Nof S.Y. Springer Handbook of Automation
Подождите немного. Документ загружается.


Automation and Control in Biomedical Systems 76.2 Theory and Tools 1365
Table 76.1 Summary of theory and tools by Section, topic(s), and advantages/disadvantages of the various methods
Section Topic(s) Pros (+) and cons (–)
76.2.1 A priori identifiability + Highlights parameter estimation concerns based on available data
− Theoretically and computationally challenging
(Weighted) least + Straightforward application, many tools available
squares − Weighting of points is subjective
Maximum likelihood + Robust and commonly employed
− Based on assumed error structure/distribution
76.2.2 Compartmental PK + Compact models, generally identifiable
− No relationship to mechanism or physiology
Population PK + Incorporates intra-/interpatient variability in model
(when justified by available data)
− Not straightforward to tailor to an individual
− Patient and data intensive
76.2.3 Physiological PK + Incorporates patient physiology explicitly
− Measurements of individual tissues are difficult
to collect (limited to animal studies)
− Large number of equations makes parameter estimation
computationally challenging
76.2.4 Biochemical networks + May provide high-resolution mechanistic detail
− May be difficult to resolve causality (cause versus effect)
− Semiquantitative (colorimetric)
76.2.5 Optimal control + Solves constrained dynamic optimization problem
− Formulation (two-point boundary-value problem, continuous
controls) must be reasonable for solution of corresponding
biomedical problem
Model predictive + Robust to mismatch between actual patient/process
control and mathematical model
+ Solves constrained dynamic optimization problem
− Suboptimal (relaxed) solution of problem
− Mathematical analysis of stability and performance
is theoretically challenging
zero mean and normally distributed with variance σ
2
i
.
While these may not be rigorously true of biomedi-
cal data sets, MLE has been shown to work well with
biomedical data, and packages are available that use
MLE for estimation [76.18, 19]. These packages also
return parameter information in the form of a confi-
dence interval or coefficient of variation. Large values
indicate poor parameter accuracy, and hence possible
structural parameter identifiability issues [76.20]orvi-
olations of the assumptions above as parameters may
not be Gaussian distributed in the modeling of multiple
individuals.
Part H 76.2
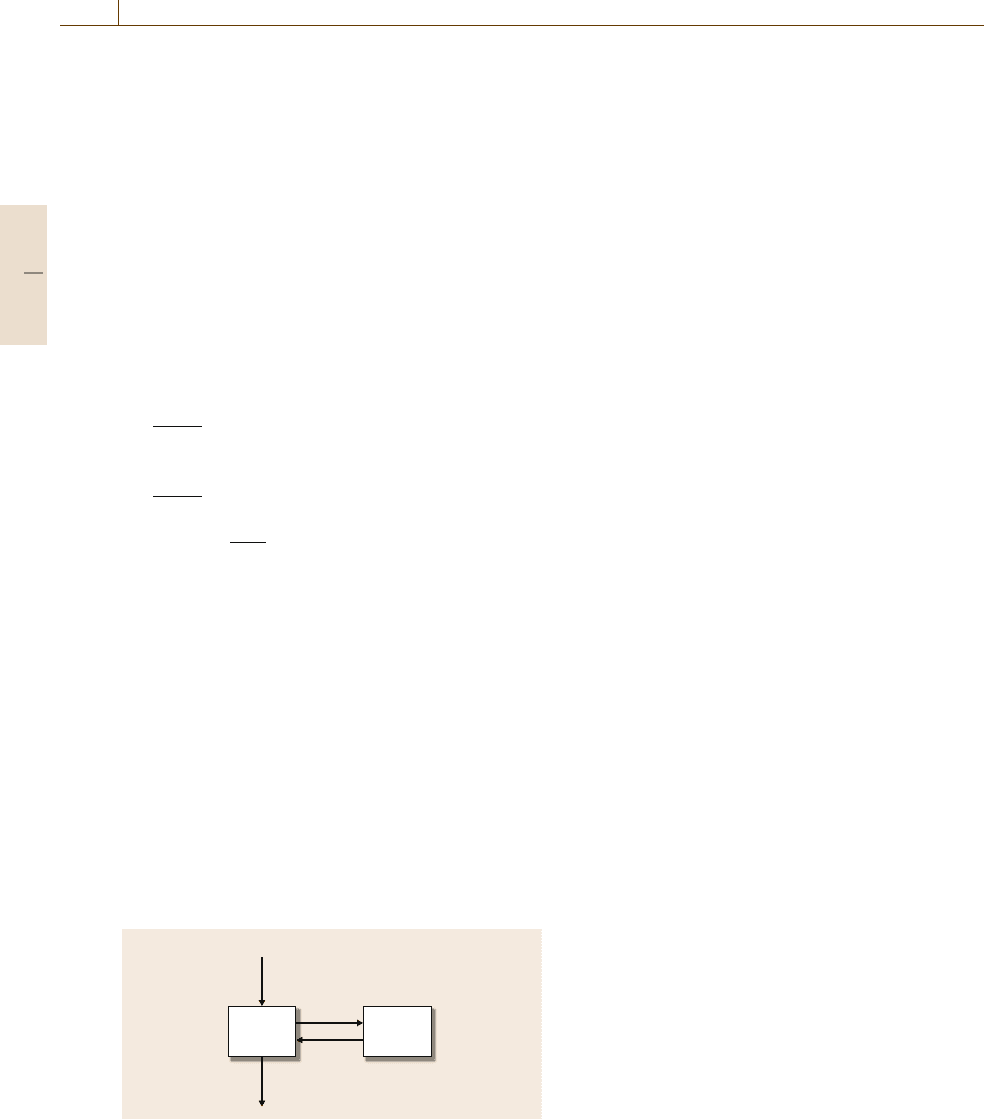
1366 Part H Automation in Medical and Healthcare Systems
76.2.2 Pharmacokinetics
PK is the study of drug administration to, distribution
in, and clearance from the body [76.4,5]. To capture the
dynamics observed after drug dosing, compartmental
models are often employed (Fig. 76.3). The adminis-
tered dose is given by D(t). Individual compartments
aremodeledasdrugmasses(x
1
and x
2
), with cor-
rection to concentration in the output equation (via
division by volume V). Clearance of the drug is from
the central compartment (rate k
cl
). Additional dynamic
character is included by adding compartments and in-
tercompartment transfer rates (k
ij
from compartment i
to compartment j). A two-compartment model can be
represented mathematically as
dx
1
(t)
dt
=−k
cl
x
1
(t)−k
12
x
1
(t)+k
21
x
2
(t)+ D(t) ,
(76.3)
dx
2
(t)
dt
=k
12
x
1
(t)−k
21
x
2
(t) , (76.4)
y(t) =
x
1
(t)
V
.
(76.5)
The output(s) of the model corresponds to measurable
concentrations of drug, as given by y(t). Additional
blocks can be added, as necessary, to capture observed
plasma dynamics related to dosing (for drugs not de-
livered intravenously) and/or absorption. The resulting
model is an empirical description of patient drug con-
centration as a function of time after administration.
These model structures are commonly employed using
available PK modeling tools such as ADAPT II [76.18],
and the FDA requires some pharmacokinetic informa-
tion for most new chemotherapeutics [76.7]. Parameters
in the model can be estimated from individual patient
data, when detailed pharmacokinetic measurements are
available. More often, models of this form are con-
structed from animal studies where multiple animals
12
D(t)
k
cl
k
21
k
12
V
Fig. 76.3 Two-compartment mamillary model used in
pharmacokinetic analysis. Plasma dynamics are captured
by the central compartment (block 1) in Eq. (76.3)
(e.g., mice or rats) are euthanized at each measuredtime
point. The mean and standard deviation of measured
drug concentrations are used as the data to which a sin-
gle model is fit. Low-order deterministic models may
not capture the observed drug concentration measure-
ments for a variety of reasons, including nonlinearity,
higher-order dynamics, and interindividual variability.
To address interindividual variability and data spar-
sity, population models are often developed [76.16,
21, 22]. In this case, a stochastic approach to model
parameterization is employed, where parameters are
composed [76.21]as
p
i
=θ
μ
+η
p
i
+θ
c
C
i
. (76.6)
Parameters (p
i
) for patient i are a function of the
population mean value of the parameter θ
μ
, interindi-
vidual variability in the parameter η
p
i
, and any known
correlative effects C
i
scaled by their population mean
correlation θ
c
. These parameter formulations are then
included in model structures akin to those in (76.3–
76.5), as replacements for the k
ij
and k
ci
parameters, for
example. Variances on the measurement noise (added
to (76.5)) and interpatient variability η
i
are specified as
part of the estimation. With this approach, it is possi-
ble to use a small number of output measurements from
a large number of individuals to characterize both the
underlying model structure (through the observed dy-
namics) andthe populationvariability(through the need
for η and θ
c
or C
i
to describe individual responses).
Software tools are available for constructing these mod-
els (including NONMEM [76.22] and SPK [76.23]).
A population model, once constructed, is representative
of the population response to a drug dose. Furthermore,
it can provide a characterization of both validated vari-
abilities (as a function of body weight, gender, race,
liver or kidney performance status, etc.) and the dis-
tribution of responses expected after dosing a group of
patients with the drug. However, it is not designed to
be predictive in the single-patient case; here it may be
of more use to couple the variabilities established in the
population model with the ability to tailor an individual
PK model.
76.2.3 Modeling Physiology
The potential of detailed physiological models was
recognized by Teorell when he began studying pharma-
cokinetics [76.4, 5], but calculational limitations made
physiological modeling of drug PK intractable in 1937.
With today’s desktop computational power, simulat-
ing physiological models of drug administration and
Part H 76.2
Automation and Control in Biomedical Systems 76.2 Theory and Tools 1367
distribution is straightforward. A physiologically-based
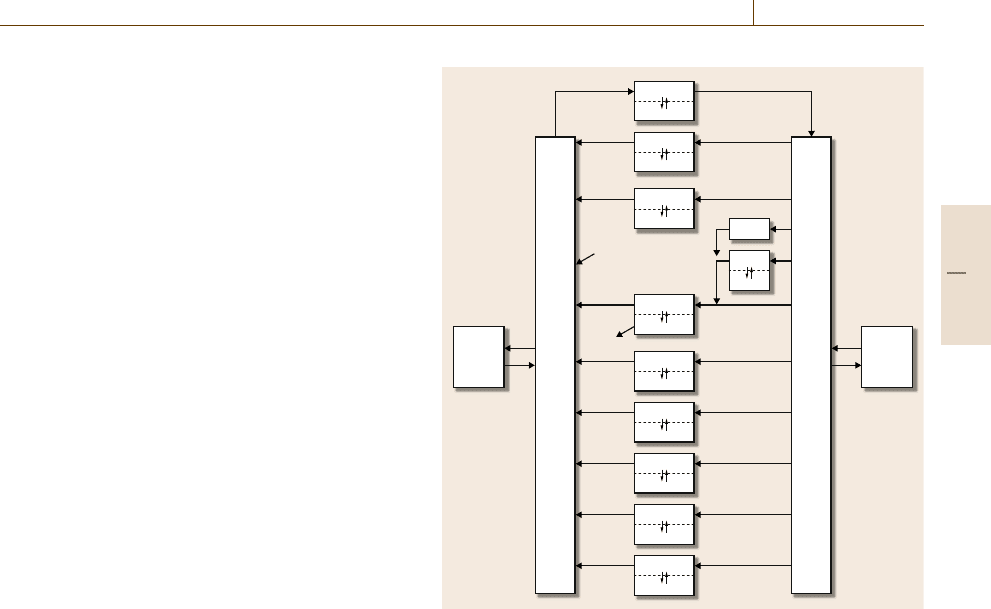
model for a cancer chemotherapy application is shown
in Fig.76.4. Each block in a physiologically-based
model represents an ordinary differential equation
that characterizes concentration in a particular tissue
or fluid, with the volume of the compartment cor-
responding to the physiological volume into which
the compound distributes. Where necessary, subcom-
partments can be added to modify tissue dynamic
response, and these are often denoted as vascular (blood
space) and extravascular (interstitial space) compart-
ments (models may also include intracellular or tissue
binding spaces as well [76.8, 24]). Transfer between
vascular and extravascular space can be via gradient-
based diffusion or active transport, and may be uni-
or bidirectional. Unidirectional arrows between tissue
compartments denote the flow of blood; the bidirec-
tional arrow pairs between red blood cells (RBCs)
and the plasma compartments represent equilibration
(transport is often fast in RBCs, such that they are of-
ten represented algebraically rather than differentially).
Metabolic elimination and clearance are shown as un-
connected arrows exiting particular (sub)compartments
(e.g., the arrow out of liver extravascular space), and
infusion into the system can be properly placed in
a physiological sense, e.g., the IV dose administered
to the venous blood compartment. Advantages of this
approach include the accuracy of the tissue connectiv-
ity and the availability of mean values for the flow and
volume parameters [76.25]. Furthermore, knowledge of
the metabolic pathway of drug clearance (e.g., liver
metabolism or kidney excretion) can be included in the
physiologically correct location. This structure changes
the identification burden to that of metabolic rates and
intratissue transport (plasma ↔interstitial fluid ↔cell)
and dynamics. Resolution of these effects can be chal-
lenging (when feasible) as the necessary information
(e.g., arterio–venous differences in drug concentration)
for identification is not typically available from in vivo
measurements. The result is that tissues are often as-
sumed to be well mixed if they are highly perfused
(e.g., kidney and liver), and only tissues where sig-
nificant transport resistances are involved are modeled
using more detailed intratissue dynamics (and then of-
ten using a compartmental approach).
The utility of these structures is of more potential
benefit when evaluating pharmacodynamic effects. Un-
like the compartmental or population models described
above, the physiological model also allows the effects
of the drug to be properly assigned to their physiologi-
cal sites of action. These outcomes can be positive (e.g.,
Lung
Brain
Heart
Liver
RBCs RBCs
IV dose
Spleen
Gut
Kidney
Venous blood
Arterial blood
Tumor
Other
Muscle
Fat
Fig. 76.4 Block structure schematic of a physiological model of
drug distribution
antitumor activity related to tumor concentration, not
plasma concentration, of an antineoplastic) or negative
(e.g., toxic side-effects of a drug affecting liver perfor-
mance status). This is a significant advantage in the use
of in vitro information for the purposes of in vivo mod-
eling, as the concentration at the site of action (effect
or toxicity) is more accurately represented by a physio-
logical model than the plasma concentration estimate of
a compartmental model.
76.2.4 Biochemical Networks
Phenotype is related to genotype at some level, mean-
ing that macroscopic observables such as physiology,
PK, etc., are driven by biological networks at a vari-
ety of scales. The field of systems biology is focused
on developing a systems-level description of biology
by studying the dynamics of cellular and organ func-
tion, rather than the traditional drill-down study of
increasingly highly resolved portions of a cell [76.26].
In order to rigorously integrate the complex behaviors
taking place at the genetic level with the macroscopic
(whole-organism) response, and to ground these hy-
Part H 76.2

1368 Part H Automation in Medical and Healthcare Systems
potheses in experimental data, requires a computational
model [76.27].
Due to the scales spanned in network analysis (from
the gene regulatory [76.28] to the metabolic [76.29]), it
is infeasible in this space to review the plethora of tools
employed. Ordinary differential equations, due to their
simplicity, are popular, and compartmental approaches
(such as those in Sect.76.2.2) are also used to sim-
plify the model structures. The systems biology markup
language (SBML) is one tool for representing biochem-
ical networks, both qualitative and quantitative [76.30].
AreviewofSBML, along with two other data rep-
resentation standards (PSI MI (Proteomics Standards
Initiative Molecular Interaction) and BioPAX), can be
found in[76.31]. The strengths of thesepackages can be
seen in the complexity of the models that are generated,
and can be simulated, as a result of complex network
modeling (see [76.29] and models at www.systems-
biology.org and www.biopax.org).
76.2.5 Model-Based Control
and Optimization
A commonly employed tool in model-based treatment
design for biomedical systems is optimal control. Math-
ematically, optimal control problems can be formulated
as follows [76.32]
min
u(t)
J(y(t), u(t), r(t)) , (76.7)
subject to:
˙
x = f(x(t), u(t), p) , (76.8)
y(t) =h(x(t), u(t), p) , (76.9)
u
min
≤u(t) ≤u
max
, (76.10)
y(t
f
) = y
fin
. (76.11)
The objective function (76.7) often takes the form of
a least-squares deviation of y(t) from some desired
trajectory r(t), and the decision variable is the u(t)
profile over 0 ≤t ≤ t
f
; for example, in the diabetes ex-
ample introduced later in this chapter, the measured
variable would be plasma glucose concentration (y(t)),
r(t) would be the desired glucose concentration (e.g.,
90mg/dl), and u(t) would be the insulindelivery profile
(or delivery rate) from the present time t to t +t
f
. Feasi-
ble system trajectories are governed by the dynamics of
the model (states x(t), parameters p), and these are in-
corporated as constraints (76.8)and(76.9). Continuing
the diabetes example, the states characterize the patient;
in a physiological model context, these states would be
the glucose and insulin concentrations in the various
physiological tissues (not all of which are measurable).
Constraints on the input magnitude can be enforced, as
in (76.10); other input constraints can also be included,
though they are not explicitly described here. Finally,
a desired final value for the controlled variable y(t
f
)
is included. The identification of a feasible treatment,
an input sequence that drives y(0) to y(t
f
), involves
the solution of a constrained two-point boundary-value
problem. Control vector parameterization [76.33]is
a common method of computer implementation, where
the timeaxis is discretized into k equal-length steps, and
the series of input levels over each of the steps become
the decision variables of the optimization problem. The
resulting input profile generally has a characteristic
bang–bang shape, switching from full on to full off at
one or more points along the time axis. The strength
of optimal control is the ability to solve nonlinear
control problems in the presence of input constraints.
A key shortcoming is the formulation of optimal control
versus the practice of medicine. The former specifies
an endpoint constraint and has no mechanism for the
inclusion of information that becomes available at in-
termediate time points. Clinical practice, on the other
hand, does not generally have an a priori specified end
time of treatment and routinely takes measurements of
patient performance status and incorporates these into
treatment decisions.
One way to take advantage of model-based control
and optimization techniques while retaining the advan-
tages of the optimal control formulation is to use reced-
ing horizon control, also referred to as model predictive
control [76.34,35]. Recedinghorizon controlis an open-
loop optimization posed and solved for a sequence of
input move changes at each time step; a typical model
predictive control formulation may be posed as
min
Δu(k|k)
Γ
y
(r(k +1|k)−y(k +1|k))
2
2
+Γ
u
Δu(k|k)
2
2
(76.12)
subject to: x(k +1|k) = f(x(k|k), u(k|k), p)
(76.13)
y(k|k) =h(x(k|k), u(k|k), p) (76.14)
u
min
≤u(k|k) ≤u
max
(76.15)
|Δu(k|k)|≤u
rate
. (76.16)
Because the model is used to predict output responses
at future times (up to N
p
steps in the future), statisti-
cal notation is employed, where y(k +1|k) is the vector
of N
p
predicted outputs at time k+1 given information
up to time k. The corresponding vector of desired out-
put values is r(k+1|k), and the degrees of freedom are
the N
u
input moves Δu(k|k). The matrices Γ
y
and Γ
u
Part H 76.2
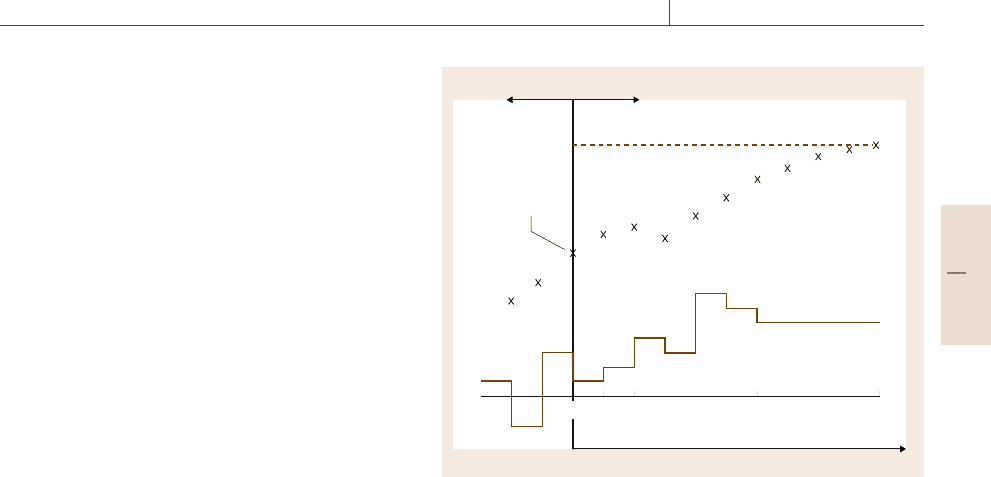
Automation and Control in Biomedical Systems 76.3 Techniques and Applications 1369
are weights to trade off tracking error (large Γ
y
)ver-
sus input move suppression (large Γ
u
). The underlying
model dynamics of (76.13)and(76.14) can be continu-
ous or discrete, as they are simulated at each time step,
but the output values employed in the calculation of
the objective correspond to the N
p
future step times.
Because the solution methodology includes the use of
an optimization routine at each time step, constraint
incorporation is straightforward, and input constraints
can be included in magnitude-constrained (76.15)and
rate-constrained (76.16) forms. Output constraints can
be included, but these may lead to infeasibilities in the
optimization problem, much as state constraints do in
optimal control. One option is to soften the constraints
by including them as additional weighted terms in the
objective function [76.36]. At each sample time, mea-
surements from the system are used to update the states
or outputs of the model, and the optimization problem
is solved. The first input move change is implemented,
and the process repeats at each following sample time
(as shown schematically in Fig.76.5). In the case that
measurable quantities are sampled at different intervals,
the update rate of the model can be variable (this is
referred to as a multirate MPC formulation [76.37]).
When measurements are not collected at each sample
time, multiple inputs in the sequence Δu(k|k) can be
implemented. The structural advantages of MPC,in-
cluding the online solution of an optimization problem
that incorporates available patient information, are sig-
nificant, but a key drawback of this control structure is
k+1 k+2 k+N
u
–1
u(k+N
u
–1|k)
k+N
p
k
Past Future
Current output
measurement
y(k)
Past
output
measurements
Model-predicted
future output values
y
ˆ
(k+1|k)
Optimal
input profile
u(k|k)
Reference r(k+1|k)
Horizon
Fig. 76.5 Receding horizon (model predictive) control implemen-
tation schematic. Model-predicted deviations from the desired
reference are minimized over a future horizon
the challenging analysis of algorithm performance. Sta-
bility guarantees, which may be required by the FDA
before an algorithm can be deployed in an ambulatory
setting, often require the use of endpoint constraints
or long prediction horizons (i.e., large N
p
or infinite-
horizon formulations), which may limit the achievable
performance.
76.3 Techniques and Applications
The potential scope for automation and control in
biomedical problems precludes a canonical review here.
Entire journals are focused on biomedical problems
from the engineering perspective (e.g., IEEE Transac-
tions on Biomedical Engineering, Annals of Biomedical
Engineering, IET Systems Biology), and mainstream
control journals (e.g., Automatica, Journal of Process
Control, Control Engineering Practice) also publish
biomedical control papers. There are also journals fo-
cused on the tools as they apply to classes of problems
(e.g., Journal of Pharmacokinetics and Pharmaco-
dynamics for PK and PD modeling tools). Beyond
the diabetes and cancer case studies discussed below,
a wide variety of problems have been addressed, such
as human immunodeficiency virus (HIV)/acquired im-
munodeficiency syndrome (AIDS), blood pressure, and
anesthesia, among others (some recent review articles
include [76.9,38,39]).
76.3.1 Type I Diabetes Modeling and Control
Diabetes has been a popular biomedical modeling and
controller design case study for almost 50years [76.40–
44]. The development of models of glucose–insulin
interaction, sometimes including additional hormones,
substrates, or contributions, has an equally long his-
tory [76.40–42, 45–56]. In order to estimate the
parameters in these models, tools from Sect. 76.2.1
are employed, and the model structures can be
of either compartmental (Sect. 76.2.2) or physiologic
(Sect.76.2.3) form. With a model in place to represent
the patient (i.e., patient=model), for use in con-
Part H 76.3
1370 Part H Automation in Medical and Healthcare Systems
troller design (i. e., model-based optimal control using
tools from Sect.76.2.5), or both (i. e., patient =model),
a control problem can be formulated. One option
is to pose a single-input single-output control prob-
lem, the formulation of which is quite similar to the
continuous processes seen in the chemical industry
for decades: a manipulated variable (insulin delivery),
with a steady-state value (pancreatic insulin release
in a healthy patient), is altered to maintain a con-
trolled variable (glucose concentration) within a small
range of its nominal value. Normoglycemia is generally
taken as circulating glucose concentrations in the range
70–120 mg/dl [76.57], with a nominal value between
80 and 100mg/dl. Insulin delivery for type I diabetic
patients, who have no insulin release from the β-cells
of the pancreas, is altered in order to control glucose
concentration. Clinical approaches at present involve
intensive subcutaneous insulin therapy [76.57, 58]or
continuous subcutaneous insulin infusion via mechan-
ical pump [76.59].
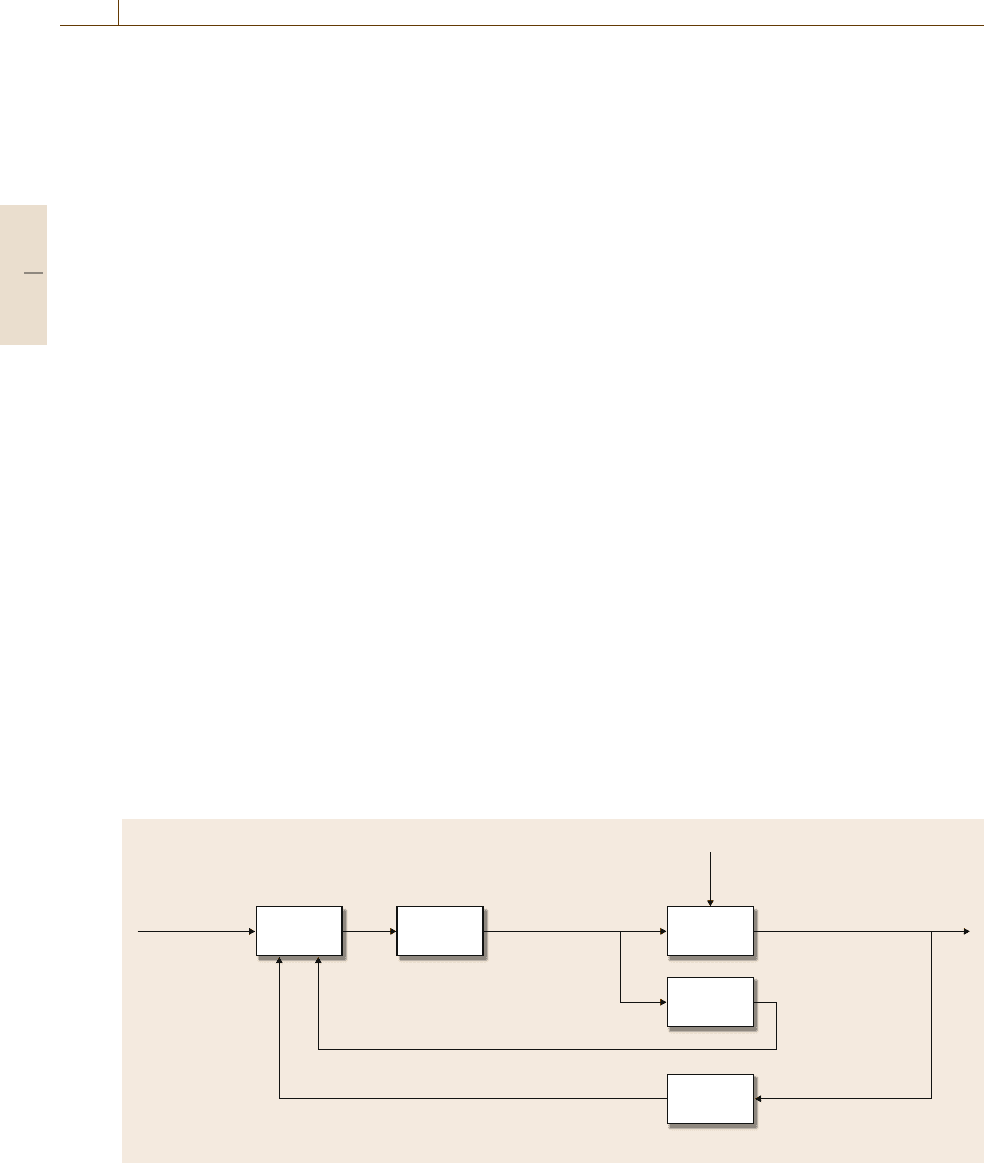
Based on this traditional control configuration and
the control schemes introduced in Sect. 76.2.5,the
glucose control problem can be posed in the model-
predictive control framework [76.42, 43], as shown
in Fig. 76.6. Using glucose concentration measure-
ments (at 10 min or faster intervals), insulin delivery
rate would be changed in order to regulate ambu-
latory diabetic patient glucose levels in response to
meal disturbances. Controller tuning would require
rapid rejection of meal disturbances (to keep glucose
levels below 120 mg/dl), but also aggressive down-
regulation of the controller so that post-meal glucose
concentrations remain above the hypoglycemic level of
Controller Patient
Patient
model
Glucose
sensor
Insulin
pump
Glucose concentrationInsulin delivery
Meals, exercise, etc.
Measurement device
Disturbances
Desired
glucose
concentration
Reference Manipulated
variable
Controlled variable
Model predicted glucose concentration
Controlled variable (model-predicted)
Fig. 76.6 Block diagram structure for model predictive control, as applied to the diabetic patient control problem
60mg/dl, which can be dangerous to patients [76.57,
58]. Furthermore, significant levels of measurement
noise accompany each glucose observation. The poten-
tially conflicting objectives of aggressive response and
noise suppression challenge model-based algorithm de-
signers. Simulation studies of control algorithms have
focused on the type of control needed [76.60], pro-
viding upper bounds on achievable control [76.42],
or evaluating the potential effects of measurement or
other disturbances on closed-loop performance [76.43].
A key hurdle atpresent isthe glucosesensor,as the FDA
has not approved any real-time glucose sensor for use in
a closed-loop control algorithm.
One focus of improving diabetes treatment via
automation is the use of run-to-run control (com-
monly deployed in the batch processing literature) in
designing insulindosing protocols[76.61,62]. Here, pa-
tient glucose concentrations are not sampled at fixed
short intervals, but instead the measurements taken by
patients over a typical day are used to improve day-
to-day performance of their glucose control. In the
context of Fig.76.6, the controller and insulin pump
are replaced by the patient (who will determine in-
sulin delivery amount) and their delivery mechanism
(pump, subcutaneous injection, etc.); Fig. 76.7 shows
a potential configuration of a wireless sensor commu-
nicating with the combination data storage device and
insulin pump. By reformulating this problem, the use
of advanced control and automation techniques has the
potential to make a rapid contribution to the treatment
of diabetic patients instead of suffering from the crit-
ical flaw in artificial pancreas deployment highlighted
above: the lack of FDA acceptance of a glucose sensing
Part H 76.3

Automation and Control in Biomedical Systems 76.3 Techniques and Applications 1371
Fig. 76.7 Diabetic patient using a wireless subcutaneous
glucose sensor communicating the measurement to the
data storage device. This device also includes the insulin
pump mechanism; note that the pump is notaltering insulin
delivery rate based on the glucose measurement unless
commanded to do so by the patient (courtesy of Dr. Cesar
Palerm, Medtronic Diabetes)
device for use in the closed loop. In fact, the run-to-run
algorithm can provide robustness to interpatient differ-
ences [76.62], which is important given the span of
patients that such an algorithm could encounter.
Changing the focus from ambulatory diabetes to
the critical-care setting, the potential for impact is sig-
nificant [76.63–65]. In postsurgical patients, glucose
control via insulin administration can dramatically re-
duce morbidity and mortality. The altered metabolic
state in thesepatients issimilar to the diabeticcondition,
in that patients are hyperglycemic with low insulin sen-
sitivity. Hence, the automated administration of insulin
to regulate glucose concentration could assist in pa-
tient recovery. Whilemeasurement noise and rate would
(likely) be unchanged from the ambulatory setting,
other challenges manifest. First, individual models of
patients are not available a priori, and population mod-
els are insufficient for use on specific patients because
individual insulin sensitivity will vary widely. Further-
more, the dynamic state of recovering patients would
lead to time-varying insulin sensitivity requiring time-
varying model parameters. A patient model updating
mechanism, such as recursive least squares (assuming
a linear parameterization of the model), is required.
Given the present legal and regulatory climate, a high-
level safety and fault-detection layer for the algorithm
would need to be developed. Alternatively, the algo-
rithm could be implemented as a semiclosed decision
support system with medical professionals affirming or
declining the recommendation of the updating algo-
rithm. In the context of bringing an automated system
to market, the decision support system in critical care
could be the most rapid approach because collecting
a nonautomated measurement and allowing medical
professionals to intervene in therapy has the lowest en-
ergy barrier to review board approval.
A concern with any insulin delivery system is the
potential for overadministration, as this could lead to
hypoglycemia and possibly death. Adding a second
channel to the closed-loop system could alleviate some
of these issues. A model-based structure using both in-
sulin and glucose has been proposed [76.66,67], where
insulin is added to reduce glucose levels, and glucose
infusion is used to elevate glucose. To keep glucose
from being administered continuously, an output reg-
ulator formulation is employed (a modification of the
MPC scheme in Fig.76.6 and Sect. 76.2.5), such that
positive glucose infusion rates are penalized at a lower
level than deviations in glucose concentration from
the reference value. The result is that glucose is in-
fused only when predicted glucose levels drop below
the basal level. While numerically superior, this ap-
proach is more relevant to use in a clinical, rather than
ambulatory, setting, as patients are unlikely to desire
a two-pump/two-needle (or two-infusion-line) device.
Also, a high-dextrose solution is an excellent culture
medium for bacteria, so sterility in the nonhospital
setting would become an even greater issue for these
patients.
As McGarry highlighted in the 2001 Banting lec-
ture, glucose and insulin are not the only important
endogenous compounds in diabetes. Type 2 diabetes
typically involves the dysregulationof fatty acids (FAs);
there is also significance of FAs in insulin-dependent di-
abetes as metabolic interactions exist with insulin and
glucose. Using the minimal model [76.41] as a basis, an
extendedminimal model ofglucose/insulin/FA has been
constructed [76.55] using data from the literature and
the tools of Sects. 76.2.1 and 76.2.2 (see the schematic
in Fig.76.8). While this model is not mechanistic, it
does capture the interactions of glucose, insulin, and
FAs; furthermore this model is a low-order parameteri-
zation, which would facilitate tailoring to an individual
patient.
Finally, meal disturbances are not the sole chal-
lenge to diabetic patients, and hence automated insulin
delivery systems. Exercise alters glucose and insulin ki-
netics [76.68,69], and models of these processes have
been proposed in both physiologic[76.54] (Sect.76.2.3)
and minimal [76.56] (Sects. 76.2.1 and 76.2.2)forms.
Part H 76.3
1372 Part H Automation in Medical and Healthcare Systems
Adipose
tissue
Liver Periphery
Periphery
I
G
F
X
IG
X
IF
X
FG
Insulin
administration
Meal
(fats)
Meal
(glucose)
Fig. 76.8 Schematic of interactions between insulin (I),
glucose (G), and fatty acids (F). Peripheral tissues con-
sume glucose and fats, while the liver and adipose tissue
serve dual source/sink roles for G and F, respectively.
Meal consumption increases glucose and fatty-acid levels,
and insulin administration drives circulating insulin level.
Dashed lines represent the effect of one compound on an-
other (G on F dynamics, for example). The X
ij
blocks
represent additional dynamics (equations) that alter the ex-
tent and duration of effect of i on j (e.g., insulin on glucose
through X
IG
)
Again, these models are more macroscopic than mech-
anistic, but they are able to capture the important
responses for the purposes of glucose regulation in di-
abetic patients. While the population-level variability
of the model parameters remains to be characterized
(which is also true of the FA models, above), the
proposed structure does allow preliminary analysis of
control structures to establish feasibility and potential
performance of automated glucose control systems.
76.3.2 Cancer Radio- and Chemotherapy
Cancer is a collection of diseases resulting from a series
of genetic mutations and characterized by an imbal-
ance between cell proliferation and apoptosis, or pro-
grammed cell death [76.71]. Ifuntreated, cancerleads to
organ failure and the death of the host organism. One in
four deathsin the USA in 2008was projectedto be from
cancer, claiming over 565650 lives [76.72]. In addition,
the total disease burden, including treatment costs and
loss of productivity, was estimated at US$ 219.2 billion
in 2007, providing both societal and monetary reasons
for improving cancer treatment [76.72]. Unlike insulin-
dependent diabetes, cancer is not a single disease, and
therefore, no silver bullet (i. e., insulin) exists. Hence,
cancers must be addressed individually; e.g., pancreatic
and breast cancers are separate diseases with different
treatment options and chemotherapeutic choices.
Accessible cancers are removed surgically. Alter-
natives or complements to surgery include systemic
chemotherapy and targeted site-specific treatment,
such as photodynamic therapy (PDT) or radiotherapy
(RT) [76.73, 74]. RT is often used in place of surgery
because the tissue surrounding the tumor is too sen-
sitive to permit surgical excision (e.g., tumors located
near the spinal column). PDT is used alone, or as an
intrasurgical procedure, to kill tumor cells located at
the tumor margin that are invisible to the human eye.
All cancer treatments are usually complemented by sys-
temic chemotherapy for two reasons: (i) small remote
metastases are often present by the time cancer is de-
tected; and (ii) chemotherapy is the only treatment that
is whole body in nature, thereby giving it the ability
to indiscriminately attack metastatic lesions before they
are clinicallydiagnosed. Hence, automation advances in
this area address targeted therapies and chemotherapy.
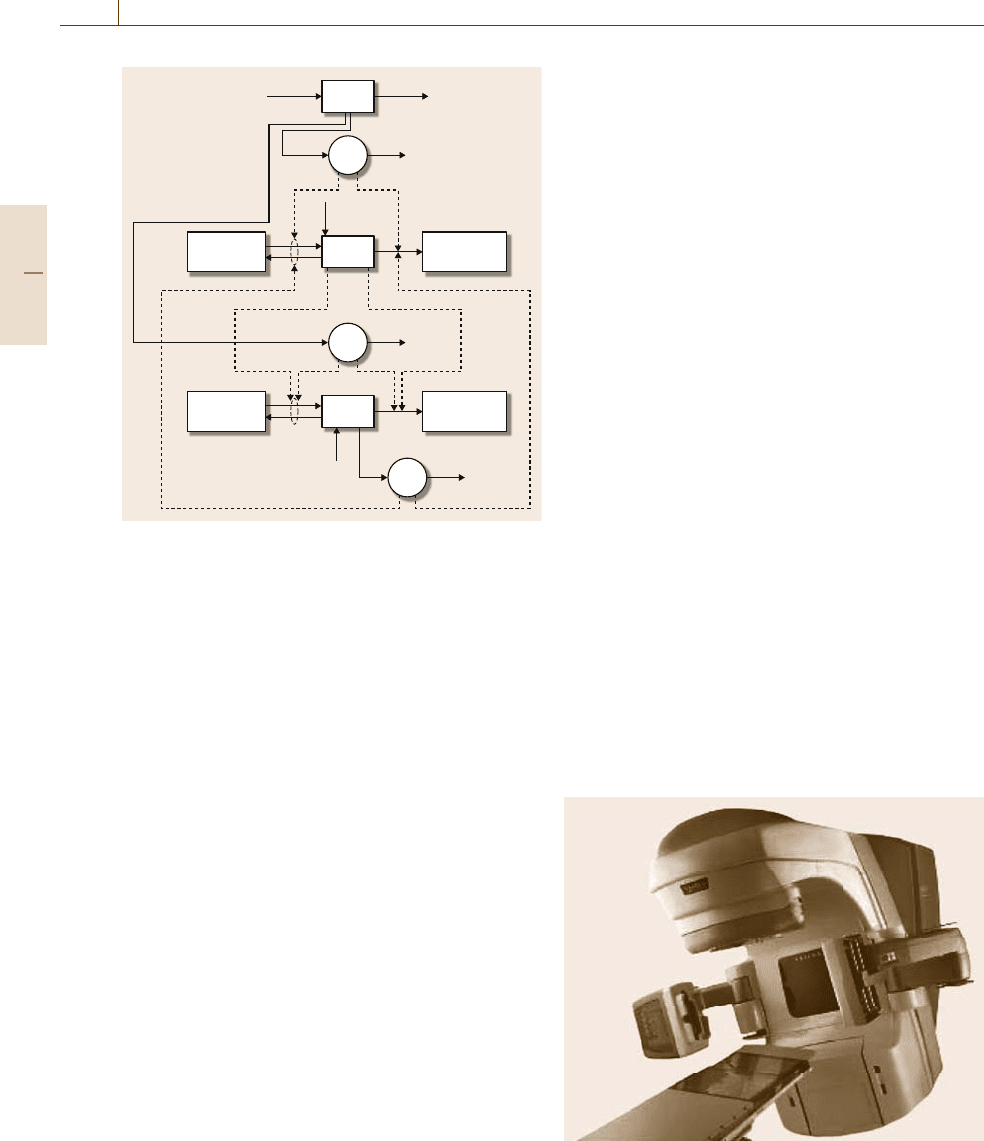
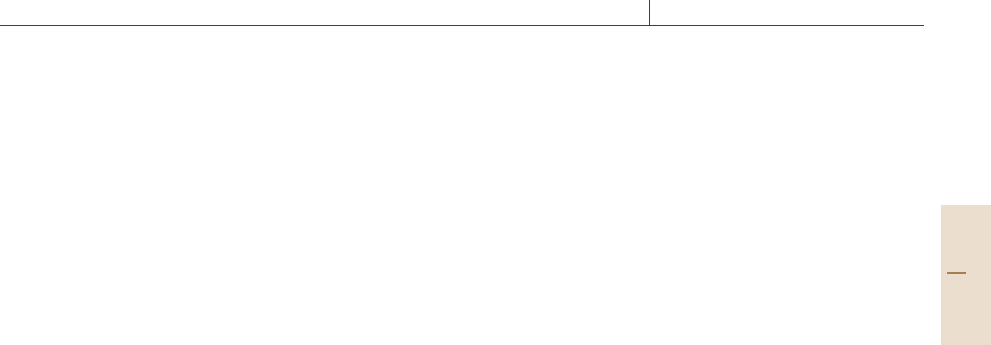
AkeyadvanceinRT, which requires a signif-
icant automation component, was intensity modula-
tion (IMRT) [76.75, 76]. A linear accelerator source
(Fig.76.9) provides radiation that has its fluence mod-
Fig. 76.9 IMRT gantry and linear accelerator source (fig-
ure originally published in [76.70])
Part H 76.3
Automation and Control in Biomedical Systems 76.4 Emerging Areas and Challenges 1373
ulated across the beam width, thereby providing pre-
scribed radiation doses to a target tissue volume. By
using a rotating beam gantry and placing the patient
on an actuated table, critical tissues can be delivered
lower doses through optimization of radiation dose to
a particular planning volume. Mathematical models are
constructed based on fundamental physics of radiation,
with refinements basedon the interactionof endogenous
absorbers and scatterers with the administered radia-
tion beam(s). Collaborative work between engineers
and clinicians (such as [76.76]) has provided advanced
offline algorithms for optimizing the treatment dose.
The state of the art in RT is image-guided radiation
therapy (IGRT) [76.73, 74]. This extends IMRT tech-
niques to include real-time adjustment of the planned
dose in order to reduce interfraction (positional changes
of anatomical objects between the fractions of a ra-
diation dose) and intrafraction (real-time motion of
tissues in response to actions such as breathing) spa-
tial uncertainties. From a calculational perspective, this
changes a mixed-integer programming (MIP) prob-
lem, as solved in [76.76], into either a mixed-integer
dynamic optimization problem [76.77, 78] or a dy-
namic control problem using the MIP solution as
a reference.
Model-based approaches to cancer chemotherapy
treatment design are an active area of research. Core
to this approach is the existence of models describing
PK (the effect of the body on the drug), untreated tumor
growth (generally using the Gompertz model [76.79],
which has been shown to capture solid-tumor progres-
sion in human patients), and pharmacodynamics (the
effect of the drug on the body, including both antitumor
effect [76.80, 81] and toxicity [76.82]). These mod-
els are constructed using compartmental (Sect.76.2.2)
or physiological (Sect. 76.2.3) approaches, depending
on the amount of data available. Population tools
(Sect.76.2.2) are also important, as the cancer-affected
population is heterogeneous. A common solution tech-
nique for the chemotherapy dosing problem is optimal
control [76.33, 83, 84] (Sect. 76.2.5), where the fol-
lowing two-point boundary-value problem is posed:
minimize tumor volume at the end of a fixed time pe-
riod subject to constraints on drug dose (magnitude),
path constraints (states, as governed by the model dy-
namics), and in some cases explicit representations
of toxicity (e.g., neutrophils [76.85]). An alternative
formulation and solution method uses mixed-integer
programming [76.86–88]; advantages of this approach
include path constraint satisfaction and extensibility of
the framework to additional constraints. The existence
of nonlinearities (beyond the bilinear kill term) in the
PK or PD models causes additional challenges for their
solution, requiring either parameterization [76.87]or
mixed-integer nonlinear programming techniques (for
details on these methods, see [76.89,90]). A key change
in formulation introduced in [76.87] is the use of a re-
ceding horizon strategy instead of the fixed final time
(as in the latter part of Sect.76.2.5). This is consistent
with clinical practice: the patient is treated until dis-
ease progression or cure, thereby making the final time
formulation clinically irrelevant.
76.4 Emerging Areas and Challenges
In a 2001 perspectives article [76.91], Morari and Gen-
tilini describe the state of process control, and in a larger
context, automation, as “... a victim of [its past suc-
cess]: it has reached a plateau and turning point.” The
remainder of their article highlights biomedical pro-
cesses as an area where, “...control engineers [can]
have significant impact,” because automation and au-
tomatic control were not commonly employed. While
there have been some changes since 2001, the potential
impact of automation and engineering on biomedical
practice remains significant. In this regard, this chapter
has introduced a set of challenges and tools for use in
melding systems engineering techniques with biomed-
ical problems. The case studies are areas in which
the author is active; additional opportunities abound
for those motivated to: (i) learn the biology, medical
practice, and automation need of a disease (e.g., inflam-
mation, infection, regenerative medicine), and (ii) work
closely with collaborators in complementary fields to
address the disease of interest (e.g., clinicians, biolo-
gists, engineers, mathematicians, etc.). Related to some
of the challenges that began this chapter, the emerg-
ing areas and challenges below represent areas where
systems engineering and automation can be employed
to advance science and disease treatment. These ar-
eas align along a key principle of model-based control:
model quality dictates theoretically achievable control
system performance [76.92]. Hence, the biomedical
goal is to construct mechanistic models, when possible,
and to understand the context of the model, which may
serve to limit the prospective use of the model to certain
scenarios.
Part H 76.4

1374 Part H Automation in Medical and Healthcare Systems
76.4.1 in vitro Physiological Systems
The basic science of biomedicine often begins with cell
culture and in vitro experiments. However, a key is-
sue in translating cell-only experimental results to live
animals is the disconnect between cells in a dish and
cells interacting. One approach to this problem has
been coculturing of cells to allow cellular crosstalk
and to allow the development of three-dimensional
structure that better mimics physiology. This is a tech-
nique used in blood–brain barrier research [76.93]to
develop in vitro tests for neurotoxicity. For PK anal-
ysis, many drugs are cleared by the cytochrome P450
enzymes in the liver but cause toxicities or effects else-
where in the body. Hence, an in vitro liver cell culture
could be used to analyze drug kinetics; one such op-
tion, although the cells are encapsulated in a polymeric
coating, is described in [76.94]. A secondary concern
beyond three-dimensional (3-D) structure is spatial het-
erogeneity, which may be more difficult to recreate
in vitro [76.95].
A potential system for in vitro testing of drug PK
would be a fully integrated physiologically motivated
cell culture system: cells of different types growing in
culture, with fluid volumes for each cell type corre-
sponding to in vivo physiological volume; fluid flow
and connectivity allowing intercell crosstalk within
and between cell types (simulated physiology); rele-
vant compartments for evaluating effect, as appropriate
(e.g., tumor cells in culture for cancer PD analysis);
and the entire system automated for sensing and con-
trol. In the context of cancer drug PK and PD,the
incorporation of tumor cells would alleviate two short-
comings commonly present in current in vitro analysis:
(i) clinicallyinvalidpharmacokinetic profiles,where the
tumor cells are bathed in a constant concentration of
drug for a period of time, and (ii) cell kill analysis
based on the aforementioned pharmacokinetic profile.
While the mathematical analysis of tumor cell kill un-
der time-dependent pharmacokinetic profiles is more
complicated than the constant-concentration approach
used presently, the use of in vitro physiological systems
could serve to reduce the number of animal studies nec-
essary in drug development while also improving the
translation of in vitro results to the toxicity and efficacy
studies that have to be performed.
76.4.2 Translating in vitro to in vivo
The transition from in vitro to in vivo provides a serious
challenge to modelers and treatment designers. Phys-
iology may provide an advantage, as the connectivity
between organs in animals is well understood. Organ
weights and blood flow rates to individual organs are
available in the literature for a variety of species, in-
cluding toxicologic man [76.96]. However, this detailed
approach assumes a model of physiological structure,
which is not the generally applied practice in clini-
cal drug development. As described in Sects. 76.2.2
and 76.2.3, compartmental models are more commonly
employed in the (pre)clinical setting due to the smaller
number of parameters, reduced mathematical complex-
ity, and the relative ease of parameter estimation. In
this less detailed structure, however, the method for
incorporating in vitro information is ad hoc. Hence,
a more mechanisticapproach is desiredwhen significant
in vitro information is available because the mapping to
the in vivo situation will be improved.
As discussed above, animals are not humans.
The compartmental and population-based PK models
often developed in animals rely upon allometric scal-
ing [76.97, 98] to address the interspecies differences
in dynamic profile. While imperfect (as witnessed by
the dearth of PK-driven model-based treatment designs
deployed in the clinic), this scaling principle can pro-
vide useful insight for selecting first-in-human doses
and times to sample during phase I trials. Translat-
ing information relies to some degree on mechanistic
accuracy at the cellular level, thereby limiting the
unknown factors to the physiologically connected tis-
sues. In some cases, carefully constructed physiological
models can be successfully scaled from animals to hu-
mans by accounting for changes in physiology, such
as body fat percentage, as seen for the lipophilic an-
ticancer drug Docetaxel [76.10]. Often this requires
assumptions, such as equivalent plasma protein binding
characteristics (potentially based on binding informa-
tion from in vitro studies), and similar mechanisms of
clearance (liver metabolism, elimination in urine, etc.).
Under these assumptions, the human physiologically
based pharmacokinetic (PBPK) model (Fig.76.4) can
be successfully constructed by using the metabolic and
clearance parameters as thedegrees of freedom in fitting
human plasma data. When inconsistencies between the
scaled PBPK model and human data manifest, relaxing
the assumptions and fitting novel mechanistic behaviors
to the human plasma profile is required. The resulting
model at the human scale can provide information that
would otherwise be unattainable in humans, such as es-
timated drug concentrations in key tissues (e.g., brain)
or those prone to toxic side-effects (e.g., liver, kidney,
white blood cells).
Part H 76.4
