Rudnick L. Lubricant Additives: Chemistry and Applications (Присадки, добавки к смазкам)
Подождите немного. Документ загружается.

94 Lubricant Additives: Chemistry and Applications
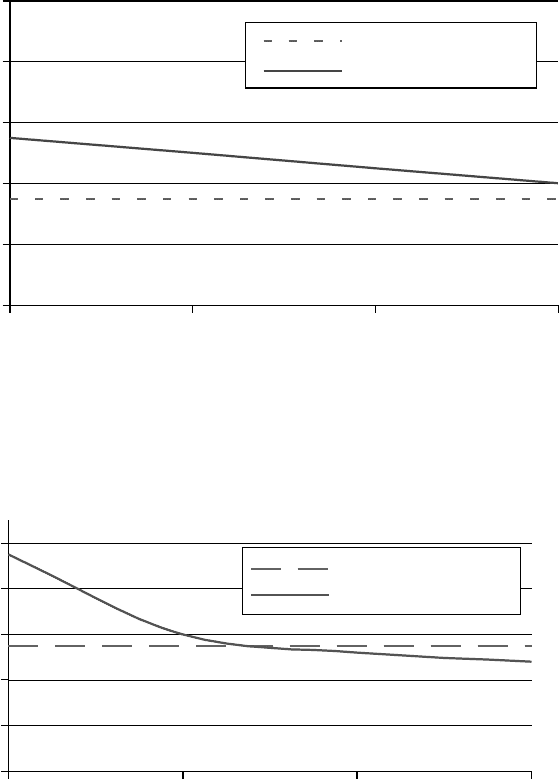
Test conditions: 40 kg, 100 rpm, 121°C, 60 min
Four-ball wear scar diameter (mm)
0
0.2
0.4
0.6
0.8
1
0204060
Ethylene oxide (wt%)
(a)
Test conditions: 100 kg, 100 rpm, 121°C, 60 min
Four-ball wear scar diameter (mm)
0
0.2
0.4
0.6
0.8
1
0204060
Ethylene oxide (wt%)
(b)
Oleyl alcohol 40 kg
Dinonyl phenol 40 kg
Oleyl alcohol 100 kg
Dinonyl phenol 100 kg
FIGURE 3.14 Effect of ethylene oxide content on wear properties 1% of additive in 100 SUS (100°F) naph-
thenic base oil.
conditions. Of greater relevance than conventional four-ball or pin and v-block tests are actual
cutting or tapping torque tests. The results given in Table 3.12 show that (1) products with a hydro-
philic–lipophilic balance (HLB) value of 11–12 give the best results and (2) in general, the further
the value deviates from this, the worse the results become. Unfortunately, no studies appear to have
CRC_59645_Ch003.indd 94CRC_59645_Ch003.indd 94 3/19/2009 6:26:57 PM3/19/2009 6:26:57 PM

Ashless Phosphorus-Containing Lubricating Oil Additives 95
been made on the nature of surface lm deposited on the metal, but the adsorption mechanism
indicated previously is probably still valid.
3.5.3.3 Amine Salts of Acid Phosphates
One amine phosphate that appeared in the patent literature as early as 1934 as a corrosion inhibitor
for aqueous systems (and is still occasionally used) is triethanolamine phosphate [114]. Formed by
the neutralization of phosphoric acid with triethanolamine, this product was widely used as a cor-
rosion inhibitor for automotive antifreeze formulations for many years [115].
In 1970, Forbes and Silver [116] reported on their work investigating the effect of chemical
structure on the load-carrying properties of different phosphorus compounds. In this case, the
structures under review were di-n-butylphosphoramidates, amine salts of di-n-butyl phosphate, and
derivatives of dialkylphosphinic and alkylphosphonic acids. The results indicated that the phos-
phoramidates were more effective load-carrying additives than the neutral phosphates, TBP and
TCP, but less active than the amine salts of di-n-butyl phosphate. The evaluation of the series
of dialkylphosphinic and alkylphosphonic acid esters indicated that the AW performance related
directly to the strength of the acid from which they had been produced (Figure 3.15), suggesting that
adsorption through the polarity of the ester group was an important step in the process.
In addition to the work carried out in hydrocarbon base stocks, some testing was also performed
in a synthetic ester. This uid enabled a comparison to be made of tetra-alkylammonium salts of
dibutylphosphate (otherwise insoluble in mineral oil), which displayed the best AW/EP properties
of all the amine phosphates tested (Table 3.13). The authors suggested that this was probably due to
the stability of the ions.
TABLE 3.12
Phosphate Ester Surfactant Ranking on Steel in a Water-Based System as a Function
of Structure
Alcohol
Phosphate
EO Units
Pin-on-Vee
Block
Rankings of
Sample
Four-Ball
Wear
Tapping
Torque
Total of
Rankings
Overall
Rating
HLB
Number
C9–16 5.5 4 1 4 9 1 13
C18 4 6 4 2 12 2 12
Nonylphenol 4 10 2 1 13 3 11
C13 10 2 9 5 16 4 14
C12 6 1 12 7 20 5 12
C8–10 6 8 7 6 21 6 11.5
C12 12 5 8 12 25 7 15
C13 6 9 6 10 25 8 11.5
C12 9 7 5 14 26 9 14
Nonylphenol 6 14 3 9 26 10 8
Phenol 6 12 13 3 28 11 15.4
Dinonylphenol 5 3 14 11 28 12 9
Nonylphenol 9.5 11 11 8 30 13 13
C13 4 13 10 15 38 14 9.7
butanediol 6 15 15 13 43 15 —
CRC_59645_Ch003.indd 95CRC_59645_Ch003.indd 95 3/19/2009 6:26:57 PM3/19/2009 6:26:57 PM
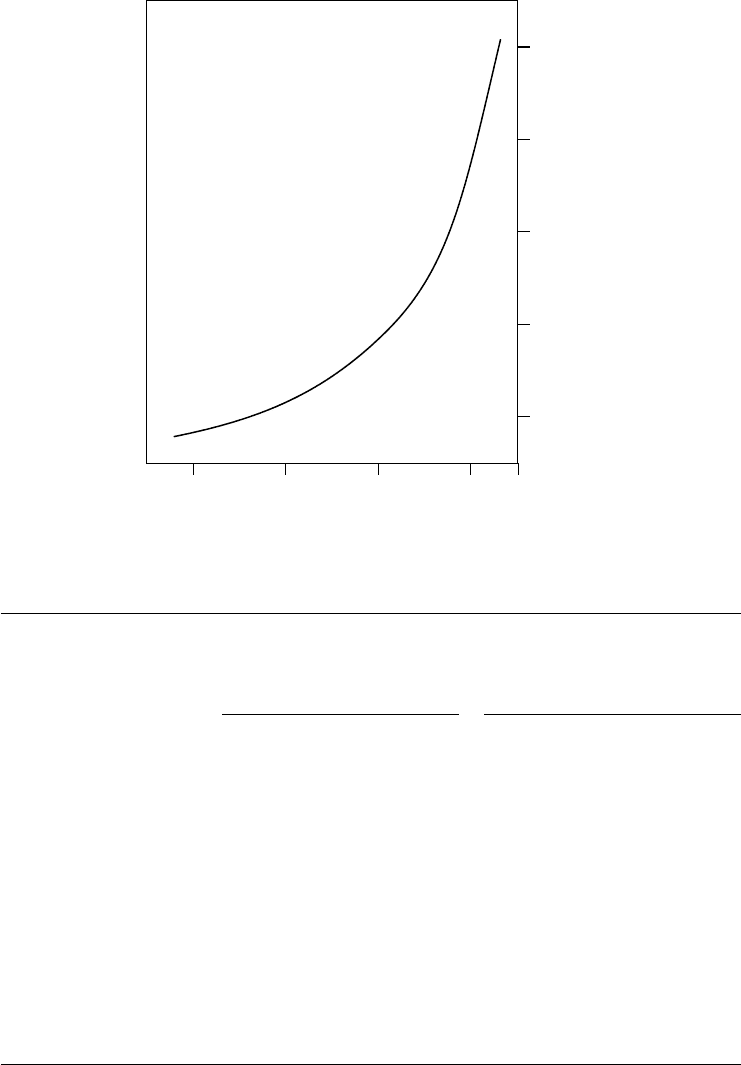
96 Lubricant Additives: Chemistry and Applications
A further study of the mechanism of amine phosphates by Forbes and Upsdell appeared in
1973 [117]. Adsorption/reaction studies of dibutyl and di-2-ethylhexyl phosphates with either n-
octylamine or cyclohexylamine and iron powder showed that both the amine and the acid phosphate
were adsorbed onto the metal surface and that the rate and extent of their adsorption/desorption
varied with chemical structure. The higher the solubility of the iron–phosphate complex formed,
3.5
3.0
2.5
2.0
1.5
pK
A
of acid
Four-ball wear scar diameter (mm)
0.3 0.4 0.5 0.6 0.65
2
5
3
4
6
1
1: n-butyl di-n-octylphosphinate
2: Di-n-butyl hexylphosphonate
3: Di-n-butyl phenylphosphonate
4: Tri-n-butyl phosphate
5: Diethyl benzyl phosphonate
6: Diethyl o-nitrophenylphosphonate
x
x
x
x
x
x
FIGURE 3.15 Effect of acid strength on AW performance. (From Forbes, E.S., Silver, H.B., J. Inst. Pet.
56(548), 90–98, 1970. With permission.)
TABLE 3.13
Four-Ball Test Results of Various Amine Dibutyl Phosphates in Diisooctyl Sebacate
EP Test AW Test
(BuO)
2
PO
2
NR
1
R
2
R
3
R
4
MHL (kg) WL (kg) ISL (kg) 30 min
WSD (mm)
after 45 min 60 min
nC
4
H
9
NH
2
40.5 130 125 0.38 0.38 0.36
nC
6
H
11
NH
2
40.0 130 115 0.37 0.38 0.39
PhNH
2
43.6 140 120 0.37 0.38 0.39
[nC
4
H
9
]NH
2
34.8 140 100 0.26 0.27 0.33
[nC
4
H
9
]
3
NH 37.8 150 110 0.37 0.38 0.42
[nC
4
H
9
]
4
N 54.4 150 150 0.25 0.25 0.26
[CH
3
]
2
[n
C
8
H
17
]
2
N 56.1 165 165 0.30 0.35 0.40
None 18.6 120 55 0.57 0.61 0.64
TCP 19.8 110 60 0.31 0.33 0.35
Note: % additive = 4 milliatoms of P/100 g of uid. (MHL, Mean Hertz Load; WL, Weld Load; ISL, Incipient Sei-
zure Load; WSD, Wear Scar Diameter.)
Source: Forbes, E.S., Silver, H.B., J. Inst. Pet., 56(548), 90–98, 1970. With permission.
CRC_59645_Ch003.indd 96CRC_59645_Ch003.indd 96 3/19/2009 6:26:58 PM3/19/2009 6:26:58 PM
Ashless Phosphorus-Containing Lubricating Oil Additives 97
the greater was the likelihood of desorption. Furthermore, good AW performance depended on high
phosphate and amine adsorption and retention of the phosphate moiety on the surface.
The conversion of a dialkyl acid phosphite to an amine phosphate and the use of the mixed
product as a multifunctional AW/EP additive, antioxidant, and corrosion inhibitor with improved
metal passivation properties were claimed in 1997 [118].
An amine salt and TCP were studied as AW agents for different synthetic esters by Weller and
Perez [119] and compared with a sulfurized hydrocarbon. The neutral ester (TCP) generally showed
an increasing amount of wear up to 1% addition before reducing at higher levels. The amine salt,
however, rapidly reduced wear to very low levels. Friction coef cients were also consistently lower
with the amine salt.
Kristen [120] reported the effect of additive interaction between amine phosphates and a phos-
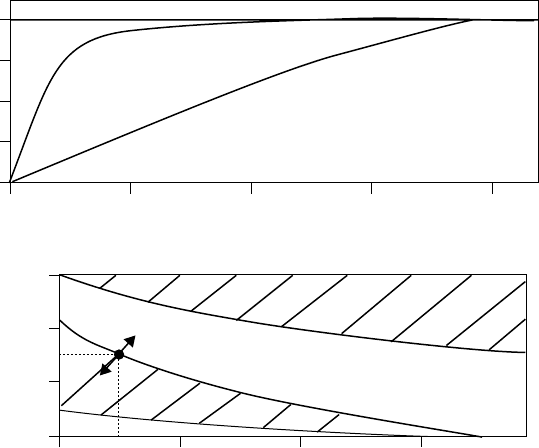
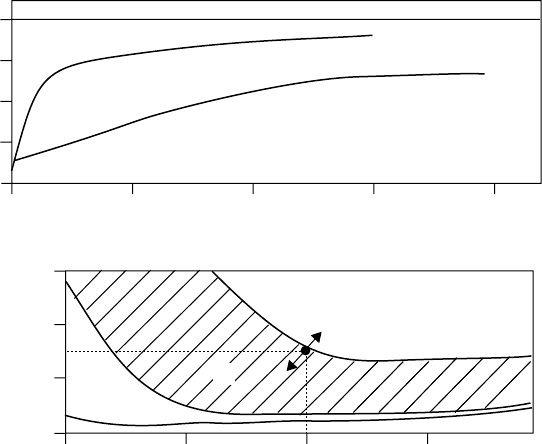
phorothionate. The additives were evaluated under FZG gear test conditions (DIN 51354). The
results showed that the additives respond differently in nonpolar and polar base stocks, speci cally
a polyalphaole n and a synthetic ester (Figures 3.16 and 3.17). In the synthetic hydrocarbon base, a
level of 0.75% amine phosphate and 0.25% phosphorothionate (or perhaps 0.5% of each) provided a
borderline FZG 12 load stage pass/fail. In comparison, 0.75% of amine phosphate ester and 1% of
phosphorothionate were required to achieve the same level of performance in the ester. Monitoring
the response of additive combinations reveals not only the most cost-effective mixtures but also any
antagonisms between additives, as were found here in the ester base at higher additive levels. Such
information is invaluable to formulators when trying to meet speci cation requirements and ensur-
ing that the performance level is consistently above the minimum limit.
Amine salts, for example, triethanolamine salts of alkyl and arylpolyethyleneoxy acid phos-
phates, are widely used in metalworking applications. Some of these products are not only
commercially available but are also produced in situ when the pH of the product is adjusted by the
addition of base to ensure the product is alkaline in use. This is to avoid corrosion and minimize
skin irritation.
0.50 1.0 1.5 2.0
0.50 1.0 1.5
Additive concentration (%)
TPPT (%)
12
10
8
6
4
>12
Amine phosphate
TPPT
FZG load stage failure
Amine phosphate (%)
1.0
0.5
0
1.5
11
12 load stage
>12
FIGURE 3.16 FZG performance of an amine phosphate and TPPT separately and in mixtures in an ISO VG
32 polyalphaole n. (From Kristen, U., Additive für Schmierstoffe, Expert Verlag, Renningen-Malmshaim,
German, 1994. With permission.)
CRC_59645_Ch003.indd 97CRC_59645_Ch003.indd 97 3/19/2009 6:26:58 PM3/19/2009 6:26:58 PM
98 Lubricant Additives: Chemistry and Applications
3.5.4 NEUTRAL ALKYL AND ARYL PHOSPHITES
3.5.4.1 Use as Antiwear/Extreme-Pressure Additives
The earliest known reference to the evaluation of phosphites as AW/EP additive is in a 1950 paper
by Davey [75]. As a result of these investigations, which also included a comparison with phosphates
and the effect of incorporating chlorine into the phosphate/phosphite molecule, it was found that
• Phosphites have superior EP properties to the phosphates, and long alkyl chains are more
effective than aryl groups.
• Evaluation of TBP and TXP revealed similar optimum concentrations of between 1 and 2%
as were found in the previous study with TCP [5].
• Polar compounds such as acids or esters improve the lubricating (AW) properties of phos-
phites and phosphates by being strongly adsorbed on to the surface.
• The incorporation of chlorine or sulfur into the molecule (or the addition of small amounts
of free sulfur) improves the EP properties. Chlorine is more effective when part of an alkyl
residue, and when sulfur is added to a P/Cl compound (e.g., a chlorinated phosphite), the EP
properties are further improved.
Following the study by Davey, a number of patents appeared claiming the use of phosphites
in lubricant applications [121–124], but it was not until 1960 that a further detailed study of
the behavior of phosphites, this time by Sanin et al. [125], was published. The study emphasized
the correlation between structure and activity, and the short-chain derivatives were found to be the
most active.
In 1993, Ohmuri and Kawamura [126] carried out fundamental studies into the mechanism of
action of phosphite EP additives. They found that initial adsorption rates of phosphorus- containing
FIGURE 3.17 FZG performance of an amine phosphate and TPPT separately and in mixtures in an ISO VG 22
pentaerythritol ester. (From Kristen, U., Additive für Schmierstoffe, Expert Verlag, 1994. With permission.)
0.50 1.0 1.5 2.0
0.50 1.0 1.5
Additive concentration (%)
TPPT (%)
12
10
8
6
4
>12
Amine phosphate
TPPT
1.0
0.5
0
1.5
Amine phosphate (%)
FZG load stage failure
12 load stage
11
10
CRC_59645_Ch003.indd 98CRC_59645_Ch003.indd 98 3/19/2009 6:26:58 PM3/19/2009 6:26:58 PM
Ashless Phosphorus-Containing Lubricating Oil Additives 99
esters depended largely on the existence of
–
OH and
–
P=O bonds in the structure. The extent of
adsorption was in uenced by the hydrolytic stability of the esters, and this process was found to
occur through reaction with water adsorbed onto the iron surface. Adsorption of the phosphites
varied depending on the degree of esteri cation; triesters were adsorbed after being decom-
posed hydrolytically to monoesters, whereas diesters were adsorbed without hydrolysis. Phos-
phite esters eventually hydrolyzed to inorganic acid regardless of the degree of esteri cation,
followed by its adsorption and conversion to the iron salt. It was suggested the adsorbing and
hydrolyzing properties of the esters depended on the arrangement of the molecules physisorbed
onto the surface.
Evaluation of a range of alkyl phosphites as EP additives in gear oils was reported by Riga and
Rock Pistillo [127]. The most effective products were those with short chains, particularly dibutyl
phosphite, which resulted in a wear layer of >1000 Å and the formation of both iron phosphate and
phosphide. Other phosphites formed only traces of phosphide, and as the chain length increased,
the resulting lm became thinner and contained less phosphorus, possibly due to steric hindrance.
Long-chain (C
12
) alkyl phosphites have also been claimed as AW additives for aluminum rolling oil
[128] and, in fact, are still used in metalworking applications.
In view of the work carried out on the use of phosphates as vapor-phase lubricants, an investiga-
tion into the effect of phosphites on the frictional properties of ceramic-on-ceramic and ceramic-
on-metal surfaces was carried out in 1997 [129]. The phosphites (and other additives evaluated) had
no effect on ceramic-on-ceramic friction; in fact, short-chain phosphites signi cantly increased
friction. When several types of metal were slid against oxide ceramics, the alkyl phosphites were
found to lower the friction for each metal except copper. Apparently, the reaction products between
copper and the phosphite had adhesive properties and increased friction.
The decomposition of trimethylphosphite on a nickel surface was also studied to obtain insight
into the initial steps in the decomposition of phosphates when used as vapor-phase lubricants [130].
The main breakdown path is the cleavage of the –P–O– bond to yield the methoxy species, which
then degrades to CO and H
2
or reacts with the nickel surface. Following heating to 700°K, the sur-
face loses adsorbed species other than phosphorus, which is seen as a simple way for the controlled
deposition of phosphorus onto a metal surface.
3.5.4.2 Use as Antioxidants for Lubricating Oils
In addition to their use as AW/EP additives, neutral (and acid) phosphite esters have long been used
as antioxidants or stabilizer for hydrocarbons. They were originally introduced as stabilizers for
rubber and thermoplastics. Trisnonylphenyl phosphite, for example, was rst used to stabilize sty-
rene-butadiene rubber in the early 1940s; this was shortly followed by patents claiming phosphites
as antioxidants for lubricants [48,122,123,131,132].
Phosphites function as decomposers of hydroperoxide, peroxy, and alkoxy radicals (reactions
3.20 through 3.22) rather than eliminating the hydrocarbyl-free radicals formed in the chain initia-
tion process. They also stabilize lubricants against photodegradation [133].
R OOH (RO) P (RO) P O R OH
1
hydroperoxide
33
1
⫹⫽⫹→
(3.20)
ROO (RO)P RO (RO)P O
1
alkylperoxy
radical
3
1
3
••
→⫹⫹⫽
(3.21)
R O (RO) P R OP(RO) RO
1
alkoxy radical
3
1
2
••
→⫹⫹
(3.22)
CRC_59645_Ch003.indd 99CRC_59645_Ch003.indd 99 3/19/2009 6:26:59 PM3/19/2009 6:26:59 PM
100 Lubricant Additives: Chemistry and Applications
This behavior as secondary antioxidants by destroying the hydroperoxides, etc., formed in the
chain propagation process results in their use in synergistic combination with those antioxidant
types that are active as radical scavengers in the initiation process, for example, the hindered phe-
nols and aromatic amines [134–138].
Phosphites are useful additives because of their multifunctionality. However, although they
are still used as antioxidants in hydrocarbon oils, their relatively poor hydrolytic stability and
the formation of acidic compounds that could affect the surface active properties of the oil have
prompted the introduction of “hindered” phosphites with better hydrolytic stability: for example,
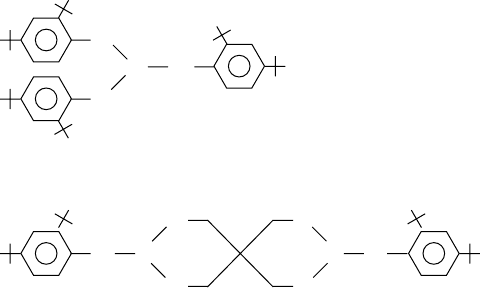
tris-(2,4-ditertiarybutylphenyl) phosphite or tris-(3-hydroxy-4,6-ditertiarybutylphenyl) phosphite,
and, where solubility permits, cyclic phosphites, for example, based on pentaerythritol such as bis-
(2,4-ditertiarybutylphenyl) pentaerythritol diphosphite (Figure 3.18). These types are claimed as
stabilizers or costabilizers for lubricating oils [139–142].
Table 3.14 [141] illustrates the signi cant improvement in oxidation stability shown by such
blends.
In common with most other types of phosphorus-containing products, neutral (and acid) phos-
phites have also been claimed as corrosion inhibitors [143,144].
3.5.5 ALKYL AND ARYL ACID PHOSPHITES
As might be predicted from the behavior of the other types of phosphorus-containing additives, the
acid phosphites have good AW/EP properties; the nonylphenyl acid phosphite is particularly effec-
tive [145,146]. When used in aviation gas turbine lubricants, the acid phosphites were sometimes
formulated in combination with neutral phosphates (TCP); blends of the two products showed syn-
ergy even when the amount of the phosphite was very low [147]. The acid phosphites are also
claimed to be corrosion inhibitors [144] and antioxidants [47,149,150].
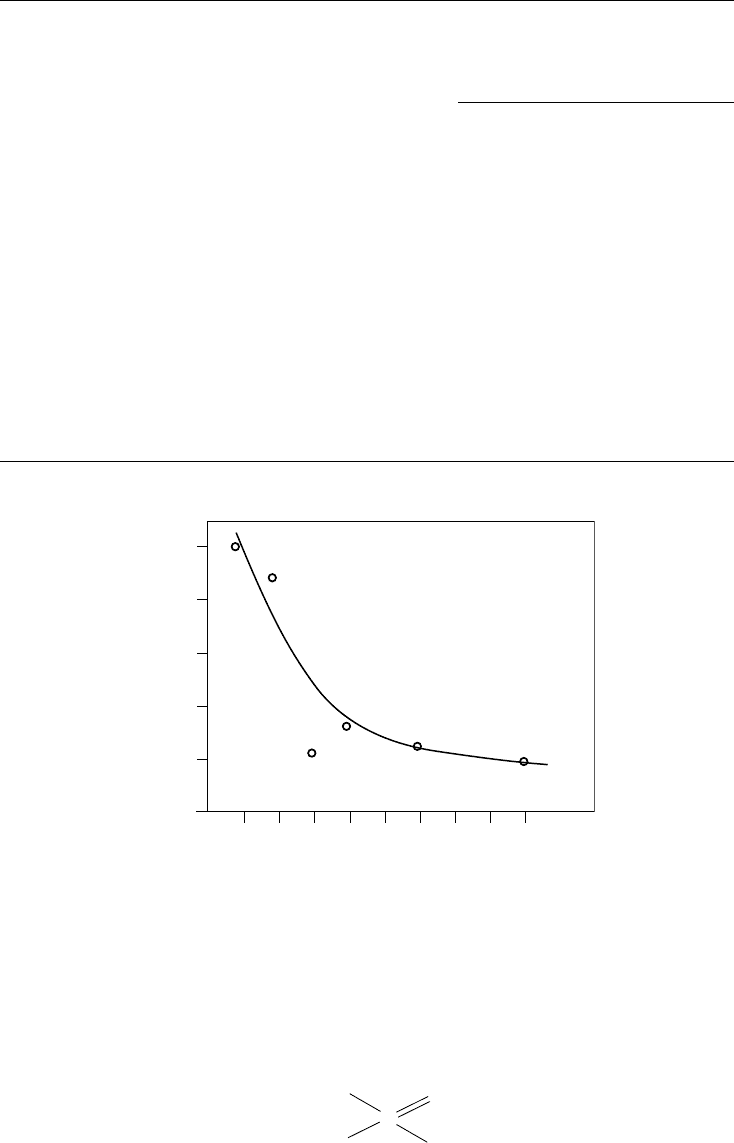
The effects of structure on the AW and load-carrying properties of dialkyl phosphites was
studied by Forbes and Battersby [151] in a liquid paraf n. AW performance was best with long-
chain compounds (Figure 3.19), whereas the short-chain (highest phosphorus content) derivatives
displayed the best load-carrying performance.
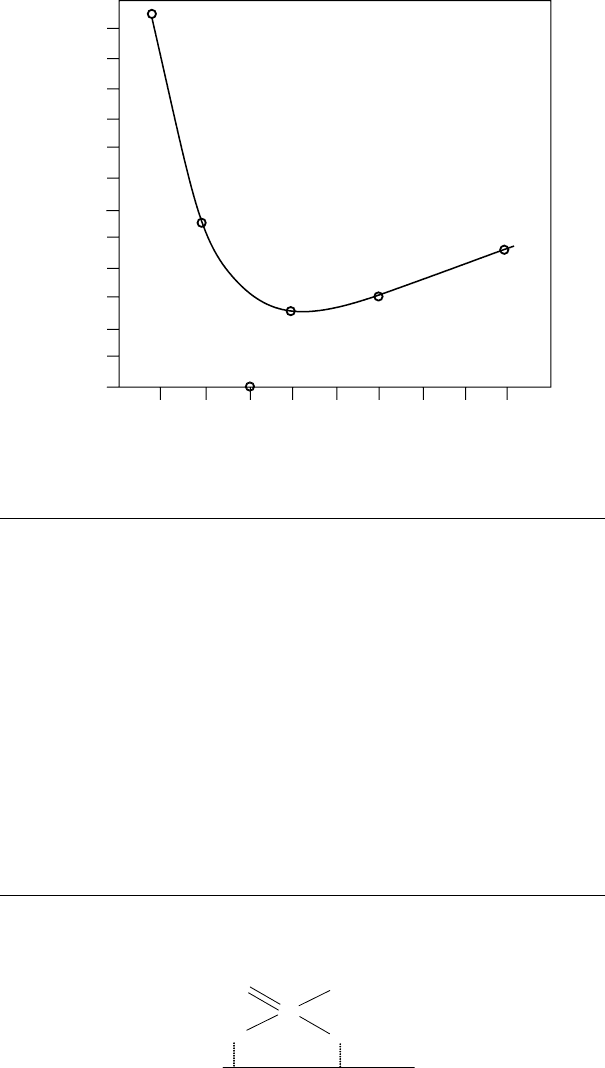
Scuf ng behavior, however, appeared to reach a minimum at about a C
8
carbon chain length
(Figure 3.20). This parallels the behavior of the neutral phosphites. Adsorption studies also showed
that the phosphorus content of the solution was depleted in the same order of the load-carrying
performance, namely, the most active products showed the highest loss from solution. The pres-
ence of water increased the uptake of phosphorus from solution. Comparison of the performance
P
PP
O
O
O
O
O
O
O
O
Tris-(2,4-ditertbutylphenyl) phosphite
Bis-(2,4-ditertbutylphenyl) pentaerythritol diphosphite
O
FIGURE 3.18 Structures of some commonly available hindered phosphites.
CRC_59645_Ch003.indd 100CRC_59645_Ch003.indd 100 3/19/2009 6:26:59 PM3/19/2009 6:26:59 PM
Ashless Phosphorus-Containing Lubricating Oil Additives 101
of the phosphites against the corresponding acid phosphate revealed that the phosphites had better
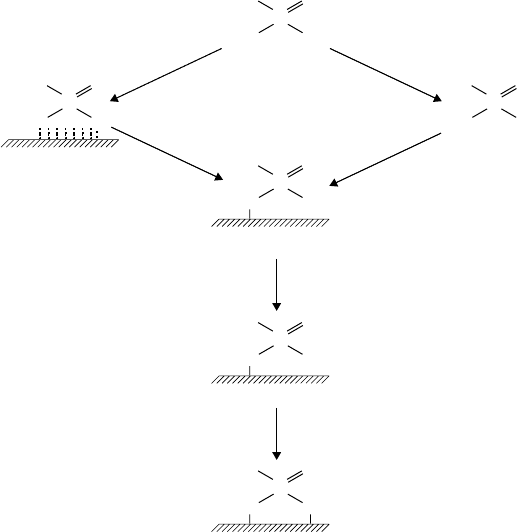
load-carrying but inferior AW behavior (see Table 3.15). The authors suggest that the activity of the
phosphites is due to an initial hydrolysis to produce the following intermediate either in solution or
on the metal surface:
HO O
P
RO
H
This reacts with the iron surface to give an iron salt that was thought to be responsible for the
AW properties of the product. Under much more extreme conditions as are found with scuf ng,
TABLE 3.14
Antioxidant Synergism between Hindered Aryl Phosphites and a Hindered Phenol
Oxidation Stability
Base
Stock Antioxidant
% Viscosity
Change
Total Acid
Number Increase
1 Hindered phenol (0.5%) 357 11.5
Hindered phosphite A (0.5%) 438 12.2
Hindered phenol (0.1%) + phosphite A (0.4%) 8.7 0.01
Hindered phenol (0.17%) + phosphite A (0.33%) 9.4 0.06
2 Hindered phenol (0.5%) 712 14.2
Hindered phosphite B (0.5%) 452 10.6
Hindered phenol (0.1%) + phosphite B (0.4%) 8.1 0.05
Hindered phenol (0.17%) + phosphite B (0.33%) 8.7 0.03
Note: Hindered phenol is tetrakis-(methylene-3,5-ditert-butyl-4-hydroxy hydrocinnamate) methane; Phosphite
A is tri-(2,4-ditert-butylphenyl) phosphite; Phosphite B is bis-(2,4-ditert-butylphenyl) pentaerythritol
diphosphite. Test conditions: IP 48 (modi ed), 200°C for 24 h, air at 15 1/h in an ISO VG 32 mineral oil.
Source: U.S. Patent 4,652,385, Petro-Canada Inc., 1987.
Ethyl
n-Butyl
2-Ethylhexyl
Lauryl
Stearyl
Cyclohexyl
Compounds blended
at 4 mmol/100g
liquid paraffin
2 4 6 8 10 12 14 16 18
0.2
0.3
0.4
0.5
0.6
0.7
Carbon chain length
Wear scar diameter (mm)
FIGURE 3.19 Effect of chain length on the four-ball AW performance of dialkyl phosphites. (From Forbes,
E.S., Battersby, J., ASLE Trans., 17(4), 263–270, 1974. With permission.)
CRC_59645_Ch003.indd 101CRC_59645_Ch003.indd 101 3/19/2009 6:26:59 PM3/19/2009 6:26:59 PM
102 Lubricant Additives: Chemistry and Applications
the aforementioned salt was thought to decompose further to give the phosphorus-rich layer of the
following type:
OH
P
OO
The authors postulated the load-carrying mechanism of phosphites given in Figure 3.21.
3.5.5.1 Amine Salts of Acid Phosphites
In 1975, Barber [152] investigated the four-ball test performance of several long-chain amine salts of
short-chain acid phosphites, which were found to be very active. Unfortunately, he did not investigate
Ethyl
n-butyl
2-Ethylhexyl
Lauryl
Stearyl
Cyclohexyl
Compounds blended
at 4 mmol/100g
liquid paraffin
2 4 6 8 10 12 14 16 18
100
120
140
160
150
130
110
210
200
190
180
170
220
Carbon chain length
Initial seizure load (kg)
FIGURE 3.20 Effect of chain length on the initial seizure loads of dialkyl phosphites. (From Forbes, E.S.,
Battersby, J., ASLE Trans., 17(4), 263–270, 1974. With permission.)
TABLE 3.15
Comparison of the Load-Carrying Properties of Dialkyl Phosphates
and Dialkyl Phosphites at 4 mmol/100 g Base Oil
Initial Seizure
Load (kg)
Wear Scar Diameter
(60 min) mm
Diethyl phosphite 225 0.70
Diethyl phosphate 160 0.43
Dibutyl phosphite 155 0.64
Dibutyl phosphate 85 0.42
Di-2-ethylhexyl phosphite 125 0.36
Di-2-ethylhexyl phosphate 80 0.29
Dilauryl phosphite 130 0.32
Dilauryl phosphate 80 0.35
Source: Forbes, E.S., Battersby, J., ASLE Trans., 17(4), 263–270, 1974. With permission.
CRC_59645_Ch003.indd 102CRC_59645_Ch003.indd 102 3/19/2009 6:27:00 PM3/19/2009 6:27:00 PM
Ashless Phosphorus-Containing Lubricating Oil Additives 103
the effect of increasing the chain length of the phosphite while reducing the length of the amine.
Most of the paper concerns the behavior of a wide range of phosphonate esters (see Section 3.16).
3.5.6 PHOSPHONATE AND PHOSPHINATE ESTERS
A large group of phosphonate esters was prepared by Barber in 1975 [152] and evaluated using the four-
ball machine. Although short-chain esters were more effective in preventing scuf ng, the most effective
products were those containing chlorine. However, even at high levels of chlorine, the performance was
still inferior to the amine phosphite reaction products reported earlier. In comparison with TCP, incipient
seizure loads were generally higher, but the weld loads were broadly similar. Unfortunately, there were
no direct comparative data under wear test conditions. A limited number of phosphinate esters were
evaluated and found (also by four-ball tests) to give similar performance to the phosphonate esters.
The activity of a range of phosphonates was studied by Sanin et al. [153], who concluded that
their effectiveness depended on their structure and the friction regime, but esters containing no
chlorine had “no effect at either low or high load.” A further study, by the same authors, of the
mechanism of activity of phosphonates again suggested the reaction of decomposition products
with iron and the formation of a protective layer. Under severe conditions, this layer is removed,
resulting in a sudden increase in friction followed by seizure or welding. Studies of the reaction of
a dibutyltrichlorophosphonate (Cl
3
CPO(OBu)
2
) indicated that a reaction took place at 405–408K
to give chlorobutane and an iron-containing polymer. At 413K, this polymer decomposed to give
FeCl
3
, which gave additional protection.
Phosphonate (and pyrophosphonate) esters, as their metal or amine salts, have appeared in the
patent literature over many years as AW/EP additives. Amine salts of dinonylphosphonate are, for
example, claimed in aircraft gas turbine lubricants [154], and dimethyltetradecyl phosphonate has
P
RO
RO
O
H
P
RO
O
O
H
P
RO
RO
O
H
P
RO
HO
O
H
Fe
Fe
(a) Hydrolysis
(b) Reaction
Reaction with iron surface
P
HO
O
O
H
Fe
P
H
O
O
Fe
O
Adsorption Hydrolysis in solution
Antiwear region
Antiscuff region
Hydrolysis
FIGURE 3.21 Mechanism of load-carrying action of dialkyl phosphites. (From Forbes, E.S., Battersby, J.,
ASLE Trans., 17(4), 263–270, 1974. With permission.)
CRC_59645_Ch003.indd 103CRC_59645_Ch003.indd 103 3/19/2009 6:27:00 PM3/19/2009 6:27:00 PM
