Popov K.I., Djokic S.S., Grgur B.N. Fundamental Aspects of Electrometallurgy
Подождите немного. Документ загружается.

52
Chapter 3
This is because the point is closer to the diffusion-layer boundary; i.e.,
the effective diffusion layer is thinner, and hence the diffusion flux and
resulting current density are larger. Obviously, this is valid if the protrusion
height does not affect the outer limit of the diffusion layer, i.e. if
The limiting diffusion current density, is given as:
c) At the tip of an irregularity, there is an additional reason for an
increased current density. The lateral flux cannot be neglected, and the
situation can be approximated by assuming a spherical diffusion current
density, given by:
where C
*
is the concentration of the diffusing species at a distance r from the
tip, assuming that around the tip a spherical diffusion layer having a
thickness equal to the radius of the protrusion tip is formed
37,38
. If deposition
to the rnacroelectrode is under full diffusion control, the distribution of the
concentration C inside the linear diffusion layer is given by
36
where Hence,
and from of Eqs. 2.29, 3.20, and 3.22
39
, it follows:
The general equation of the polarisation curve for a flat surface is given
by Eq.
2.28:
3.
Surface Morphology of Metal Electrodeposits
53
The current densities and to different points of the electrode surface
can then be obtained by substitution of in Eq. 2.28 by appropriate values
from Eqs. 3.19 and 3.23 for the side and the tip of the protrusion, around
which spherical diffusion layer is formed, respectively. Hence:
and
If a diffusion layer around the tip of a protrusion can not be formed (see
3.2.1,3.2):
The effective rate of growth of the side elevation is equal to the rate of
motion of the side elevation relative to the rate of motion of the flat
surface
31
. Hence:
Substitution of from Eq. 3.24 and j from Eq. 2.28 in Eq. 3.27 and
further rearrangement gives
39
:
54
Chapter 3
if, and or in the integral form:
where is the initial height of the local side elevation just as in the
previous case (Eq. 3.16), Q is given by Eq. 3.17, and:
According to both mechanisms (Eqs. 3.16 and 3.29), an increase in the
surface coarseness can be expected with increasing quantity of deposited
metal for the same deposition current density, as well as with increasing
current density for the same quantity of electrodeposited metal.
In the same way, the propagation rate of the protrusion tip can be
obtained by substituting from Eq. 3.25 and j from Eq. 2.28 into Eq. 3.27,
where and are substituted by and h, on further rearrangement the
following expression is obtained:
It should be noted that Eq. 3.31 is valid only if the radius of the
protrusion tip is sufficiently large to make the surface energy term
negligible
37
.
It is obvious from Eqs. 3.28 and 3.31 that
because and which means that the tip propagation
protrusion will be larger under spherical diffusion control.
3.2.1.2
Physical Simulation
To test the validity of the above equations, and Popov
40,41
carried
out experiments on diffusion-controlled metal electrodeposition on a well-
defined, triangularly shaped surface profile, through a diffusion layer of
3. Surface Morphology of Metal Electrodeposits
55
well-defined thickness
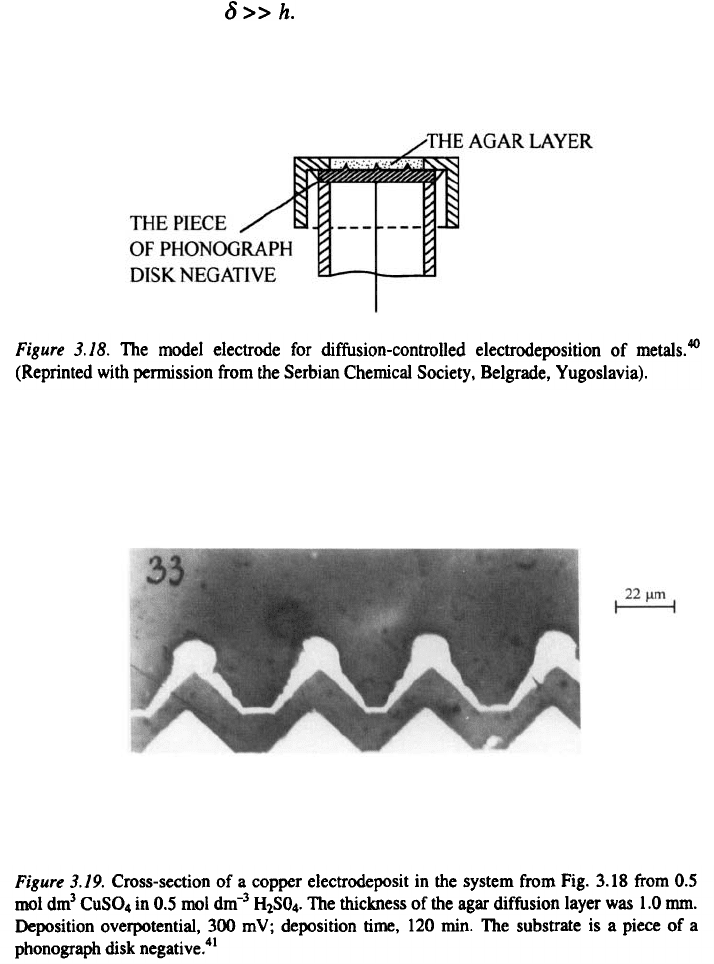
A phonograph disk negative was used as the
substrate upon which a layer of an agar-containing copper sulfate-sulfuric
acid solution was placed and left to solidify, as illustrated in Fig. 3.18.
As current was passed and the layer was depleted of copper ions, an
increase in the height of the triangular ridges was observed. Metallographic
samples were made in wax and the cross sections of the deposit were
photographed under a microscope, Fig. 3.19 was thus obtained
41
.
In accordance with the discussion presented in Section 3.2.1.1, three parts
of the surface can be seen in Fig. 3.19: the flat part of the electrode and the
sides and the tips of irregularities, providing an excellent physical illustration
of the mathematical model.
56
Chapter 3
The effect of deposition time at a given current density, i.e., the effect of
the quantity of electrodeposited metal, on the protrusion height is obvious,
the larger the deposition time, the larger is the surface coarseness
41
.
3.2.1.3
Real Systems
3.2.1.3.1
Optimum current density
for
compact
metal
deposition
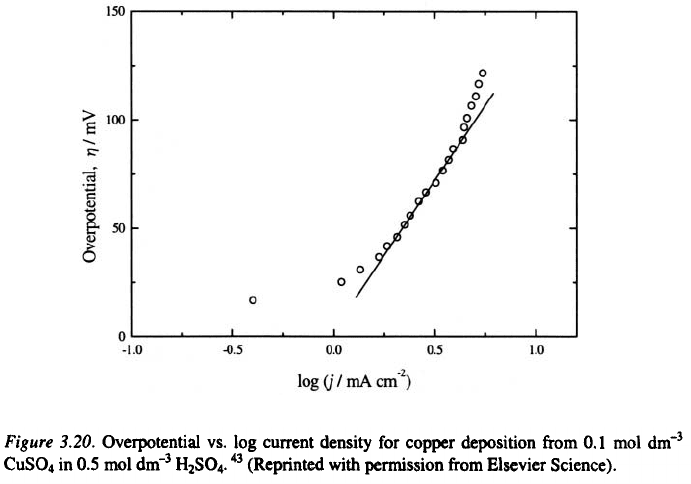
The Tafel plot for copper deposition is given in Fig. 3.20.
The surface coarseness for a fixed quantity of electrodeposited metal in
mixed activation–diffusion controlled deposition increases strongly with
increasing current density
42,43
.
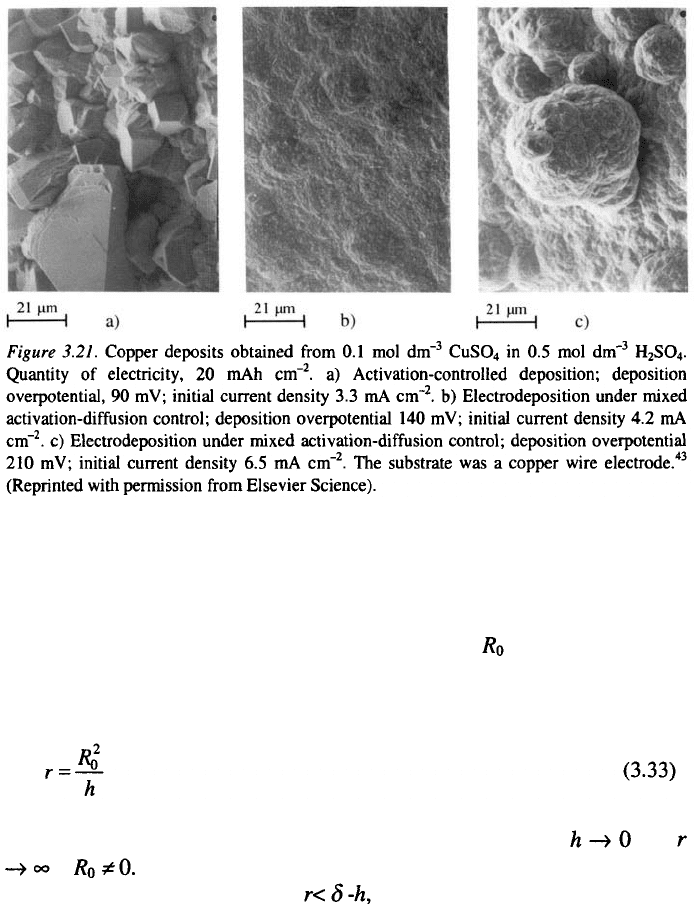
Activation-controlled deposition of copper produces large grains with
relatively well-defined crystal shapes. This can be explained by the fact that
the values of the exchange current densities on different crystal planes are
quite different, whereas the reversible potential is approximately the same
for all planes
44
. This can lead to preferential growth of some crystal planes,
because the rate of deposition depends only on the orientation, which leads,
to the formation of a large-grained rough deposit. However, even at low
degrees of diffusion control, the formation of large, well-defined grains is
not to be expected, because of irregular growth caused by mass-transport
limitations. Hence, the current density which corresponds to the very
beginning of mixed control (a little larger than this at the end of the Tafel
3. Surface Morphology of Metal Electrodeposits
57
linearity) will be the optimum one for compact metal deposition, as follows
from Fig. 3.20.
All the above facts are illustrated in Fig. 3.21
43
.
3.2.1.3.2
Cauliflower like forms
It can be seen from Fig. 3.21c that the surface protrusions are globular
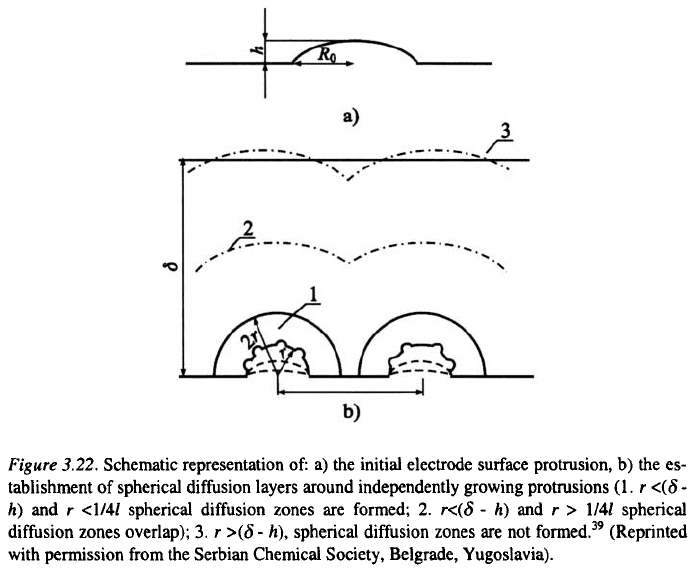
and cauliflower-like. If the initial electrode surface protrusions are ellip-
soidal, they can be characterized by the base radius and the height h as
shown in Fig. 3.22a.
The tip radius is then given by:
The initial electrode surface protrusion is characterized by and
if In this situation, a spherical diffusion layer cannot be formed
around the tip of the protrusion if and linear diffusion control occurs,
leading to an increase in the height of the protrusion relative to the flat sur-
face according to Eq. 3.29. When h increases, r decreases, and spherical
diffusion control can be operative around the whole surface of protrusion, if
58
Chapter 3
it is sufficiently far from the other ones, as illustrated by Fig. 3.22b. In this
situation, second-generation protrusions can grow inside the diffusion layer
of first-generation protrusions in the same way as first-generation
protrusions grow inside the diffusion layer of the macroelectrode, and so on.
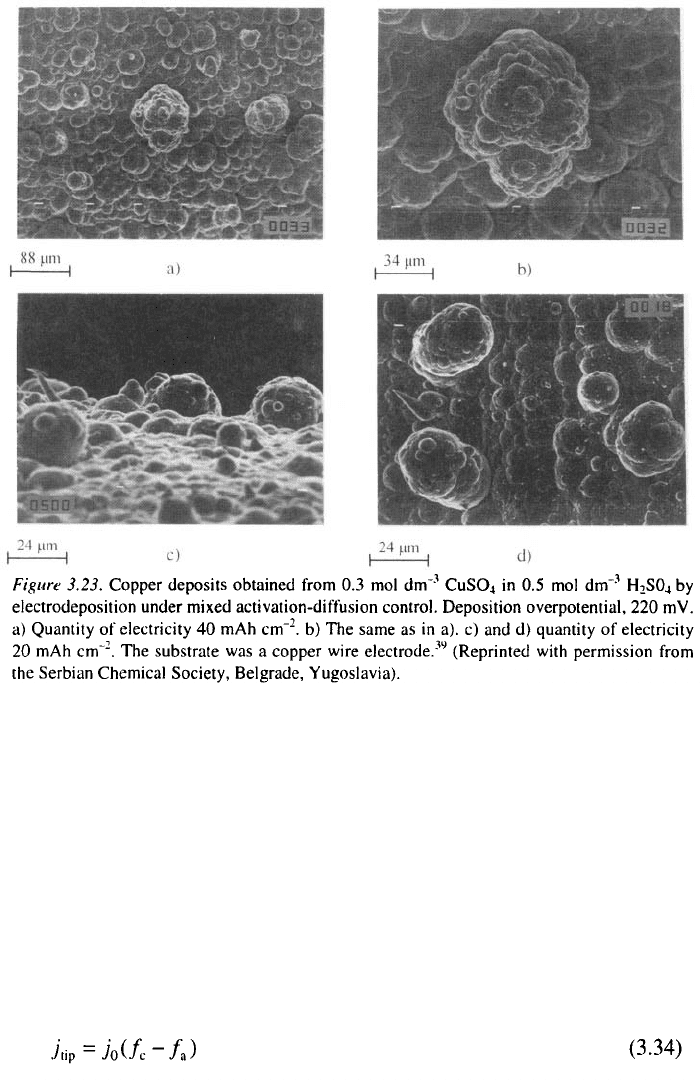
A cauliflower deposit is formed under such conditions, as is shown in
Fig. 3.23. It can be seen from Fig. 3.23a that the distance between the
cauliflower grains is sufficiently large to permit the formation of spherical
diffusion zones around each of them. At the same time, second-generation
protrusions grow in all directions, as shown in Fig. 3.23b and c. This
confirms the assumption that the deposition takes place in a spherically
symmetric fashion.
To a first approximation, the rate of propagation can be taken to be
practically the same in all directions, meaning that the cauliflower-type
deposit formed by spherically symmetric growth inside the diffusion layer of
the macroelectrode will be hemispherical, as is illustrated in Fig. 3.23a-c.
This type of protrusion is much larger than that formed by linearly
symmetric growth inside the diffusion layer of the macroelectrode (Fig.
3.23a-c), as is predicted by Eq. 3.32.
3. Surface Morphology of Metal Electrodeposits
59
This is because a spherical diffusion layer cannot be formed around
closely packed protrusions, their diffusion fields overlap and they grow in
the diffusion layer of the macroelectrode.
3.2.1.3.3
Carrot like forms
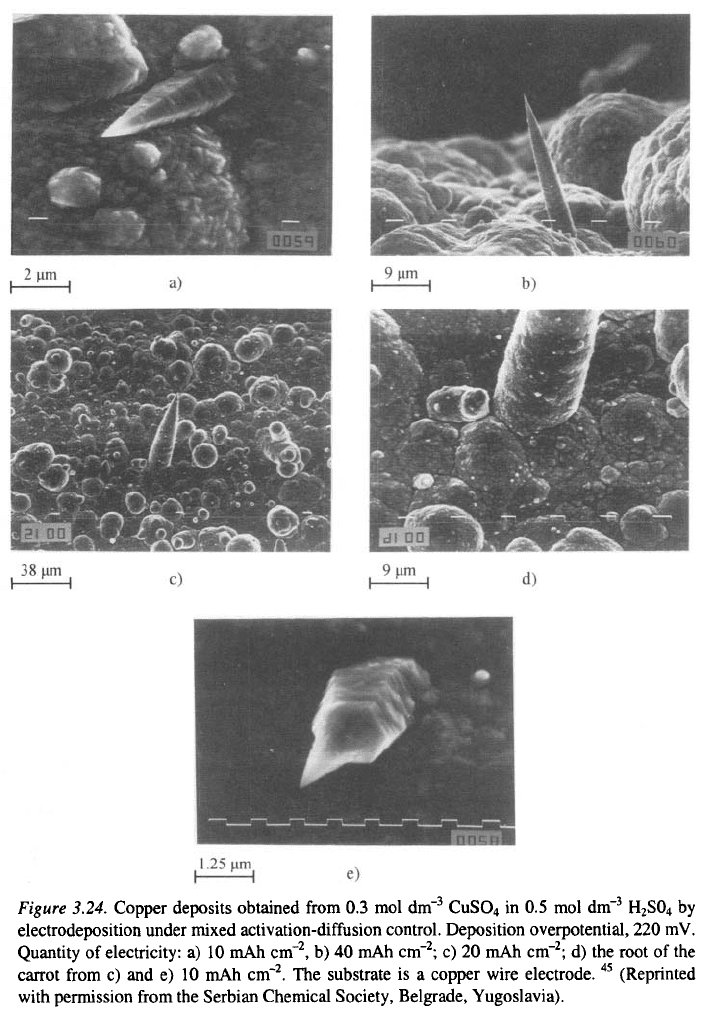
It can also be seen from Fig. 3.23c,d and 3.24 that the growth of some
protrusions produces carrot-like forms, another typical form obtained in
copper deposition under mixed activation-diffusion control. This happens
under the condition r/h << 1, when spherical diffusion control takes place
only around the tip of the protrusion, as is illustrated in Figs. 3.17 and 3.24.
In this case Eq. 3.25, can be rewritten, if the surface energy effect is
neglected, in the form:
60
Chapter 3
meaning that deposition on the protrusion tip can be under pure activation
control at overpotentials lower than the critical one for the initiation of
dendritic growth (see section 3.3.2).
3. Surface Morphology of Metal Electrodeposits
61
This happens if the nuclei have a shape like that in Fig. 3.24a. The
assumption that the protrusion tip grows under activation control is
confirmed by the regular crystallographic shape of the tip
45
just as in the case
of grains growing on the macroclectrode under activation control (see Fig.
3.21a).
The maximum growth rate at a given overpotential corresponds to
activation-controlled deposition. As a result, the propagation rate at the tip
will be many times larger than that in other directions, resulting in
protrusions like that in Fig. 3.24b. The final form of the carrot-like
protrusion is shown in Fig. 3.24c. It can be concluded from the parabolic
shape that such protrusions grow as moving paraboloids in accordance with
the Barton-Bockris theory
37
, the tip radius remaining constant because of the
surface energy effect. It can be concluded from Fig. 3.24d that thickening of
such a protrusion is under mixed activation-diffusion control because the
deposit is seen to be of the same quality as that on the surrounding
macroelectrode surface. It can be seen from the Fig. 3.24e that activation
control takes place only at the very tip of the protrusion.
Some of the new nuclei are precursors of carrot-like protrusions,
depending on their crystal orientation and position relative to the already
growing protrusion
32
. In this case, they are in the form of small hexagonal
pyramids, as shown in Fig. 3.24e. Based on their morphology and because
copper has a face-centred cubic crystal structure, it is reasonable to assume
that they are truncated by a high-Miller-index plane. According to Pangarov
et al
3-5
, the orientation of nuclei is related to the applied overvoltage. It is
reasonable to expect that the appearance of precursors of carrot protrusions
have its own overvoltage range. Obviously, such kind of protrusions can
produce short cuts and deposition of copper must be carried out at
overpotentials lower than this at which carrot like protrusion can be formed.
3.2.2
Smooth surfaces
3.2.2.1
Basic Facts
It is well known
31
that in electrolytes containing specific substances as
additives, a phenomenon opposite to the ones described so far can occur, i.e.
a more rapid metal deposition at recessed points of the surface than at
elevated points. This causes levelling of the surface irregularities as is
illustrated in Fig. 3.25.
The fact that this phenomenon is only observed at microprofiles not
exceeding in amplitude necessitated the introduction of the concept
of “microthrowing power” as a category different from ordinary throwing
