Popov K.I., Djokic S.S., Grgur B.N. Fundamental Aspects of Electrometallurgy
Подождите немного. Документ загружается.

42
Chapter 3
density. The value of the deposition overpotential is larger than in the case of
cadmium and the crystallisation overpotential is lower, resulting in a
decrease in the zero nucleation zone radius, and hence a considerably larger
nucleation rate. A further decrease in the exchange current density value, as
in the case of Ni, leads to the situation shown in Fig. 3.10. A surface film is
formed, but it is porous, probably due to hydrogen co-deposition.

3. Surface Morphology of Metal Electrodeposit
s
43
Hence, a decrease in the value of the exchange current density of the
deposition process enhances thin surface metal film formation on inert
substrates due to an increase in the nucleation rate and a decrease in the
radius of the zero nucleation zones. As a result of this, a compact surface
metal film is formed with a smaller quantity of electrodeposited metal, and
its coarseness and porosity decrease with a decreasing exchange current
density. On the other hand, at sufficiently negative equilibrium potentials
and low hydrogen overpotential for an inert substrate, decreasing the
exchange current density of the deposition process can produce a porous
deposit due to hydrogen co-deposition.
3.1.5
Deposition from complex salt solutions
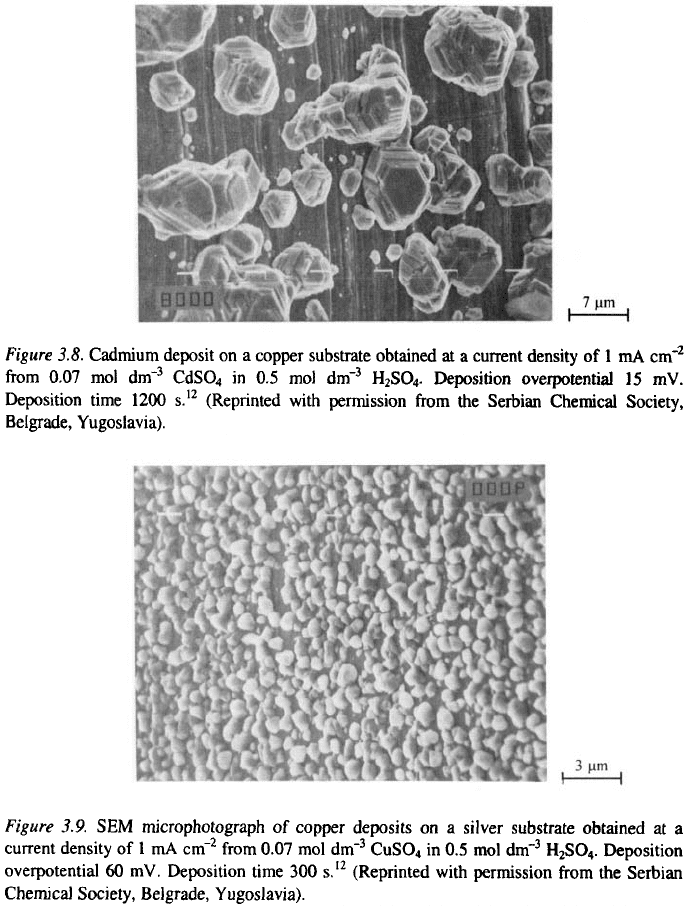
The silver deposits obtained from a nitrate bath at overpotentials
corresponding to an initial current densities of and from the ammonium
complex salt bath at an overpotential corresponding to the current density of
are presented in Fig. 3. 11
11
. The solutions are the same as in section
3.1.2.2. It can be seen that the deposit obtained from the nitrate bath consists
of a small number of nuclei and that boulders are formed even at
which leads to the formation of a non-compact deposit.
On the other hand the deposit obtained from the ammonium complex salt
bath is microcrystalline.
The poor microthrowing power of deposits obtained from nitrate
solutions at smaller current densities can be explained in the following way.
44
Chapter 3
For the deposition overpotential is given by:
according to Eq. 2.41, where:
and, for and from Eqs. 3.11 and 3.12.
Thus, at low current densities, poor microthrowing power is expected. In
the ammonium complex salt solution, For and Eqs. 3.11,
3.12 and 2.37 are valid which means that N > 0. Hence, for deposition at low
current densities, decreasing lead to increasing coverage of an inert
substrate for a given quantity of deposited metal.
3.1.6 Deposition in the presence of adsorbed additives
3.1.6.1
Inorganic compounds
In order to illustrate the effect, silver was deposited onto Ag and Pt
substrates from aqueous solutions containing in 100 g
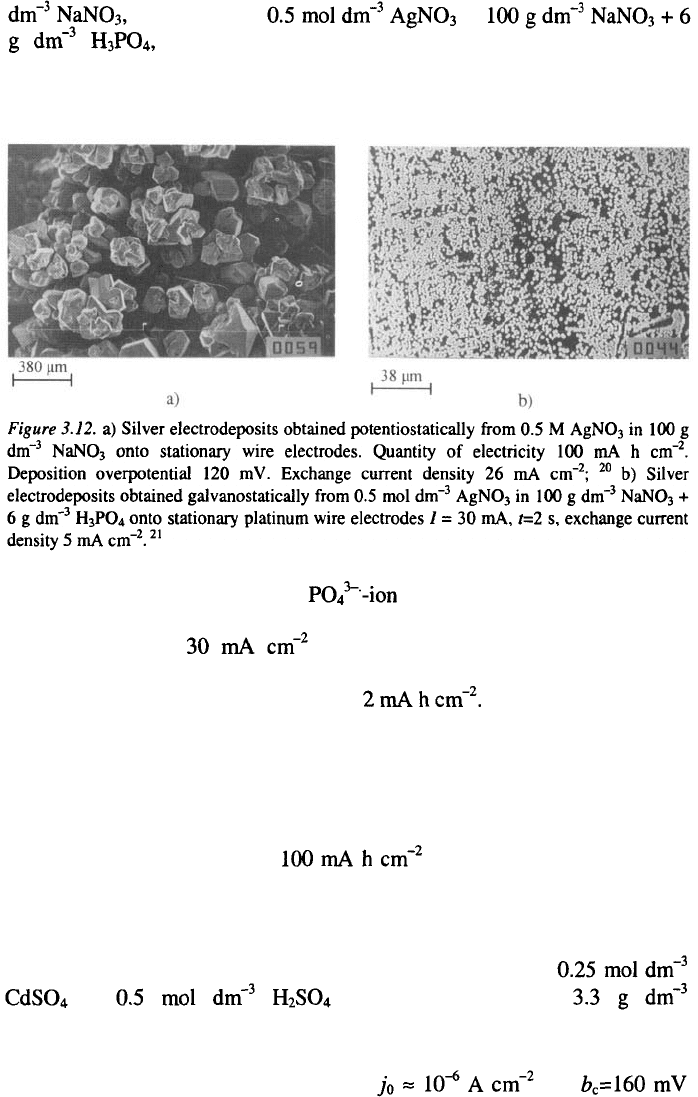
3. Surface Morphology of Metal Electrodeposits
4
5
Fig.
3.12a
and in
Fig 3.12b. Galvanostatic and potentiostatic deposition
conditions were applied in an open type cell
20-22
.
Silver deposits formed from a containing electrolyte on to a Pt
substrate with galvanostatic current pulses are shown in Fig. 3.12b. At a
current density of (i.e. under the optimal film deposition
conditions, as determined in Ref. 20), almost complete surface coverage was
achieved even with a charge quantity of
This is probably due to the possibility of further nucleation occurring
immediately next to the already existing nuclei, as a result of the smaller
values of the radii of the nucleation exclusion zones. Obviously, this is due
to the decrease of the exchange current density for the deposition process.
For comparison, in phosphate-free nitrate solution, a compact Ag film had
not been deposited even after had been passed through the
cell, as can be seen from Fig. 3.12 a.
20
3.1.6.2
Organic compounds
The electrolyte used in all experiments was a solution of
in to which was added
poly(oxyethylene alkylphenol) (9.5 mol ethylene oxide)
23
.
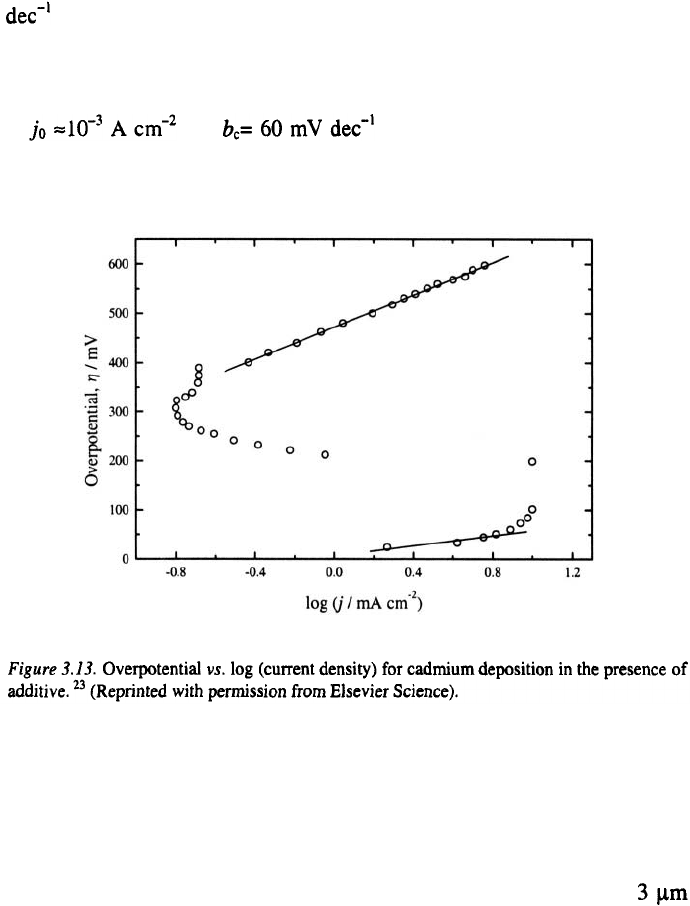
The overpotential-log(current density) plot is given in Fig. 3.13. A
well-defined Tafel line characterised by and
46
Chapter 3
was observed at higher potentials also. This phenomenon is explained
by the formation of a film of the organic additive which completely covers
the cathode at sufficiently negative potentials
24,25
. Tafel linearity was also
observed over a short overpotential range at low overpotentials. The values
of and obtained in this case are close to
the values expected for deposition from a pure solution
26
.
It has recently been shown
27
that the optimum plating overpotential and
current density are determined by the upper limit of the validity of the
Tafel equation for the deposition process (see also section 3.2.1.3.1). In
this case, as can be seen in Fig. 3.13, the optimum deposition
overpotentials are about 530 mV and 40 mV in the presence and the
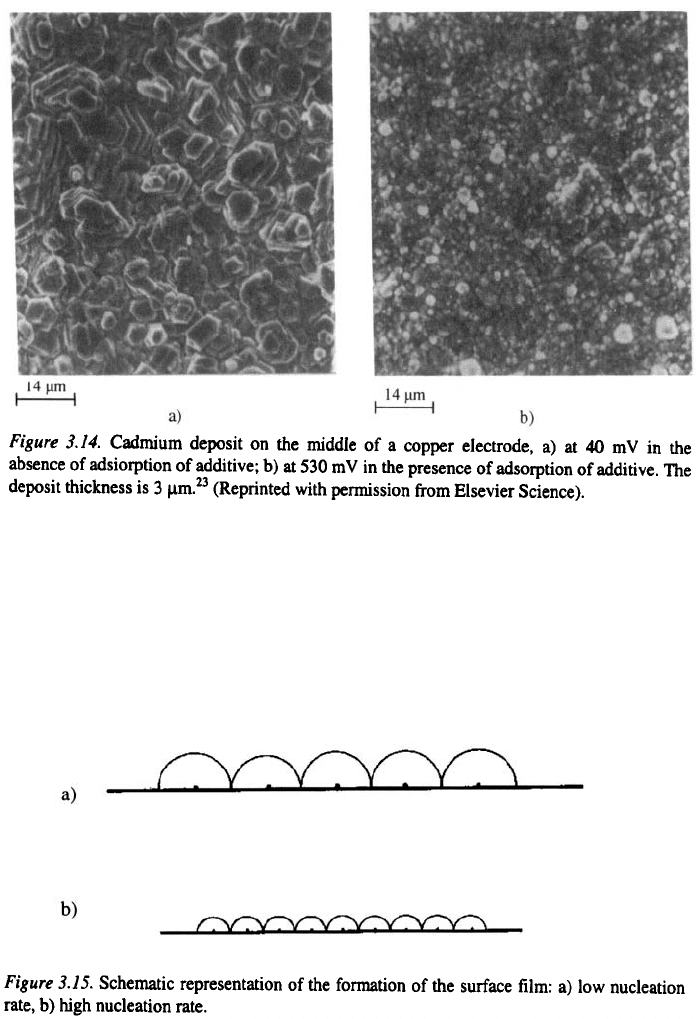
absence of adsorption of the aditive respectively. Cadmium deposits
thick obtained at 40 mV and 530 mV are shown in Fig. 3.14. It can be seen
that the deposits obtained at 40 mV have a large grain size, whereas those
obtained at 530 mV are fine grained, due to the larger overpotential.
3. Surface Morphology of Metal Electrodeposits
47
3.1.7
Conclusions
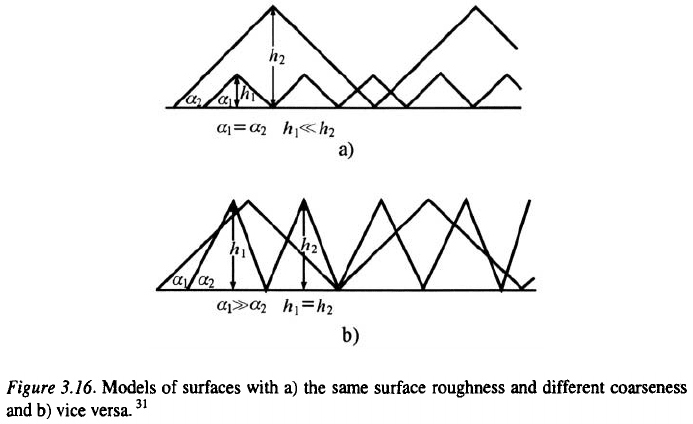
A surface metal film on an inert substrate is formed by the coalescence of
growing grains developed from corresponding nuclei, as is illustrated in Fig.
3.15, whereby the surface properties of the inert substrate are changed to
those of the electrodeposited metal.
48
Chapter 3
It is obvious that the larger nucleus density, the thinner is the thickness of
the metal film required to isolate the substrate from the solution. At the same
time a thinner surface film will be less coarse than a thicker one. This means
that a smoother and thinner surface film will be obtained at larger deposition
overpotentials and nucleation rates, i.e. by electrodeposition processes
characterized by high cathodic Tafel slopes and low exchange current
densities.
It is obvious that the discussion concerning the effect of the value of
exchange current density on the nucleation exclusion zone radius is not
connected to the mechanism of surface film formation but to the mechanism
of nucleation itself and the saturation nucleus number density. Papers
dealing with three-dimensional growth and related phenomena
28
are mainly
concerned with the determination of the mechanism of the formation of a
surface film and are unimportant from the point of view of the estimation of
the deposit thickness required to isolate a substrate from an electrolyte
solution. For this purpose, the saturation nucleus density, or better to say the
distribution of the distances between nearest neighbours is much more
important. It is obvious that half of the largest distance between nearest
neighbours
8,29
(as illustrated by Fig. 3.15) is the radius to which each grain
must grow to produce a nonporous thin metal film. It is clear that the
distribution of the distances between nearest neighbour crystallites is the
most important dependence in the treatment of the thin metal film formation
on an inert substrate. From the corresponding histograms, as shown by
Milchev et al
8,29
, it is possible to estimate the radius of the nucleation
exclusion zones, as well as the maximum distance between nearest
neighbour crystallites, which determines the thickness of a deposit required
to isolate the substrate from the electrolyte solution, as illustrated in Fig.
3.15. If the distance between the nearest neighbour crystallites is smaller
than the grain radius the deposit will overlap resulting in coarse deposit
growth initiation.
Apart from the nucleation density, the preferential orientation of the
nuclei is important in surface metal film formation. As the deposit becomes
free of the influence of the substrate structure on thickening, instead of the
formation of a randomly oriented grain structure, a preferred crystal
orientation can develop, which gives a definite texture to the cross section of
the deposit
30
. Texture can be expressed in terms of degree of orientation of
the grains constituting the deposit.
It is to note a the theoretical approach to the problems of deposit
orientation was successfully developed by Pangarov et al
3-5
. Using this
3. Surface Morphology of Metal Electrodeposits
49
theory, it was possible to determine the preferred orientation as a function of
overpotential from silver single crystals
4
to nickel and iron thin films
3,5
.
3.2 THICK COMPACT METAL
ELECTRODEPOSITS
After formation of a thin surface metal film on an inert substrate further
deposition takes place in the same way as on a massive electrode of the
metal, the ions of which were reduced to form the electrodeposit. The final
thickness of the electrodeposited surface layer varies from the order of ten
micrometers in electroplating, to many times larger in electrowinning and
refining of metals. The surface of deposits obtained in electroplating must be
smooth and bright, in the other cases the surfaces of the deposit surface has
to be as smooth as possible. How this can be achieved will be revealed in
this section.
3.2.1
Coarse surfaces
3.2.1.1
Mathematical models
Any solid metal surface that represents a substrate for metal deposition
possesses a certain roughness. In addition, it may appear coarse or smooth,
and this is not necessarily related to the roughness. Fig. 3.16 shows cases of
surfaces with a) the same roughness and profoundly different coarseness and
b) vice versa.
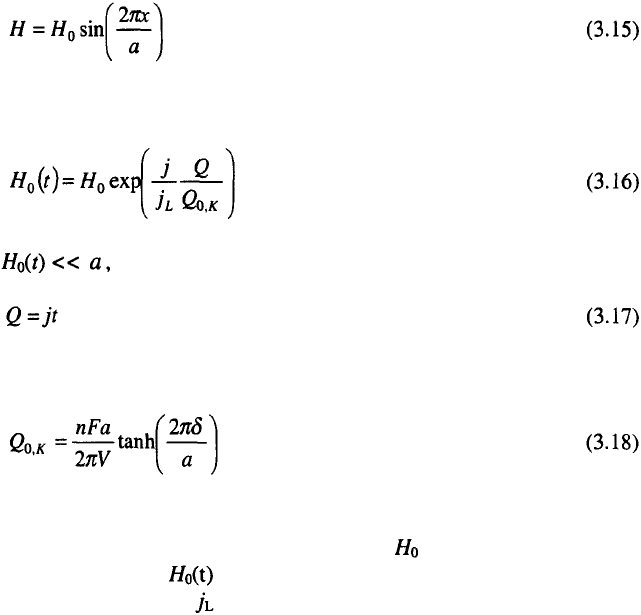
50
Chapter 3
It is the level of coarseness which determines the appearance of metal
deposits, while even with considerable roughness, if below the visual level,
the surface may appear smooth.
It is convenient to define the surface coarseness as the difference in
thickness of the metal at the highest and lowest points above an arbitrary
reference plane facing the solution. In early models used to describe the
surface by periodic functions this is equal to twice the amplitude of the
function
31,32
.
Historically, it was first established that under certain conditions of
dissolution the surface coarseness tends to decrease
33
. Krichmar
34
was the
first to point out that in some cases of deposition, under conditions
somewhat analogous to those in which the coarseness decreases, the opposite
effect occurs; i.e., in prolonged cathodic reduction, under conditions at
which, the process is close to being under complete diffusion control,
amplification of the surface coarseness occurs.
Taking a sinusoidal profile for the electrode surface:
Krichmar
34
obtained the relationship:
for where
and
In the above equations a is the wavelight of the sinusoidal profile, F is
the Faraday constant, H is the local elongation, is the initial amplitude of
the sinusoidal profile, is the amplitude of the sinusoidal profile at time
t, j is the current density, is the limiting diffusion current density, V is the
molar volume of metal, n is the number of electrons, Q is the quantity of
3. Surface Morphology of Metal Electrodeposits
51
electricity, t is the time, x is the co-ordinate normal to the plane of the
electrode and is the thickness of the diffusion layer.
Simpler mathematics were used in another, independently derived theory
of the same phenomenon put forward by et al
35
, and Diggle et al
36
. A
somewhat simplified treatment will be given here.
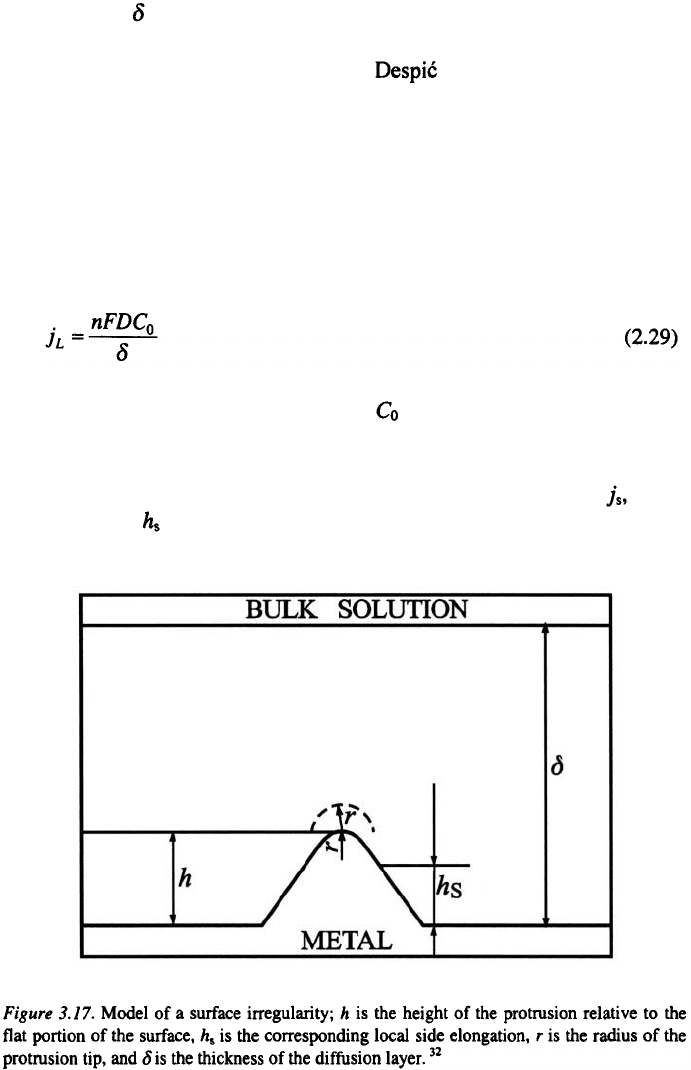
Consider the model of the surface irregularity shown in Fig. 3.17. The
surface irregularity is buried deep in the diffusion layer, which is
characterized by a steady linear diffusion to the flat portion of the surface.
The current densities at the various parts of the surface are as follows.
a) At the flat part of the surface, the limiting diffusion current density is
that for steady-state linear diffusion, i.e.,
where D is the diffusion coefficient and is the bulk concentration of the
depositing ions.
b) At the side of an irregularity, even when a possible lateral diffusion
flux supplying the depositing ions is neglected, the current density, at any
point of height must be larger than the current density, j, at the flat part of
surface.
