoxicity is also under scrutiny. As a result, detailed
investigations into both the toxicity and the ecotoxicity have been carried out on alkyl and aryl phos-
phate esters. The results are summarized in recent publications [59,167], and most are available in the
safety data sheets associated with different product types. The data demonstrate a relatively low (but
variable) order of toxicity and ecotoxicity. No signi cant risks in handling are anticipated, provided
the manufacturer’s guidance, which is essentially the same as for mineral oils, is followed.
The concerns over TCP arose as a result of the o-cresol content in the feedstock as tri-ortho-
cresyl phosphate (TOCP) was found to be a signi cant neurotoxin. Initially, the level of o-cresol in
the feedstock was high (up to ∼25%) and signi cant amounts of TOCP were present in the nished
product. Although the initial focus was on TOCP, it was later acknowledged that any isomer con-
taining the o-cresyl moiety was neurotoxic (e.g., mono-o-cresyldiphenyl phosphate was said to be
10 times more neurotoxic than TOCP [168]). For these reasons, the o-cresol content of the feedstock
used in the manufacture of TCP has been progressively reduced over time. In recent years, production
has moved to the use of 99% minimum m- and p-cresol. Levels of o-cresol in the feedstock are now
frequently <0.05%, and the TOCP content can be as low as parts per billion. Mackerer et al. [169]
TABLE 3.19
Principal Applications for Ashless Phosphorus-Containing AW/EP Additives
Application
Triaryl
Phosphate
Trialkyl
Phosphate
Amine
Phosphate
Acid
Phosphates
Alkyl/Aryl
Phosphites
Automotive
AT F
Gear oil
Power steering
Shock absorber
Electric motor
Industrial
Hydraulic oils
Gear oil
Turbine oils
Compressor oils
Gas oil
Universal tractor
Metalworking
Grease
Way oils
Circulating oil
Vegetable oil
Aircraft
Piston engine
Turbine engine
Grease
CRC_59645_Ch003.indd 108CRC_59645_Ch003.indd 108 3/19/2009 6:27:01 PM3/19/2009 6:27:01 PM

Ashless Phosphorus-Containing Lubricating Oil Additives 109
estimate that the toxicity of the TCP now available commercially is ∼400 times less than that of
material available in the 1940s and 1950s, and a recent evaluation of the organophosphorus-induced
delayed neurotoxicity (OPIDN) of a commercial aviation gas turbine oil containing TCP was nega-
tive [170]. However, in view of past concerns, the use of TCP is now largely restricted to aviation gas
turbine oils. Most general industrial applications that require an aryl phosphate AW additive now use
the isopropylphenyl or, to a lesser extent, the tertiarybutylphenyl variants. In standard tests, neither
of these types display OPIDN from acute oral ingestion. There are, however, some differences in
the toxicity and the ecotoxicity behavior between the different aryl phosphates. For example, the
reproductive toxicity of the synthetic aryl phosphates, together with TXP, was recently studied in rats
(according to Organisation for Economic Co-operation and Development [OECD] method 422). Both
the isopropylphenyl phosphate and the TXP showed adverse effects at moderate to high dose levels,
but these were reversible when exposure ceased. The TBPP (produced according to reaction 3.2) did
not display any adverse effects.
Differences are also seen in ecotoxicity behavior. Owing to the high TPP content in the lower-
viscosity grades of the synthetic phosphates (particularly ISO VG 22 and 32), these products have
the worst ecotoxicity behavior. The tertiarybutylphenyl phosphates normally have a higher TPP
content than the corresponding grade of IPPP and therefore, of the synthetic phosphates, possess
relatively worse ecotoxicity. By comparison, the IPPPs generally show satisfactory behavior in these
tests. One ISO VG 46 isopropylphenyl phosphate-based AW/EP additive has, for example, been
approved by the German Environment Agency (Umweltbundesamt) for use in rapidly biodegradable
hydraulic uids, products that are eligible for the “Blue Angel” environmental award [171].
Another difference between the product types is displayed in biodegradability tests. The tests
were carried out according to OECD method 301F (Manometric Respirometry). In this test, bio-
degradation is measured as the net oxygen uptake over that occurring in blank tests containing only
TABLE 3.20
Guidance on the Selection of AW and EP Additives
Required Characteristic
Good AW
Performance
Good EP
Performance
Combination of AW
and EP Performance
Non-phenolic additive Neutral alkyl
phosphates; dialkyl
alkyl phosphonates
Acid alkyl phosphates;
acid alkyl
phosphonates and
their salts; neutral
and acid alkyl
phosphites
Mixtures of neutral and
acid phosphates, etc.
Good hydrolytic stability TXP, dialkyl alkyl
phosphonates
Acid alkyl
phosphonates and
their salts
—
Good oxidation stability Neutral tertbutylphenyl
phosphates
Hindered aryl
phosphites
—
Low foaming/air release Neutral phosphates,
dialkyl alkyl
phosphonates
Neutral phosphites —
Good toxicity performance Neutral tertbutylphenyl
phosphates
Acid IPPPs —
Good ecotoxicity
performance
Neutral IPPPs — —
Multifunctionality, for
example, rust inhibition,
antioxidant
Neutral and acid
phosphites, acid
phosphates
Acid IPPPs
CRC_59645_Ch003.indd 109CRC_59645_Ch003.indd 109 3/19/2009 6:27:01 PM3/19/2009 6:27:01 PM
110 Lubricant Additives: Chemistry and Applications
inoculated medium. The extent of biodegradation is calculated from the mass of test material added
to the test vessels and its theoretical oxygen demand for complete biodegradation. The test was car-
ried out in triplicate on the ISO VG 46 grades of different types of aryl phosphates manufactured
according to reaction 3.2. The results are summarized in Table 3.21.
The results are initially in the order of their hydrolytic stability, but it is interesting that TXP,
after a slow start, eventually reaches the same level as the synthetic uids and might have exceeded
them had the test been extended.
In view of these data, the tertiarybutylatedphenyl phosphate would be regarded as readily biode-
gradable (Pw1), whereas the TXP and IPPP would be classi ed as inherently biodegradable (Pw2).
Despite of relatively benign ecotoxicity of the higher viscosity grades of aryl phosphates, all
these products are classi ed as marine pollutants because of the UN Marine Pollutant Classi cation.
However, because they are used at low concentrations, they are unlikely to contribute signi cantly to
the nished product’s ecotoxicity.
The toxicity of other phosphorus-containing compounds is less well documented. Drake and
Calamari [172] indicate that dialkyl alkyl phosphonates generally have a low level of acute toxicity,
which decreases with increasing chain length, apparently a general observation for these classes of
compounds. As with alkyl phosphates, certain short-chain products can be skin irritants. No clues
were found to their environmental behavior, but in view of the absence of phenolics and improved
hydrolytic stability, it might be surmised that sh toxicity could be good but biodegradability would
be inferior to that of the phosphates. Neutral phosphites, particularly the alkyl phosphites, would be
expected to have good toxicity and biodegradability behavior, but their ease of hydrolysis, which is
the factor assisting the biodegradation, would probably result in poor aquatic toxicity. The future of
the nonylphenyl phosphites is uncertain; the U.S. National Toxicology Program currently lists non-
ylphenol as an estrogen mimic and also as a thyroid disruptor. The acid phosphates, acid phosphites,
and their salts, particularly amine salts, are likely to be classi ed as irritants and, due to their ease
of hydrolysis, may again be toxic to aquatic organisms. In all cases, it is essential that reference be
made to the health and safety information provided by the manufacturer.
3.8 THE FUTURE FOR ASHLESS PHOSPHORUS-BASED
LUBRICATING OIL ADDITIVES
Although ashless phosphorus-containing additives are used in many industrial applications, there
are certain market segments where they have not, to date, been successful. These are principally
in automotive engine oils where the use of ZDDP dominates due to a combination of price and
multifunctionality and in gear oils where sulfur continues to be the EP additive of choice. However,
the use of chlorine as an EP additive, particularly in metalworking applications, is in decline for
environmental reasons and is expected to be slowly substituted by P/S combinations. The use of sul-
fur alone in applications requiring high EP performance may also move to P/S mixtures to reduce
the total sulfur level and the ability to more readily “tailor” the balance of AW and EP performance
TABLE 3.21
OECD 301F Biodegradability Data on Different
Types of Aryl Phosphates
Product (ISO VG 46
Base Stocks)
% Biodegradability After
10 Days 28 Days 68 Days
TXP 5 29 70
IPPP 18 47 65
TBPP 25 62 72
CRC_59645_Ch003.indd 110CRC_59645_Ch003.indd 110 3/19/2009 6:27:02 PM3/19/2009 6:27:02 PM
Ashless Phosphorus-Containing Lubricating Oil Additives 111
to the application. The potential in other market segments including those traditionally using ZDDP
is discussed in greater detail in the following:
3.9 LUBRICATING OIL FORMULATIONS (GENERAL)
The current trend toward the use of groups II and III mineral oil base stocks for general industrial appli-
cations, with improved antioxidant response but inferior lubricity as a result of the removal of aromat-
ics and sulfur compounds, could encourage the wider use of phosphorus. The lack of competition for
the surface, which has previously been shown for TCP in stocks containing naphthenics and aromatics,
should result in the increased activity of phosphorus compounds. Their use may also be bene cial due
to their ability to aid the dissolution of additives that might otherwise have limited solubility.
In Europe, legislation (Directive 2000/769/EC) implemented in 2004 requires a substantial
reduction in sulfur dioxide (SO
2
) emissions from the combustion of waste materials including waste
oil. This may result in a move to lower sulfur levels in lubricating oils (including metalworking oils)
and a possible replacement by phosphorus to restore the level of AW/EP performance.
3.10 HYDRAULIC OILS
In recent years, there has been a move toward the use of ashless hydraulic oils. This is mainly for
two reasons. First, as a result of the sensitivity of ZDDPs toward moisture and the resulting deposi-
tion of zinc oxide/sul de. This deposit can adversely affect the lterability of the oil and reduce oxi-
dation stability. Second, there is increasing concern regarding the environmental behavior of heavy
metals. Regulatory controls, however, are likely to extend further to cover metals such as zinc, as
in the Great Lakes Initiative between the United States and Canada. As the zinc cannot be easily
removed from waste at the ef uent plant, there has been a focus on the reduction in use levels.
Concern has also been expressed in certain countries regarding the smell of sulfur arising from
the degradation of the ZDDP when the hydraulic oil is used, for example, in elevators (Dixon, R.,
Shell Global Solutions, Private Communication, November 2007).
3.11 AUTOMOTIVE ENGINE OILS
Vehicle emissions legislation (e.g., in the United States, Europe, and Japan) now exists to control
and substantially reduce the levels of particulates, hydrocarbons, carbon monoxide, and oxides of
nitrogen in the engine exhaust. The engine manufacturers have met these requirements by a variety
of design changes that impact the composition of oils and fuels in the following ways:
• The introduction of catalytic converters to oxidize the hydrocarbon and carbon monoxide
components to carbon dioxide and water, and reduce the nitric oxide (NO) to nitrogen, has
been very successful in reducing emissions. When they operate at their normal operat-
ing temperature and optimum level of ef ciency, they are almost 100% ef cient and most
of the remaining emissions occur in the time before the catalyst reaches “light-off” tem-
perature. Many studies into reducing this period to achieve yet lower emissions have been
conducted. Although much success has been achieved, further progress may be hindered
by the formation of a deactivating lm on the catalyst surface by the phosphorus from the
ZDDP antioxidant and AW/EP additive. As a consequence, there is pressure to reduce the
phosphorus content of engine oils to minimize catalyst fouling. Currently, oil speci ca-
tions such as ILSAC GF-4 and ACEA Cx limit the phosphorus content of both diesel and
gasoline engine oils to 0.05–0.09% with the actual level being linked to the amount of
catalyst used and the expected service interval. Further reductions below 0.05% are being
considered, but there is a concern that such a low level could adversely affect the durabil-
ity of certain engine parts, for example, the valve train and timing chain, as reducing the
ZDDP content also reduces the wear protection. However, a recent study [173] suggests that
CRC_59645_Ch003.indd 111CRC_59645_Ch003.indd 111 3/19/2009 6:27:02 PM3/19/2009 6:27:02 PM
112 Lubricant Additives: Chemistry and Applications
the behavior of phosphorus compounds in wear and catalyst tests varies according to the
way in which phosphorus is incorporated into the molecule. Further work reports that
it is possible to achieve improvements in catalyst protection (and fuel economy) by
reducing the ZDDP content and then adding a metal-free phosphorus-containing AW addi-
tive [174].
• In an attempt to increase fuel economy, the so-called fuel-ef cient lubricants are being
developed. These are usually lower-viscosity products (since energy losses decrease with
viscosity), sometimes complemented by the use of friction modi ers. However, low-viscos-
ity oils may cause increased wear of some engine components, and the necessity for improv-
ing the wear protection is being studied. The ILSAC GF-5 speci cation, for example, will
necessitate the use of some form of friction modi er to guarantee the required level of
economy. Currently, molybdenum compounds or long-chain esters are under evaluation, but
other approaches (e.g., the use of functionalized viscosity modi ers) are also being studied
as the ability of these additives to deliver reduced friction over long periods is uncertain
(Mainwaring, R., Shell Global Solutions, U.K.). ZDDP is linked to increased friction and
therefore reduced ZDDP levels may also be required.
• To reduce the particulate (soot) levels in exhaust gas, the diesel engines in many
passenger cars and trucks need to use particulate lters. These lters can also remove the
ash-containing residue produced from metallic fuel and lubricant additives, and if they are
not occasionally cleaned, they will block causing a deterioration in engine performance.
The engine builders, however, are trying to preserve or even extend service intervals and
consequently are interested in reducing the ash content of the oil. Although ZDDP is not
the only source of metals in the oil, a reduction in zinc content will follow automatically
from any reduction in the phosphorus content (as long as ZDDP remains in formulations)
and will therefore help to reduce engine oil ash content (Mainwaring, R., Shell Global
Solutions, U.K.).
• One of the techniques used to remove the soot from the particulate lters (and thereby
maintain an acceptable engine back pressure) has been to oxidize the deposited carbona-
ceous material by nitrogen dioxide (NO
2
). This is obtained from the exhaust gas by catalyti-
cally oxidizing the NO component. The oxidation of the soot to carbon dioxide effectively
removes carbonaceous lter deposits, and the NO
2
is such a powerful oxidant that it enables
the process to be carried out at a relatively low temperature (∼250°C). Unfortunately, the
catalyst used to oxidize the NO preferentially oxidizes any SO
2
in the exhaust, thereby
reducing the ef ciency of the NO conversion. Additionally, the sulfur trioxide (SO
3
) formed
passes through the trap in the gas phase and is converted there into sulfuric acid by the
water in the exhaust. The sulfuric acid (monitored as “sulfates”) contributes, as droplets,
to the overall level of particulate emissions and is clearly undesirable if the exhaust gas
is inhaled. Any reduction in sulfur content by lowering ZDDP levels in engine oils also
reduces the phosphorus content arising from this additive. Supplemental phosphorus may
therefore be needed.
• Increased emphasis on fuel economy led some manufacturers to introduce direct injection
strati ed charge gasoline engines. Conventional catalysts cannot remove oxides of nitrogen
(NO
x
) in these “lean-burn” engines, and, as a result, NO
x
storage catalysts have been devel-
oped in which the oxides are stored as nitrates by reaction with barium sulfate contained in
the catalyst coating. When the barium-containing sites become saturated, the engine switches
to stoichiometric or slightly rich operation at which temperature the nitrates break down and
release the NO
x
, thus promoting its reduction through the conventional route of reaction
with hydrocarbons and carbon monoxide. Unfortunately, barium sites react preferentially
with any sulfur oxides present, reducing their ability to “store” NO
x
. As a consequence,
there is again pressure to reduce the fuel sulfur content. However, these levels are already
being lowered (see “Fuels” below), and at such levels, the engine oil begins to be a signi cant
CRC_59645_Ch003.indd 112CRC_59645_Ch003.indd 112 3/19/2009 6:27:02 PM3/19/2009 6:27:02 PM
Ashless Phosphorus-Containing Lubricating Oil Additives 113
contributor to exhaust “sulfur” content.A debate has therefore arisen regarding the future
level of lubricant sulfur, and diesel engine manufacturers have already expressed interest in
lubricants with a sulfur level as low as 0.2%— considerably below the current value of ∼1%
(Mainwaring, R. Shell Global Solutions, Private Communication, January 2008).
In 1999, the European Union (EU) issued emission requirements for heavy-duty diesels that
anticipated signi cant reductions in NO
x
, CO, unburnt hydrocarbons, and particulates over
the period from 2001 to 2008. The greatest challenge was to lower NO
x
while at the same time
reducing particulates as measures to correct the former normally resulted in an increase in the
latter. In addition, in reducing NO
x
, the ef ciency of the diesel engine would be impaired, and
the result would be an increase in fuel consumption and CO
2
emissions. However, a technique
called selective catalytic reduction (SCR) has now been developed and adopted by several
engine manufacturers in the EU [175]. This involves injecting an aqueous solution of urea
(CO(NH
2
)
2
) into the exhaust stream where it degrades to carbon dioxide and ammonia (NH
3
).
The NH
3
then reduces the NO
x
to nitrogen (N
2
) and water on a tungsten/vanadium catalyst. It
is obviously important to avoid NH
3
being released into the atmosphere, and another catalyst
is required to oxidize any residual NH
3
while avoiding oxidation of the nitrogen.
To date, SCR (low NO
x
but high particulates) has generally been favored over diesel partic-
ulate lters (high NO
x
but low particulates) for reducing emissions by many EU manufactur-
ers. It allows engines to operate more ef ciently—indeed suf ciently so as to more ef ciently
and more than offsets the cost of the urea. However, although it is an effective technique,
there are concerns about its size, weight, guaranteed availability throughout Europe, and its
ef cacy at the lower temperatures encountered in the exhaust of light-duty applications. These
problems have so far prevented its application to passenger cars. The effects on pollution if
urea is not used in a system designed for its use and how the solution would be made available
to the ordinary motorist are currently the subject of further investigation and debate.
3.12 FUELS
• As a result of the concern regarding the direct and indirect impacts of fuel sulfur on
engine emissions, there is pressure to reduce the sulfur content. In the EU, a limit of
50 ppm maximum sulfur in diesel fuel was introduced in 2005, with a further reduction to
10 ppm in 2008 for gasoline engines and in 2009 for diesel engines. At such low levels, it
may be necessary to restore the lubricity of the fuel by additives, and incorporating a small
amount of phosphorus has been shown to be effective [176].
• Considerable concern currently surrounds carbon dioxide emission and its connection with
global warming. Within the EU, the auto producers have reached a voluntary agreement
with the commission to achieve a carbon dioxide emission target of 140 g/km by 2008 with
a further reduction to 130 g/km by 2012. This will encourage a move toward more fuel-
ef cient vehicles, particularly those that are smaller and lighter, and perhaps also to thinner
lubricants requiring even better AW protection.
In the United States, the Senate has ruled that carbon dioxide emission from trucks are a
pollutant rather than a “by-product” of combustion. This is expected to promote reductions
in carbon dioxide levels and hence encourage the use of lower viscosity, more fuel-ef cient
diesel engine oils (Mainwaring, R., Shell Global Solutions, Private Communication, Janu-
ary 2008).
3.13 CONCLUSIONS
Ashless phosphorus-containing additives are available in a wide range of structures and perfor-
mance. Although most are used as AW and EP additives for industrial oils, they can also function as
antioxidants, rust inhibitors, metal passivators, and detergents. In some cases, the multifunctionality
CRC_59645_Ch003.indd 113CRC_59645_Ch003.indd 113 3/19/2009 6:27:02 PM3/19/2009 6:27:02 PM
