Popov K.I., Djokic S.S., Grgur B.N. Fundamental Aspects of Electrometallurgy
Подождите немного. Документ загружается.

292
Chapter 12
12.1 ENVIRONMENTAL CONCERNS IN THE
ELECTROWINNING AND ELECTROREFINING
FROM AQUEOUS SOLUTIONS
Metals produced by the electrolysis from aqueous solutions include Cd,
Cr, Cu, Mn, Co, Ni and Zn. Most of these metals are electrowon from
solutions containing sulfuric acid, although metals such as cobalt and nickel
can also be produced from chloride-type acidic electrolytes. Metals are
deposited on cathodes, while anodic reactions, depending on the anions
present in the electrolyte, usually include oxygen (sulfate solutions) or
chlorine (chloride solutions).
The evolution of oxygen in the case of sulfate electrolytes is
accompanied by lowering of the pH due to sulfuric acid formation.
Consequently, an electrowinning process, which is carried out from a sulfate
solution, results in the generation of sulfuric acid. In the case of
electrowinning from chloride electrolytes, chlorine is generated at the anode
and removed through suitable hoods in order to avoid environmentally
objectionable fuming.
The environmental issues in the electrowinning technologies include the
proper treatment of waste solutions. These solutions contain significant
amounts of sulfuric acid in addition to metal that is electrowon, and traces of
other heavy metals, which may have a negative impact on the environment.
After the electrolysis the sulfuric acid (e.g. processes involving
electrowinning or electrorefining of Cu, Zn or Ni) is usually recovered and
concentrated from residual solutions. This sulfuric acid is reused in the
process. The residual solution, after the electrowinning or electrorefining is
treated in combustion evaporators, and about 70 % of acid is recovered for
recycle. The evaporators perform quite satisfactorily; however,
disadvantages include high-energy and high-maintenance costs. Ion-
exchange resins, in which ions are adsorbed, are also considered, however,
these systems involve large amounts of water and the recovered acid is
therefore weaker than acid in the feed material.
Another process for recovery and concentration of sulfuric acid includes
electrodialysis technology.
1,2
In electrodialysis technology, the ion-exchange
membranes are used for the separation process. The ions selectively
permeate exchange membranes by migrating from one site to the next under
the influence of electrical current. The ion-exchange membranes are
arranged into continuous operation and do not require periodic stripping as
with ion-exchange resins. Membranes must have a high degree of
permselectivity (a selective permeability to a specific type of ion).
Electrodialysis systems with monovalent anionic and cationic permselective
membranes are used for the removal of monovalent ions such as chlorides,

12. Environmental Issues
293
fluorides, sodium, potassium etc. The monovalent permselective membranes
retain divalent ions (e.g. etc.) in the dilute or electrolyte
stream. The results showed a very good recovery of sulfuric acid from an
acidic nickel sulfate stream by electrodialysis, where more than 80 % of the
acid were recovered.
For the removal of heavy metals from residual solutions, chemical,
biological, or electrochemical methods are applied.
3-6
The products or
removal are either returned back in the electrowinning process or sold as
various salts.
12.2
ENVIRONMENTAL CONCERNS IN THE
MOLTEN SALTS ELECTROLYSIS
Environmental concerns in molten salt electrolysis (e.g. electrowinning
of aluminum, magnesium etc.) depend on the metal and production
conditions. In aluminum production pollution problems arise due to cell
design and operation. The following facts are of great concern:
i)
ii)
iii)
Fluoride emission
Hydrocarbon fumes, and
Dusting problems
Sources of fluoride emission include the gaseous compounds generated at
the anodes and melt vapor pressure (i.e., Due to presence of
moisture, fluorides hydrolyze, forming the HF gas. The modern aluminum
plants utilize cells where the gases produced during electrolysis are collected
and adequately scrubbed. Dry scrubbers are used in the treatment to catch
particulates and adsorb HF on alumina, and fed back into the cell.
The hydrocarbon fumes originate from anode baking and they are
generally disposed by burning. The emission of highly polluting gases
during the manufacture of the carbon anodes and cathodes is carefully
controlled. Dusting problems due to alumina handling are usually solved
with hoods and exhaust systems, which collect the dust. The dust is
separated by cyclones or filter bags etc. and recycled to the process.
The spent linings are the largest volume of waste in the production of
aluminum. In order to prevent contamination of the environment, these
waste materials should be properly stored to avoid leaching of toxic
constituents such as cyanides and fluorides. The research is directed toward
recovering valuable components from the waste and destroying the cyanide.
The recovered materials are recommended for use in the manufacture of
cement or mineral wool.

294
Chapter 12
Other toxic constituents in the aluminum electrowinning industry include
polyaromatic hydrocarbons, sulfur dioxide and hydrogen fluoride.
Polyaromatic hydrocarbons are formed in industrial cells during the baking
process.
7
These compounds are known as carcinogenic agents.
In addition to alumina - cryolite melts other molten salts electrolysis
systems represent important health hazard requiring significant safety
precautions. These systems include alkali metals e.g., lithium, sodium and
potassium, magnesium etc. Inappropriate handling of alkali metals can result
in serious injuries such as burns, blindness and even fatalities. The hazards
arise due to the tendency of alkali metals to vigorously react with water, or
to oxidize in air. In the reaction of sodium with water, hydrogen, sodium
hydroxide and significant amount of heat are produced. This combination
results in the explosion when air is present. The common fire extinguishers,
such as water, and should never be used, since all these
compounds react with alkali metals. Pure metallic sodium is usually stored
under organic solvents. In working with sodium or other alkali metals, face
shields, hard hat, hoods and multiple layers of flame-retardant protective
clothing are recommended.
In the plants where production of metals is carried out from molten
chlorides, the main anodic reaction is the chlorine evolution reaction.
Production and utilization of chlorine is considered safer than its
transportation. The chlorine is usually collected and used in different
industrial processes. Major processes utilize chlorine and create hydrogen
chloride as a by-product, which is used in industrial fields. Chlorine is
produced as the main anodic product in the electrolysis of fused It is
either recycled in the process or sold commercially. Chlorine is stored and
transported as a liquefied gas in cylinders under pressure. Exposure to
chlorine causes irritation of the eyes and the mucous membrane of the
respiratory tract. for humans in 30 minutes is estimated at about 840
ppm, while amounts causing severe symptoms in 30 to 60 minutes are
estimated at 40 to 60 ppm.
12.3
ENVIRONMENTAL CONCERNS IN THE
ELECTROPLATING TECHNOLOGIES
In the electroplating technologies safety concerns are directed towards
the handling and exposure to chemicals and solutions of various toxicity and
waste treatment. A typical electroplating technology involves steps, which
are outlined in the block diagram presented in Figure 12.1. As this block
diagram shows, a typical electrodeposition technology includes steps such as
degreasing, pickling, cleaning and electroplating. It is worth noting that
similar types of operations and environmental concerns are involved in the

12. Environmental Issues
295
electroless plating. Some of these steps are repeated several times. and order
of these operations (steps) can be changed, depending on the substrate and
on the metal.
In order to remove residual chemicals on the surface of the substrate
between these steps, rinsing with water is involved. Rinsing minimizes
contamination, however, it increases the volume of waste solutions. The last
rinse, prior to drying is very important since any traces of residues can lead
to staining and even corrosion. This rinsing is carried out with hot deionized
water, and sometimes with methyl/ ethyl alcohol, for faster drying. For
efficient rinsing, spraying with water is very useful, and environmentally
very important since it reduces the volume of waste solutions. Depending on
the metal or alloy being plated, and on the nature of the substrate, operations
in the electroplating technology include different solutions and substances,
which are more, or less toxic. It is generally accepted that most of the
substances used in this technology are dangerous up to various degrees.
The waste solutions in the electroplating technologies are divided into the
following types:
296
Chapter 12
(i)
(ii)
(iii)
(iv)
Solutions containing impurities immiscible with water (e.g. greases,
oils) and organic solvents used,
Acidic or alkaline solutions containing heavy metals,
Solutions containing cyanides and
Solutions containing chromates.
Whenever possible preventive measures should be taken in order to mini-
mize the volume of waste solutions. This is usually achieved with the intro-
duction of additional equipment (e.g., tanks) as well as prolonged time of so-
me operations (e.g., rinsing). Obviously, the preventive measures require
extra investment, although this may lead to a very successful treatment.
Treatment of waste solutions is usually carried out by chemical or physical
methods.
The degreasing solvents (chloroform, methylene chloride,
tetrachlorethylene, carbon tetrachloride etc.) are often inflammable,
however, they are sources of harmful gases. In this operation, oils, separated
from substrates can produce suspended and deposited solids, which may
require the introduction of an additional step (e.g., filtration) in order to
separate them from degreasing solvents. Emulsified oils are separated with
aluminum sulfate, at a pH of about 5 to 6, while solutions are agitated with
bubbled air. The scum formed at the surface is removed, and residual
solution treated with other waste solutions.
Cleaning processes with biological separation are also used in the electro-
plating and surface finishing industry.
8
These processes operate at a low
temperature and low pH and are applied on metals such as Al, Zn, Cu and
Fe. The advantage of the cleaning processes with biological separation is
that metals are not etched. Solutions used in the degreasing process are
slightly alkaline and contain bacteria, which destroys the oil that exists on
the metallic parts. Decomposed oil particles and bacteria are continuously
separated from the cleaning solution as sludge in significantly smaller
volumes than the associated with traditional cleaning methods.
Pickling solutions are acidic and they contain metal ions. The most
commonly used reagents for the pickling process are hydrochloric and
sulfuric acids. Acids containing fluorides are used for a pre-treatment of
silicon containing alloys. These acids produce corrosive fumes and adequate
ventilation is required. In order to protect the environment a proper disposal
of waste pickling solutions is applied in the industry. Acidic waste solutions
used in the pickling or non-cyanide waste acidic solutions used for zinc,
nickel and copper plating are removed into separate tank or in a tank with
alkaline waste solutions. In this step, the neutralization of solutions may take
place, although, with an addition of lime, sodium carbonate or sodium
hydroxide, the heavy metals are precipitated at pH 8.0 to 8.5. The amount of
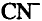
12. Environmental Issues
297
required chemicals is determined on the basis of chemical analysis of metal
content (e.g. Cu, Ni, Fe, Cd, Zn, Cr etc.) in the waste solutions. After the
precipitation, suspensions are filtered to separate the sludge and dispose the
water.
Plating solutions may contain cyanides, chromates, heavy metals (copper,
cadmium, lead, zinc and nickel). Most of these substances are toxic and also
carcinogenic. Among substances used in plating technologies the following
substances are considered as toxic and/or carcinogenic: cadmium, chro-
mium, nickel, lead, mercury, cyanide and their compounds, organic solvents
such as benzene, carbon tetrachloride, chloroform, toluene, xylene etc.
Significant amount of research is funded to investigate proper technologies
for the treatment of waste solutions, or to replace some of processes
involving cyanides, chromates etc., with alternative, environmentally
friendlier technologies. Among these alternatives, cyanide free solutions for
gold, silver or copper electroplating should be mentioned. Also, research
towards replacement hard chromium
9
and cadmium plating is carried out.
Substitutes for hard chromium plating, up to certain levels include Ni-Mo,
Ni-W and Ni-P plating. However these technologies involve substances,
which are on the list of cancerogenic or toxic chemicals. On the other hand,
physico-chemical properties of alternative coatings are frequently not
comparable to those of hard chromium. For the replacement of cadmium
substitutes are being sought in alternatives such as zinc, or Ni-Zn, or Sn-Zn
alloys. However more research is required until cadmium plating will fully
be replaced with other solutions.
Cyanides are well known as true non-cumulative protoplasmic toxic
compounds. They react with enzymes of the blood that regulate oxygen
transfer to cellular tissues. Exposure to cyanide solution causes severe
complications and even death. The toxicity of cyanide solutions is a result of
the free cyanide ion, or HCN. Hydrogen cyanide can enter the body by
inhalation, oral ingestion or skin absorption.
Hydrogen cyanide undergoes an exothermic polymerization (at pH 5 to
11, especially in the presence of water or heat). This reaction can become
explosively violent.
Work with cyanide solutions is carried out in a extremely well ventilated
fume hood, and with special safety equipment, which includes air-masks,
face-masks, rubber gloves, plastic aprons etc..
Cyanide solutions are used in plating of Cu, Zn, Au, Ag etc. Small
amounts of cyanide solutions can be decontaminated in reactions with
sodium hydroxide (pH > 12) and then with ferrous sulfate. The resulting
ferrocyanide is relatively non-toxic.
Disposal of waste cyanide solutions is carried out with chlorine or
sodium hypochlorite. With larger quantities, such as industrial waste cyanide

298
Chapter 12
solutions, treatment is carried out in alkaline media, according to the
following reactions:
The less toxic sodium cyanate, NaCNO, is further destroyed with
sufficient amount of chlorine:
In this process, the pH is maintained above 10 in order to avoid formation
of nitrogen trichloride or cyanogen chloride.
With smaller volumes, sodium hypochlorite is used instead of chlorine.
Other methods for destruction of cyanides include hydrogen peroxide, ozone,
permanganate, biological decomposition
10
, removal by ion exchange and
recovery, lime-sulfur reaction to give sulfate, electrochemical methods
11
etc.
Chromium plating is widely used for different engineering and decorative
applications. However, compounds used in chromium plating (especially
those of Cr(VI)) are recognized as very toxic and cancerogenic. Consequen-
tly, at a working place the regulations require safety glasses, rubber gloves
and plastic aprons.
The treatment of waste solutions from chromium plating is based on a
two-stage process. In the first, the Cr(VI) is reduced to Cr(III) and in second
stage, the Cr(III) is precipitated as hydroxide and disposed as sludge. The
reduction of Cr(VI) is carried out in the acidic medium (pH<3), by adding
sulfuric acid. Several compounds are used as reducing agents of Cr(VI),
species. These reducing agents include sodium bisulfate, sodium thiosulfate,
sulfur dioxide, aluminum powder etc. After completed reduction, the waste
solution containing Cr(III) species is collected for the precipitation. The
precipitation is carried out with hydrated lime or sodium carbonate. The
settlement of the sludge is improved by adding aluminum sulfate, ferric
chloride etc. After the settlement, the sludge is separated (e.g. by decanting)
and disposed. The sludge should not contain oxidizing agents (e.g.
manganese dioxide, hypochlorite, chlorine or some organic oxidizing
substances). These compounds can convert Cr(III) to Cr(VI). Recoveries of
Cr(VI) compounds or as chromium metal are also recommended.
Physico-chemical methods of treatment of residual solution from
electroplating process include evaporation, ion exchange, reverse osmosis,
electrodialysis and electrolytic metal recovery.
The current status shows that in developed nations significant attention is
paid towards researching and finding solutions related to the environmental
concerns in the electrometallurgy field. It is expected that this research and
12. Environmental Issues
299
investment in improvement of safety standards will continue to develop in
the future for the safe and healthy environment in rapidly growing modern
technology. It is certain that electrometallurgy related technologies will
change as new information is uncovered. Adapting to these changes in terms
of pollution prevention can be accomplished in an analytically sound
manner.
12.4 FURTHER READINGS
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
11.
Baltazar V., Harris G.B., White C.W. The Selective Recovery and Concentration of
Sulfuric Acid by Electrodialysis. Hydrometallurgy 1992; 30:463-481.
Nicol M. J. Progress in Electrometallurgy research and Applications. JOM 1993; 45
(4):55-58.
Jütner K., Galla U., Schimeder H., Electrochemical Approaches to Environmental
Problems in the Process Industry. Electrochim. Acta 2000; 45:2575-2594.
Njau K.N., Woude M. vd., Visser G.J., Janssen L.J.J. Electrochemical Removal of
Nickel Ions from Industrial Water, Chem. Eng. Journal, 2000; 79:187-195.
Yu B., Zhang Y., Shukla A., Shukla S.S., Dorris K.L. The Removal of Heavy Metals by
Sawdust Adsorption – Removal of Copper. J. Hazard. Materials 2000; B80:33-42
.
Townsley C.C., Ross I. S., Atkins A.S. “Biorecovery of Metallic Residues from Various
Industrial Effluents Using Filamentous Fungi.” In Fundamental and Applied
Biometallurgy, Lawrence R.W., Branion R.M.R., Ebner H.G., eds., pp.
279 – 289,
Amsterdam: Elsevier, 1986.
Grjotheim K., Krohn C., Malinovsky M., Matiašovsky K., Thonstad J., Aluminium
Electrolysis, Fundamentals of the Hall-Héroult Process, Second Edition, Düsseldorf:
Aluminium Verlag, 1982.
Westerlud M., Clarin L. A Non-Dumping Water-Based Cleaning Process that Does not
Require Rinsing. Plat. Surf. Finish. 1996; 83 (6):32-33.
Wynn P.C., Bishop C.V. Replacing Hexavalent Chromium. Plat. And Surf. Finish. 2001;
88 (2):12-14.
Hofseth C.S., Chapman T.W. Indirect Electrochemical Process at Rotating Disk
Electrode: Catalytic Alkaline Cyanide Oxidation. J. Electrochem. Soc. 1992; 139:2525.
Whitlock J.L., Mudder T.I. “The Homestake water Treatment Process: Biological
Removal of Toxic Parameters from Cyanidation Waste Waters and Bioassay Effluent
Evaluation.”, In Fundamental and Applied Biometallurgy, Lawrence R.W., Branion
R.M.R., Ebner H.G., eds., pp. 327-339, Amsterdam: Elsevier, 1986.
This page intentionally left blank
Index
301
