Popov K.I., Djokic S.S., Grgur B.N. Fundamental Aspects of Electrometallurgy
Подождите немного. Документ загружается.


252
Chapter 10
active surfaces without an external source of electrical current and by the
chemical reduction of metallic ions in an aqueous solution containing a
reducing agent. Autocatalytic deposition is defined as a process for
deposition of metallic films by a controlled chemical reaction that is
catalyzed by the metal or alloy being deposited.
3
If the metal ion, is
reduced by the reducing agent ion, the process can be simply described
by the following reaction:
Although the term electroless deposition broadly describes all processes
of metal and alloy deposition without an external source of electrical current,
it should be noted that this term is commonly used the for autocatalytic
deposition process. Consequently, in this chapter, the term electroless
deposition is used only for the autocatalytic deposition processes.
The development of electroless deposition is mainly connected
with Ni or Cu deposition. However, other electrolessly depositable
metals and/or alloys such as Ag, Au, Co, Sn, AuSn, NiWP, etc. have
also been studied because of their important applications.
10.2 SOLUTIONS FOR ELECTROLESS DEPOSITION
All solutions for electroless metal deposition have many similarities, but
depending on the metal or alloy to be deposited, there are also some
differences. Typically, a solution for electroless metal deposition is consisted
of the following components:
(i)
(ii)
(iii)
(iv)
Source(s) of metal ions
Complexing agent(s)
Reducing agent(s)
Stabilizer(s) and inhibitor(s)
Table 10.2. presents sources of metal ions in electroless deposition of
common metals. Generally speaking, the metal ion sources can be any water-
soluble salts such as sulfates, chlorides, acetates, cyanides, etc. The nature of
the metal ion source is usually determined by the stability of the solution,
properties of the deposited films, and also by environmental issues.
The majority of complexing agents used in electroless metal deposition are
organic acids or their salts, with a few exceptions of inorganic ions such as
or Ammonia and ions, in the case of nickel solutions,
are mainly used for pH control. The choice of the complexing agents is
dependent, first of all, on the nature of the metal ion used for deposition. The
10. Metal Deposition without an External Current
253
principal functions of complexing agents are: buffering action, prevention of
precipitation of hydroxides and salts, and reduction of the concentration of free
(aquo) metal ions. In addition, complexing agents affect the rate of reduction
and the properties of metal deposits. In some cases, complexing agents
apparently form strong complexes with metallic contaminants, thereby making
them less susceptible to react with reducing agents.
Complexing agents used for the electroless deposition of common metals
are listed in Table 10.3. Commercial solutions for nickel electroless
deposition operate in the pH range 4.5 to 6. The complexing agents are most
effective in this pH range. However, in the electroless deposition of Cu, Au,
Ag, Pd and in some cases Ni, solutions with pH > 8 are used.
The choice of reducing agent depends on conditions of electroless
deposition and, of course, on the metal or alloy being deposited, including
their physico-chemical properties. Use of reducing agents containing
phosphorus or boron leads unavoidably to the incorporation of these
elements, which can dramatically affect the properties of the metal deposit.
On the other hand, electroless deposition of pure metals is also possible
using reducing agents such as hydrazine or formaldehyde.
Hypophosphite is mainly used for the electroless deposition of Ni, Co, Pd
and their alloys. The deposits are not purely metallic as they usually contain
phosphorus. Utilization of hypophosphite in electroless metal deposition is
254
Chapter 10
considerably less than 100 %. The reduction reaction takes place only at
certain surfaces such as metals of the group VIII (Fe, Co, Ni, Rh, Pd, and
Pt). It also takes place on Au.
The most studied reaction among electroless processes is definitely
deposition of Ni with hypophosphite. The overall reaction for nickel
deposition with hypophosphite can be represented as:
Generally, if the concentration of hypophosphite is increased, the
phosphorous content in NiP alloy is increased.
Electroless deposition of Cu with hypophosphite is still doubtful. In the
presence of nickel, however, NiCuP alloy films have been deposited
successfully.
4
Boron-containing reducing agents used in electroless Ni deposition are
mainly borohydrides and amine boranes. Deposits usually contain 90 to 99 %
metallic phase, depending on the composition of the solution and operating
conditions. The rest is usually boron and other occluded reacting agents. The-
boron containing reducing agents are used for electroless deposition of
common metals, such as Ni, Co, Pd, Pt, Au, Ag and their alloys.
The electroless deposition of Ni with borohydride takes place in alkaline
solutions. Theoretically, each borohydride ion can reduce four nickel ions:
However, experimental results show that one mole of borohydride
reduces approximately one mole of nickel ion.
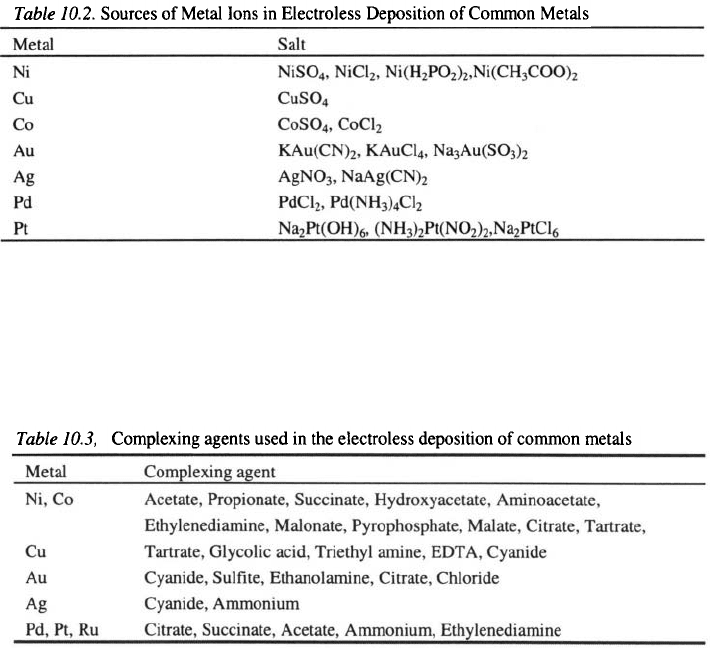
Gorbunova et al. investigated the conditions for electroless nickel-boron
deposition using sodium borohydride as a reducing agent.
5
They found that
an increase in the concentration in solution without a stabilizer or
with stabilizers such as lead chloride, 2-mercaptobenzothiazole or thallium
nitrate, leads to an increase in the rate of Ni-B deposition (Figure 10.1).
Using a solution containing as a stabilizer gives a faster rate of Ni-B
deposition.
Using borohydride as the reducing agent, gold-based alloys (Au-Ag, Au-
In) and metals such as Pt, In and Co were deposited.
5
Whereas borohydrides such as are completely ionic, the amine
boranes are covalent compounds. The electrons in the amine boranes are
displaced toward the boron atom, while the nitrogen atom displaces positive
charge as is illustrated by the following formula:
10. Metal Deposition without an External Current
255
In practice, the application of aminoboranes is limited to dimethylamine
borane, Dimethylamine borane (DMAB) is used for the
electroless deposition of Ni, Cu, Co and Ag. In alkaline and neutral
solutions, the preceding chemical reaction of dimethylamine borane with
ions can be represented as:
The acid-catalyzed hydrolysis of dimethylamine borane occurs according
to the following equation:
Based on experimental results, in the electroless nickel deposition the
molar ratio of nickel ions reduced to DMAB molecules consumed during the
process is approximately 1:1.
256
Chapter 10
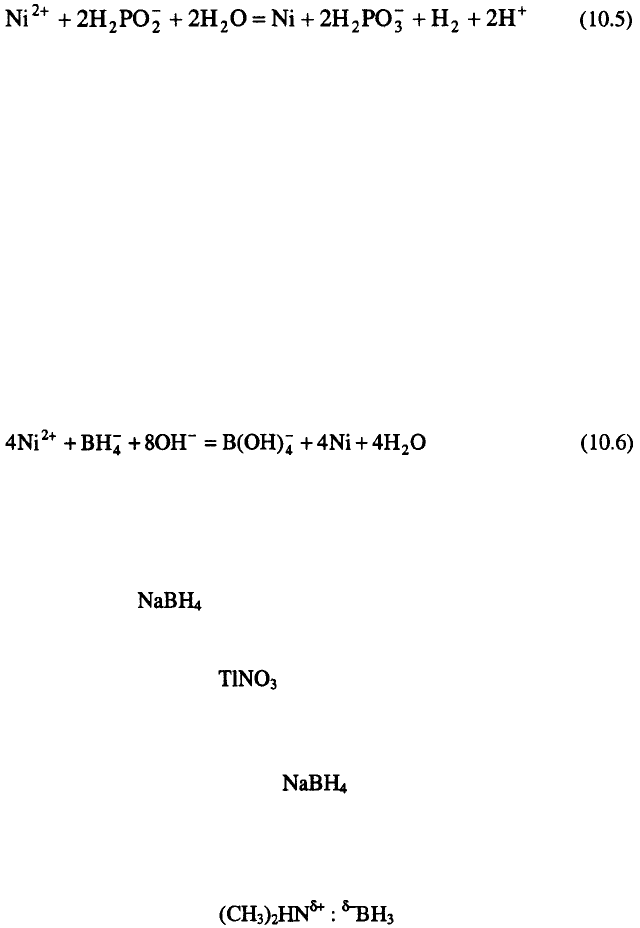
Parlstein and Weightman investigated electroless deposition of Co with
DMAB from acid solutions.
6
Dependence of deposition rate on DMAB
concentration is presented in Figure 10.2. As illustrated in this figure, the
rate of Co deposition increases almost linearly up to DMAB concentration of
about A further increase in DMAB concentration results in a rapid
decomposition of the solution.
Formaldehyde is mainly used for electroless copper and silver deposition;
however, there are reports that this reducing agent can also be used for
electroless deposition of AuCu alloy or Co.
An overall reaction for electroless copper deposition with formaldehyde
is described as follows:
Dumesic et al. studied electroless copper deposition from an EDTA
alkaline solution using formaldehyde as a reducing agent.
7
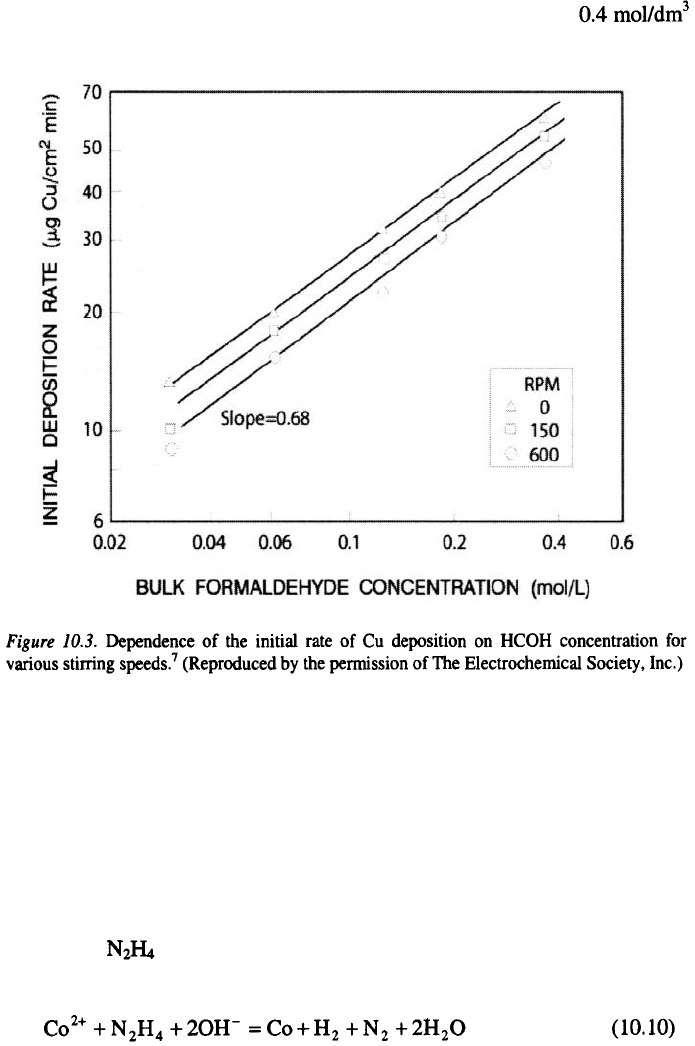
They reported
10. Meted Deposition without an External Current
257
that an increase in the formaldehyde concentration from 0.03 to
leads to a linear increase in the initial deposition rate (Figure 10.3.).
Electroless Ag deposition with formaldehyde is fast, but either a cloudy
film of silver metal is obtained or peeling occurs. From other metals, as
mentioned earlier, the electroless Co deposition is carried out using
formaldehyde as a reducing agent.
8
Hydrazine has long been recognized as a very powerful reductant of
metallic ions, and has been used for electroless deposition of metals and
alloys. Examples include electroless Cu, Ni, Co, Au, Ag, Pt-group of metals
and their alloys, NiSnW, NiFe and alloys resembling stainless steel.
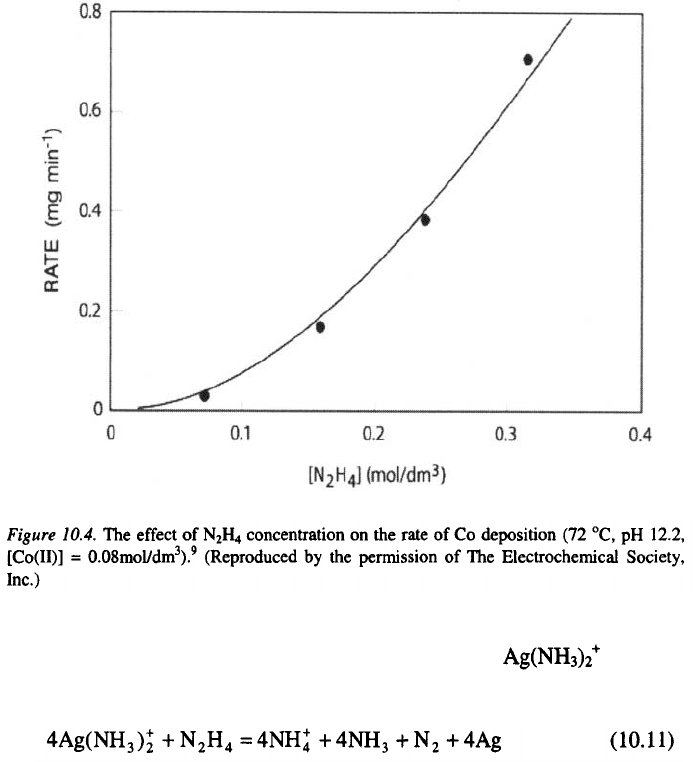
The rate of electroless deposition of Co with hydrazine, increases with an
increase in concentration, which is presented in Figure 10.4.
9
The net
reaction for electroless deposition of Co with hydrazine is described as:
Similarly to other reducing agents, increasing hydrazine concentration
leads to an increase in the rate of electroless deposition.
258
Chapter 10
Hydrazine is often used in the spray method for mirror production as the
deposition rate is fast. The deposition of Ag from an complex
solution can be described with the following reaction:
Stabilizers are chemical compounds used in electroless deposition of
metals in order to avoid the decomposition of the solution. Addition of these
compounds to the plating solution assures, under proper conditions,
operations over an extended period of time. Bath decomposition occurs as a
precipitation of metallic particles in the bulk solution. These particles act as
a highly efficient catalyst for further metal reduction because of their large
surface area. The choice of a stabilizer depends on the metal being deposited
and its compatibility with the process.
Stabilizers, used in the electroless deposition of Ni, have been divided
into the following classes:
2
10. Metal Deposition without an External Current
259
I)
II)
III)
IV)
compounds containing elements such as S, Se, Te;
compounds containingoxygen
heavy metals cations and
unsaturated organic acids (maleic, itaconic acid, etc.)
The concentration of stabilizers is very important since it determines the
rate of deposition. An increase in the concentration of stabilizers of classes I
or II above 2 ppm may completely inhibit the deposition reaction. The
concentration of class III stabilizers is in the range to
and the concentration of class IV stabilizers is in the range
to
10.3 MECHANISTIC ASPECTS OF ELECTROLESS
DEPOSITION
In spite of relatively intensive study of the electroless deposition of
metals and alloys, there is still some disagreement in the treatment of
mechanistic aspects of these processes. In order to explain electroless
deposition of metals and alloys, five different mechanisms have been
proposed, as follows:
1.
2.
3.
4.
5.
"atomic hydrogen" mechanism
"hydride ion" mechanism
"pure electrochemical" mechanism
"metal hydroxide" mechanism
"uniform" mechanism
These mechanisms involve various attempts to explain electroless
deposition. However, according to some experimentally observed
characteristics, it is difficult to use any one of these mechanisms for a general
explanation of an electroless deposition process.
10.3.1
The Atomic Hydrogen Mechanism
The atomic hydrogen mechanism was developed for electroless Ni
deposition with hypophosphite. Brenner and Riddell
2
postulated that the
atomic hydrogen reduces ions and acts by heterogenous catalysis at the
catalytic Ni surface. Atomic hydrogen is generated by the reaction of
hypophosphite with water, and is then desorbed at the catalytic surface
according to the equation below:
260
Chapter 10
At the catalytic surface, the adsorbed hydrogen reduces ions:
The atomic hydrogen mechanism fails to explain many of the features of
electroless deposition such as the simultaneous hydrogen evolution reaction.
In this mechanism, deposition of phosphorus and involvement of hydrogen
evolution reactions are explained as side reactions. Furthermore, this scheme
does not explain why the stoichiometric utilization of hypophosphite is
always less than 50 %.
10.3.2 The Hydride Ion Mechanism
In the hydride ion mechanism, the hypophosphite acts as the donor of
hydride ions. The hydride ion is the reducing agent of both and
ions. This mechanism, was modified by Lukes
10
who applied it to both
acidic and alkaline solutions. In acidic solutions, formation of the hydride
ion was described by the reaction:
In alkaline solutions, the formation of Lukes described by the
following reaction:
Two hydride ions from the above reactions can then react with or
one ion with either a hydrogen ion or water, to form Ni metal and
In broad terms, the hydride ion mechanism can then be described by the
following general equations:

10. Metal Deposition without an External Current
261
where RH is formaldehyde, hydrazine or hypophosphite as previously.
From Lukes’ theory arises a question of the reality of a hydride ion
formation having a standard reduction potential of - 2.08 V in a
hypophosphite solution with standard potential of -1.57 V.
11
Both potentials
are reported for pH=0. The change-over from standard conditions to those in
which metals are reduced by hypophosphite does not alter the difference
between these potentials. On the other hand, the existence of hydride ions in
an alkaline medium, even in an intermediate state, appears very unlikely.
10.3.3 The Electrochemical Mechanism
The so-called electrochemical mechanism was first proposed by Brenner
and Riddell
2
and later modified by other researchers. In this mechanism,
electroless deposition is considered to result from mixed anodic and cathodic
reactions. In the case of electroless Ni deposition, the oxidation of
hypophosphite with water generates electrons, and is considered as the
partial anodic process:
with E° =0.50 V.
The electrons formed in the above reaction are utilized in the coupled
cathodic processes for deposition of Ni and P:
with E° = - 0.25 V, and
with E°= - 0.50 V.
According to the electrochemical mechanism, the evolution of hydrogen
gas is a result of the secondary reaction, which follows:
with E°=0.00 V.
