Popov K.I., Djokic S.S., Grgur B.N. Fundamental Aspects of Electrometallurgy
Подождите немного. Документ загружается.

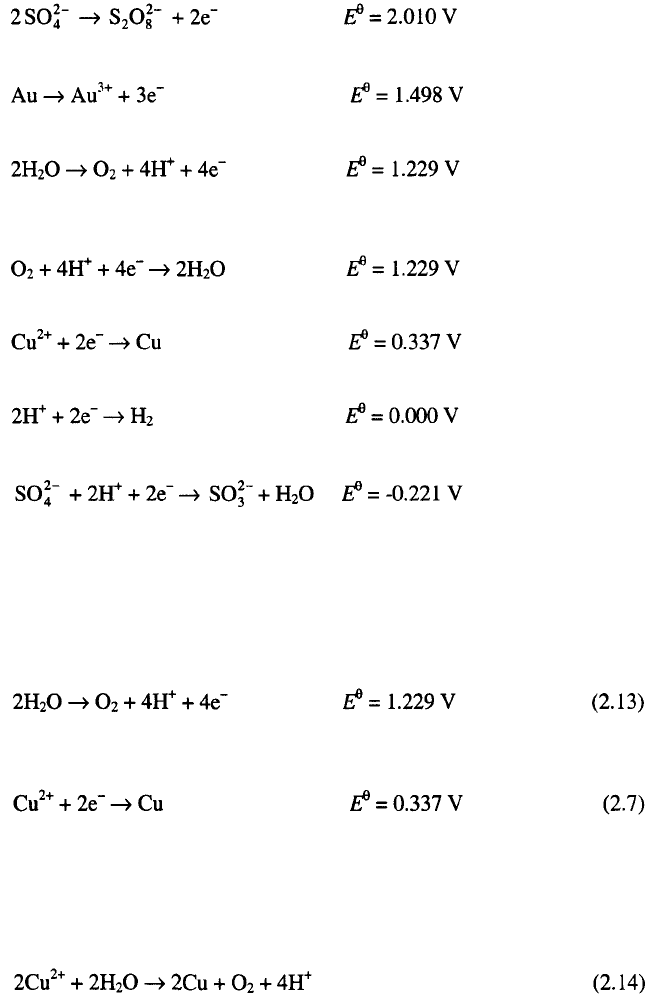
12
Chapter 2
and on the cathode (Cu)
According to the rule derived in the conclusion from section 2.3.1, on the
anode the oxygen evolution reaction will occur, and on the cathode copper
ions will be reduced to the metal phase, because the most positive catodic
reaction can be neglected due to the low oxygen concentration in the
electrolyte solution. Hence the reaction:
will take place at the anode and the reaction
at the cathode.
Obviously, the minimum external cell voltage for electrolysis to occur in
this case is 0.893 V.
The overall reaction in the cell is
It follows from the Eq. 2.14 that in a cell with an insoluble anode the
concentration of depositing ions decreases and the hydrogen ion concentra-
tion increases during electrolysis.
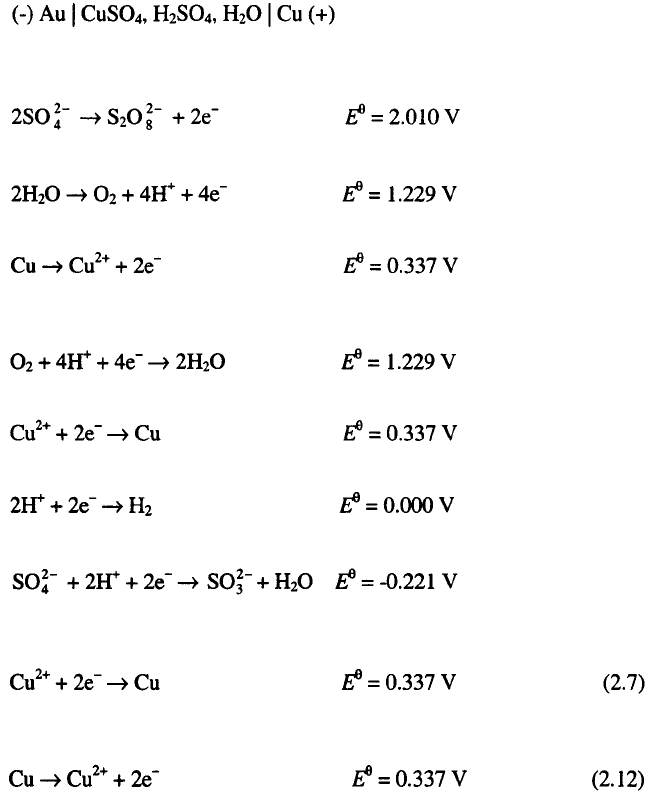
2. Definitions, Principles and Concepts
13
The mechanism of the extraction of metals from ionic solutions and the
essenc
e
of the electrowinning process are well explained by Eq. 2.14.
2.3.3
A cell with a soluble anode
In the cell:
the following reactions are possible on the anode (Cu):
and on the cathode (Au):
Hence, the reaction
occurs on the anode, and
at the cathode, and so the composition of the electrolyte solution remains
constant if the anode is made of pure copper and oxygen is removed from
the solution. The lowest cell voltage at which electrolysis can start in this
cell is zero.
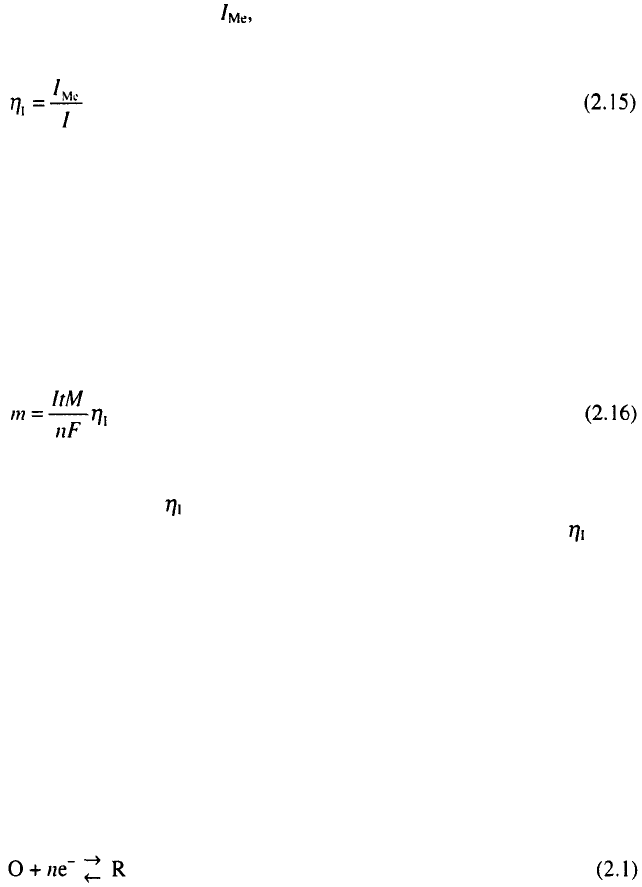
14
Chapter 2
It is obvious that electrorefining processes are based on electrolysis in
cells with soluble anodes.
2.3.4
Current efficiency
Metal deposition can be accompanied by any other cathodic reaction,
most frequently hydrogen evolution.
This leads to the situation in which metal is deposited but metal
deposition uses only a part, of the total current, I, through the cell. The
current efficiency
indicates which part of the total current is used for the deposition of metals.
It is a very important parameter of an electrodeposition process
3
.
2.3.5
Faraday’s law
Faraday’s law relates the quantity of electricity passed through the cell
and the quantity of chemical substances which react on the electrodes. It
states that the mass of metal, m, electrodeposited on the cathode is given by
where / is the total current, t is the deposition time, M is the molar mass of
the deposited metal, is the current efficiency and nF is the number of
Faradays per mole of consumed ions. It follows from Eq. 2.16 that can be
easily determined by measuring the electrodeposited mass of metals and
supplied quantity of electricity
1,2
.
2.3.6
The current density – overpotential relationships
2.3.6.1 Basic equations
The most complete discussion of the current density – overpotential
relationship was given by Bockris
4
. The approach suitable for use in metal
electrodeposition will be given here.
The general form of current density – overpotential relationship in
electrodeposition of metals for the reaction
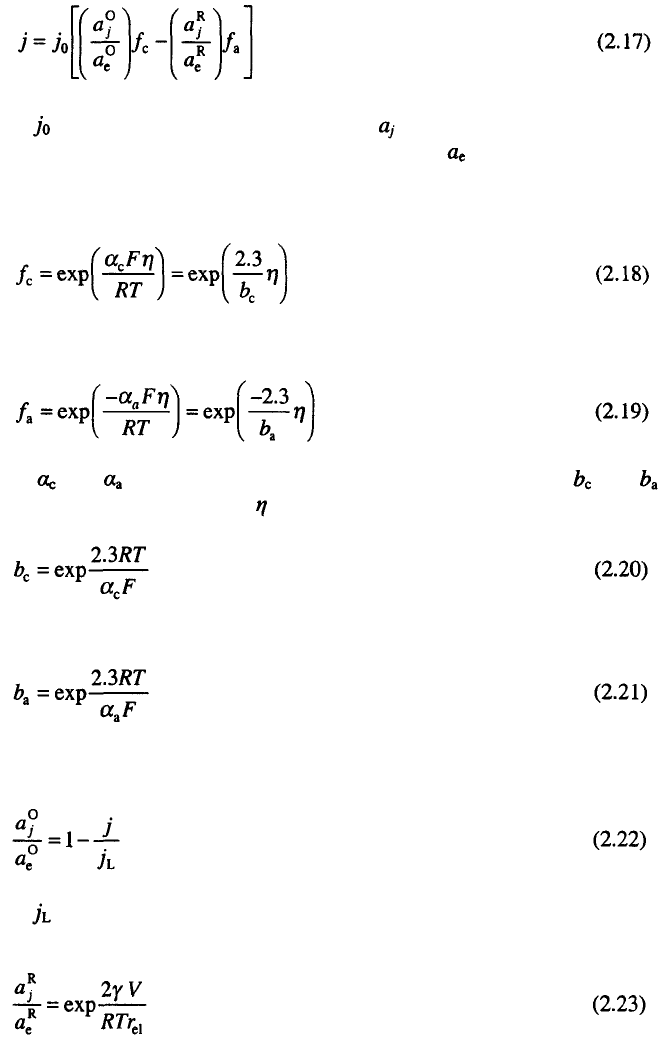
2. Definitions, Principles and Concepts
15
taking cathodic current density and overpotential as positive, is given by
where is the exchange current density and is the activity of the oxidized
(O) or reduced (R) state at a current density j and is the activity in the
equilibrium state.
5
On the other hand
and
where and are the cathodic and anodic transfer coefficient, and
corresponding Tafel slopes and is the overpotential and
and
The ratio of the activities for the cathodic reaction may be written as:
where is the limiting diffusion current density and for the reverse anodic
reaction as:
5
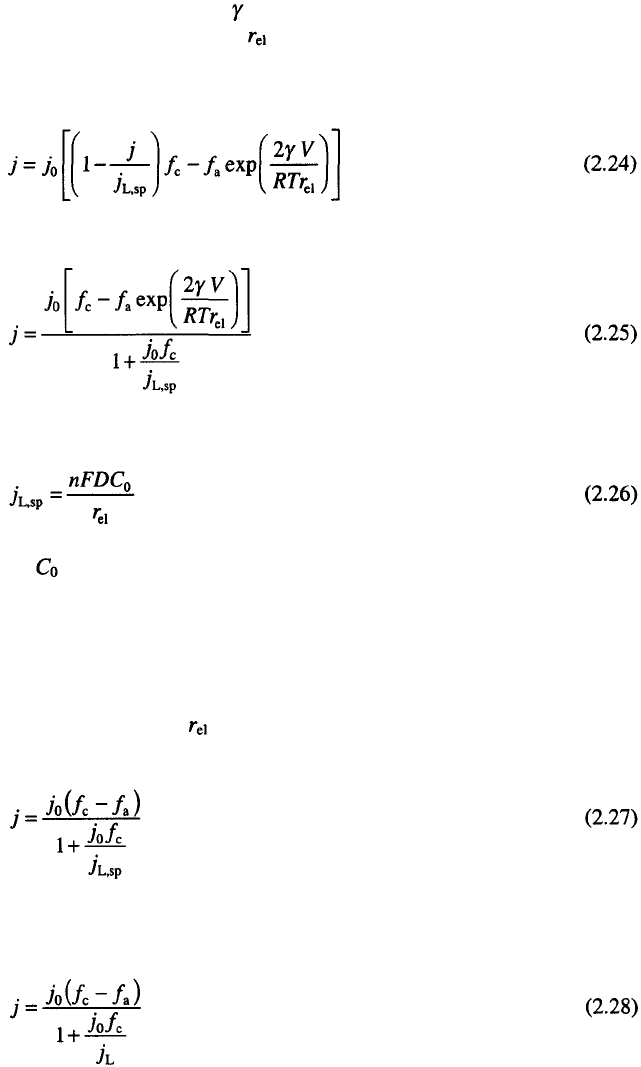
16
Chapter 2
taking into account the Kelvin term which becomes appreciable at low values
of electrode radii
6
. In Eq. 2.23 is the surface energy, V is the molar volume of
the electrodeposited metal and is the radius of the electrode. Eq. 2.23 is
valid for two electron reactions
5
, other possibilities are discussed in Ref. 7.
For a spherical electrode Eq. 2.17 can be written as:
or
where
where is the bulk concentration and D diffusivity coefficient of a
depositing ions.
A somewhat modified Eq 2.25 is necessary for an understanding of
electrodeposition on the tip of dendrites inside the diffusion layer of a
macroelectrode, especially in the case of electrodeposition at a periodically
changing rate
7-9
.
For sufficiently large to make surface energy term negligible Eq. 2.25
can be rewritten in the form:
For a sheet electrodes and sufficiently large spherical electrodes Eq. 2.25
becomes:
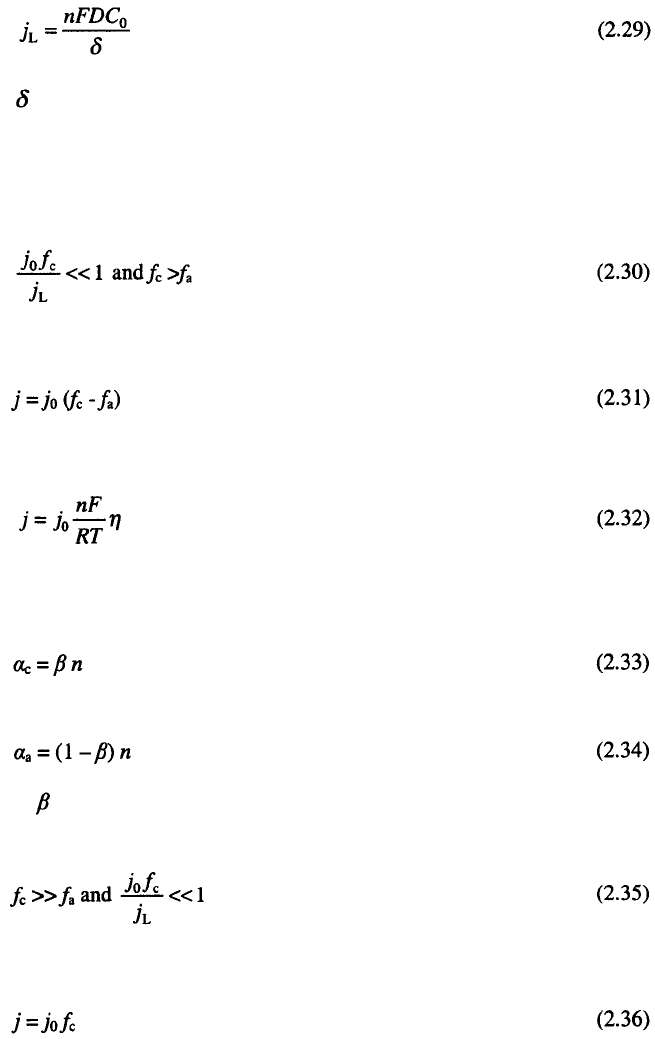
2. Definitions, Principles and Concepts
17
where
and is the diffusion layer thickness
3
.
2.3.6.2 Some approximations
Equation 2.27 is valid generally but it is more convenient to use some
approximative relations derived from them, so for flat surface if
Eq. 2.28 can be rewritten in the form:
which becomes
at very low overpotentials by expanding the exponential terms in Eq. 2.31
and retaining the two terms of expansion of each exponential terms, if
and
where is a the symmetry factor.
When
the relation:
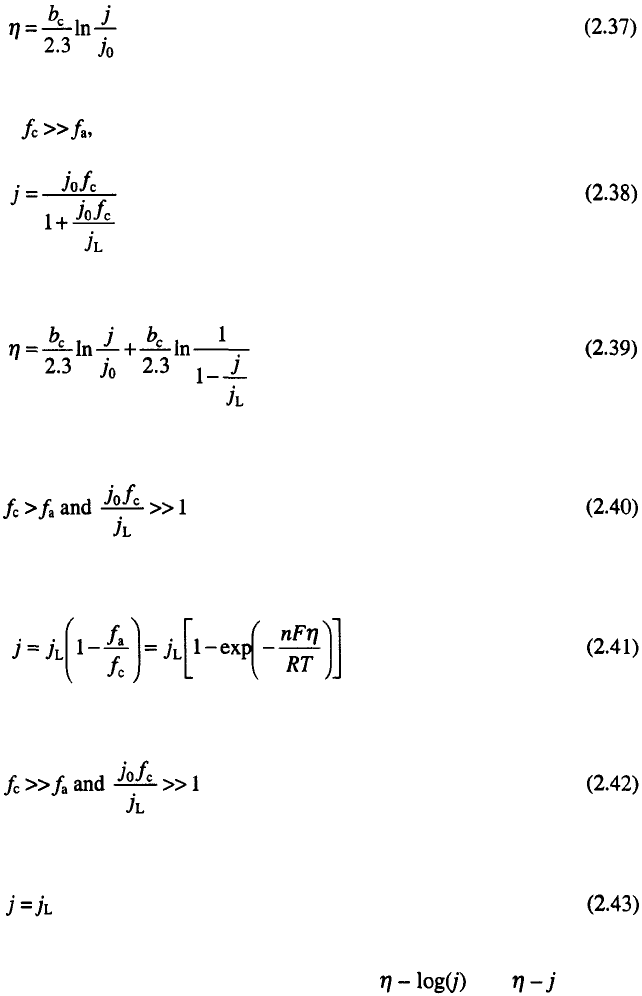
18
Chapter 2
or
is valid.
or
For
Eq. 2.28 can be rewritten in the form:
which is valid if Eqs. 2.33 and 2.34 are valid and finally, if
Eq. 2.39 becomes
The range of validity of Eqs. 2.31 and 2.32, 2.36 and 2.37 and 2.38 and
2.39 and 2.43 can be easily determined from and plots. The
equations 2.36 and 2.37 are valid from the beginning to the end of Tafel
linearity (Tafel line). At lower overpotentials, Eqs. 2.31, and 2.32 are valid
If Eq. 2.28 becomes:
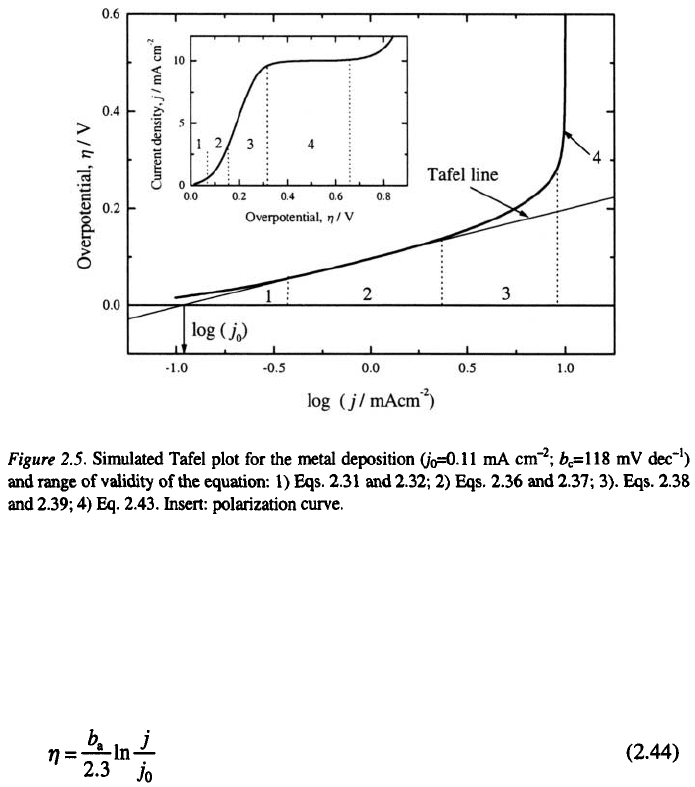
2. Definitions, Principles and Concepts
19
and at higher ones, Eq. 2.38, 2.39 and 2.43. In the Fig. 2.5 the simulated
polarization curve for cathodic metal electrodeposition together with the
Tafel plot and the range of validity of mentioned equations are shown.
Usually, in electrochemistry the marked regions are called: 1 and 2-
activation controlled region, 3- mixed activation-diffusion controlled region,
4- pure diffusion controlled region.
2.3.7
The cell voltage
It is to be noted that the difference of cathodic and anodic overpotentials
is the sum of their absolute values. Hence, if anode overpotential is
considered in the anode Tafel region the absolute values of overpotentrials
and current density should be used, and equation
is valid.
There are not mass transport limitations in anodic dissolution of metals
and there is not equation analoguous to Eq. 2.39.
20 Chapter 2
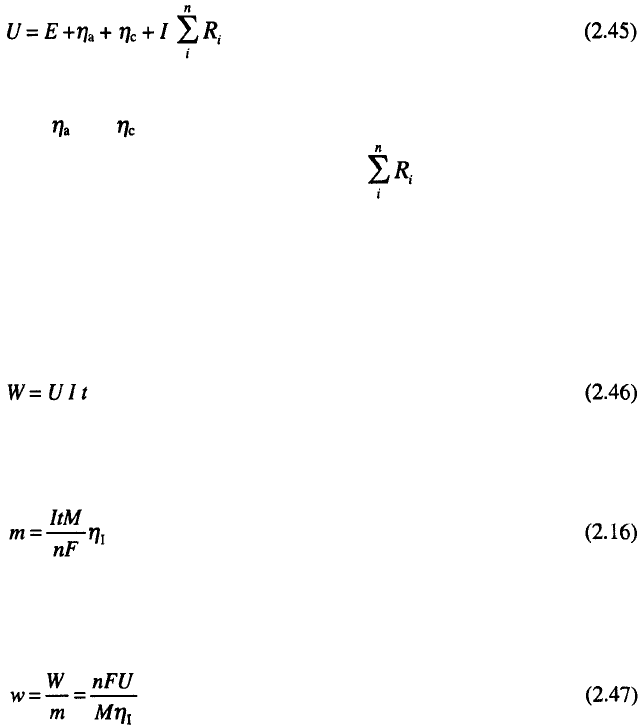
It is now possible to define the cell potential in electrolysis. The cell
voltage U of a driven electrochemical cell is given by:
where E is the equilibrium potential difference between the anode and the
cathode, and are the absolute values of the anodic and cathodic over-
potentials respectively,
I
is the current, and is the sum of the Ohmic
resistance of the electrolyte, electrodes, contacts and connecting wires.
2.3.8
Specific energy consumption
The electrical work W required to deposit a quantity m of metal on the
cathode is given by:
On the other hand, the quantity of deposited metal and the required
quantity of electricity for deposition are related by the equation:
By combining Eq. 2.16 with Eq. 2.46, the specific energy consumption,
w, is then given by:
The specific energy consumption, w, is the most important energetic
parameter in metal electrowinning and electrorefining technologies.
2.4
SOME ASPECTS OF
ELECTROCRYSTALLIZATION
Figure 2.6 shows the originally published in-situ STM images of copper
clusters which were formed during electrodeposition on Au(111) substrate
10
.
The top image shows the gold substrate at a potential positive of the
Nernst potential for bulk copper deposition. This area of the surface is
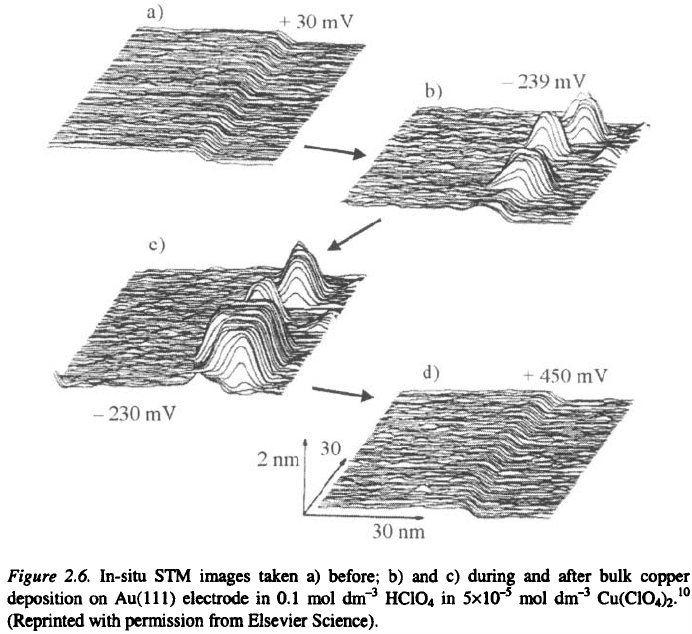
2. Definitions, Principles and Concepts
21
characterised by two atomically smooth terraces separated by a monoatomic
high step edge. Upon stepping the electrode potential to a value negative of
the Nernst potential, distinctive copper clusters form at the step edges. By
contrast, the terraces in Fig 2.6b remain free from copper clusters. The
clusters are seen to grow from Fig. 2.6b to the subsequent image 2.6c. These
STM images are a visual verification of the important role which defects can
play in the initial stages of electrocrystallization. Clearly, they are in good
accordance with the textbook model of Kossel and Stranski
3,10
. Nucleation
occurs preferentially at the step edges, where an ad-atom is more high
coordinated with the surface than an ad-atom on the atomically flat surfaces.
The energetics of copper nucleation on flat gold terraces are clearly less
favorable, occurring only at longer times or higher overpotentials
10
.
Hence, the monoatomic high step edges, the microsteps, are required for
continuos metal electrocrystalization. Possible sources of microsteps on a sur-
face are shown in Figs. 2.7 and 2.8, i.e. the low-index planes, two dimensional
nuclei, emergent screw dislocations and indestructible reentrant groves.
7,11
