Middleton G.V. (Ed.) Encyclopedia of Sediments and Sedimentary Rocks
Подождите немного. Документ загружается.


h'LANAR AND PARALLEL LAMINATION
535
U.';^;:---^.*Vfss:^->;&ft^SMiia'^
Figure Pll (A) Planar laminae thought to have formed on an upper slage plane bed. Permo-Triassic St Bees Sandstone, UK, Ltiw-angle laminae
labeled c. Sh.irp Iwses to relatively Ihitk laminae labeled d. Discontinuous (Iruncatedl laminae labeled e ilrom Besl and Bridge, 1992).
i.Hi Modern beach laminae from Virginia, tJSA, IC) Planar laminae composed of very line grainstone (lighl) and mudstone idarki. Middle
Ordovician St.
P,iul
Group, Maryland, USA (photo courtesy
ol"
Robert Demiccol, (Di Wavy and crinkled
p.ir,illel
laminae istroma(olite) composed
of firainstone idarki and dolomilic limeslone (light), Upper Cambrian Conococheague Limestone, Maryland, USA (photo courtesy of Robert
Demicco), (E) Planar, parallel laminae in dolomitlc mudstone. Lower Proterozoit Wittenoom Dolomite, Western Ausiralia Iphoto courtesy of
Robert Demicto), (F) Parallel laminated calcium carbonaie crusts on Pleistocene limestone, Florida Keys, USA (pholo tourtesy of Robert
Demicco),
boundaries, referred to as primary
citrrenl liticctlion
or
lineation. The ridges are a tew grains high and have a spacing
of about
1
cm.
Laminae commonly occur in sets
(Uiiuina.sci.s)
that are
recognizable because of changes in texture, composition, or
orientation of laminae (Figure PI I). Sets of planar and parallel
laminae normally have bounding surfaces that are morc-or-less
parallel to the laminae within them. If the anyte between the
laminae and the laminasct boundary exceeds a few degrees, the
sedimentary structure is referred to as cniss laminalion or
inclined
lamitiarion.
However, some sets of planar lamination
do contain low-angle inclined laminae in plaees.
The term lamina has been used to denote a thin sediment
layer produced by momentary fluctuations in current velocity
and sediment transport rate (Otto. 193S, and many others).
Such genetic implications for the term lamina should be

536
PLANAR AND PARALLEL LAMINATION
avoided,
as it cannot be known eonckisively how all laminae
fonn.
Indeed, planar and parallel laminae may form in
different ways in dilTerent depositiona! environments. Their
origin is also different depending on sediment size. Some
examples are given beiow, but the list is not exhaustive.
Origin of planar laminated sand under
unidirectional currents
Upper-stage plane beds occur beneath fast, shallow unidirec-
tional water flows in which there is appreciable transport of
bed load and suspended
load.
Deposition on upper-stage plane
beds gi\es rise to planar lamination (ligure Pll(A)). Most
early explanations for the formation of planar laminae beneath
upper-stage plane beds involved the influence of turbulent
variations in bed shear stress and sediment transport (reviewed
by Bridge. 1978: Allen, 1982. 1984). Turbulence, and
specifically the turbulent bursting process linked to large
coherent structures in the flow (Bridge. I97,S: Allen. 1984:
Cheel and Middleton. 1986). cannot explain the thickness,
lateral extent and size grading within planar laminae.
However, the migration of low-relief bed waves can (Jopling,
1964.
1967; Bridge and Best. 1988. 1997; Paola
elal..
1989;
Best and Bridge, 1992). The tine-grained bases of laminae are
due to deposition from suspension on the distal lee sides ofthe
bed waves. Most low-relief bed waves do not have avalanche
faces,
and the grain size of
bed
load tends to decrease from the
erest to the toe of the lee side, resulting in the coarsening
upward parts of laminae. However, the largest low-relief bed
waves may have a short lee-side avalanche face at the base of
which coarse sediment accumulates. This relatively eoarse
sediment lies with sharp contact on the underlying laminae. A
predominance of fining-upward laminae may result from
preferential preservation of the lower parts of the largest bed
waves that moved over the bed. Low-angle, inclined laminae
within the thicker planar laminae are due to turbulent
fluctuations in sediment transport on to the depositional Ice
side ofthe bed waves (Figure PI I(A)). Parting lineation results
from the imprint of high-speed and low-speed streaks within
the viscous sublayer of hydraulically smooth and transitional
turbulent boundary layers (Allen, 1982).
Transcurrt'nl lamination
is the name given to sets of more-
or-less planar lamination that are produced by the migration
of low-relief
bed
waves and/or those with a very small angle of
climb (Allen. 1982). Transeurrent laminae have also been
associated with migration oi" low-relief ripples (in water and
air) and dunes, bed-load sheets, and antidunes (.lopling. 1967;
Smith,
1971; Relneck and Singh, 1973; McBride
ciaf.
1975;
Bennett and Bridge, 1995).
(Fitzure Pll(B)), This is the well-known
becicli
laminalion
(Cntton,
1969; Reineck and Singh, 1973; Allen. 1982), The
character of sets of these laminae can be influenced by tides,
storms or storm seasons. It is uncertain whether low-relief bed
waves play any role in the formation of laminae by wave
currents.
Origin of planar and parallel
laminated mud and sand
Lamination of mud and very fine sand involves episodic
deposition from suspension, provided that the mud was not
transported as sand-sized pellets (Figure PI 1(C)). The laminae
will be planar if they are deposited on a planar surfaee, and if
deposition rate does not vary appreciably in space. The
laminae arise because of temporal ehanges in the sediment
supply or the ability of the flow to suspend sediment of a
particular type, I-or example, changes in the turbulence
intensity of water currents may be associated with the passage
of individual floods over floodplains and lakes, seasonal
variations in sediment supply and transport rate in lakes
(e.g.,
glacial
varves).
passing of gravity waves, periodic storms
at sea. and tidal currents that vary over a number of different
titneseales (reviews in Reineek and Singh, 1973; Smith
elal.,
1991;
Demicco and Hardie. 1994). The general term for these
types of laminae is rhyiltmiie. Although turbuleni fluctuations
in suspended sediment transport (time scale of seeonds or less)
have been Invoked to explain planar laminae in muds (review
in Bridge. 1978). it is unlikely that a recogni/able mud layer
could be deposited in such a short period of time.
Parallel,
nonplanar lamination in muds and sands occurs
where the sediment is periodieally draped over a surface with
preexisting topography. Carbonate mud laminae deposited on
cyanobacterial mats during storms or high tides give rise to
parallel,
nonplanar laminated
.stromalatitcs
(Figure PI 1(D)).
Chemically and biologrcally formed planar laminae
Planar laminae may be formed by seasonal variations in the
type and abundance of the tests of microorganisms
(e.g..
foraminifera, diatoms) that accumulate on the bed of the sea
and lakes. Variations in the nature of
ehemical
precipitation of
minerals in some depositional environments may also give rise
to planar lamination
(c.g,.
laminated calcium carbonate
crusts). Some lamination in muds is manifested only by color
changes, associated with changes in chemical composition that
could be primary or diagenetic (Figure PI 1(E), (F)).
John S. Bridge
Origin of planar laminated sand under
reversing currents
LJppcr-stage plane sand beds also occur under high-velocity
water currents that reverse in direction periodically, particu-
larly associated with gravity waves in shallow water. Deposi-
tion under these plane beds also gives rise to planar
lamination.
However, under these eonditions, the deposition
of laminae is probably due to the unsteady sediment
transport associated with the passing of individual waves. In
the surf zone of sandy beaches, the swash and backwash
associated with wave breaking gives rise to planar laminae
with characteristic concentrations of heavy minerals
Bibliography
Allen.
J,R,L,. 1982,
SeJinienttiry
Striicturi.'.'i:
Their
Character and
Physi-
cal
Basis.
Developments in Sedimentology 30. Amsterdam: Elsevier
Science Publishers,
Allen.
J.R.L,. 1984, Parallel l;tmin:ition developed from upper-stage
pkme beds; a modi:l based on the larger coheretu slruetures of the
turbulent boundary layer,
SeitinieniaryGeoloi^y.
39: 227-242,
Bennett, S,J,, and Bridge.
.I.S..
1995, The geometry and dynamics of
low-relief bed forms in heterogeneous sediment in a laboratory
channel,
and their relationship lo water flow and sediment
transport,
J<nirnal(if.Sedinicnlary
Re.\eareh.
Series A, 65: 29 39,
Best,
J.l.,.
and Bridge, LS,.. 1992, Tlie morphology and dynamics of
low ampliliidc bedvvaves upon upper stage plane beds and the
preservation of planar laminae,
.Scdimenlolo)>y.
39: 737 752,

POREWATERS IN SEDIMENTS
537
Bridge, J.S,, 1978. Origin of horizontal lamination under turbulent
boundary layers. Sedimentary Geology, 20: 1-16.
Bridge, J.S,, and Best, J.L,, 1988. Flow, sediment transport and
bedform dynamics over the transition from upper-stage plane beds:
implications for the formation of planar laminae. Sedimentology,
35:
753-763.
Bridge, J.S,, and Best, J,L,, 1997, Preservation of planar laminae
arising from low-relief bed waves migrating over aggrading plane
beds:
comparison of experimental data with theory. Sedimentology,
44:
253-262.
Cheel, R.J., and Middleton, G.V., 1986. Horizontal lamination formed
under upper flow regime plane bed conditions. Journal of Geology,
94:
489-504.
Clifton, H.E., 1969. Beach lamination: nature and origin. Marine
Geology, 7: 553-559.
Demicco, R.V,, and Hardie, L.A., 1994, Sedimentary Structures and
Earty Diagenetic Features of Shallow Marine Carhonate Deposits.
SEPM Atlas Series No.I, 265pp.
Jopling, A.V., 1964. Interpreting the concept ofthe sedimentation unit.
Journalof Sedimentary Petrology, 34: 165—172.
Jopling, A.V., 1967, Origin of laminae deposited by the movement of
ripples along a streambed: a laboratory study. Journal of Geology,
75:
287-305.
McBride, E.F,, Shepherd, R.G,, and Crawley, R.A., 1975. Origin of
parallel near-horizontal laminae by migration of bed forms in a
small flume. Journal of Seditnentary Petrology, 45: 132-139.
McKee, E.D., and Weir, G.W., 1953. Terminology for stratification
and cross-stratification in sedimentary rocks. Bulletin ofthe Geolo-
gical Soeiety of America. 64: 381-390.
Otto,
G.H,, 1938, The sedimentation unit and its use in field sampling.
Journal of Geology, 46: 569-582.
Paola, C, Wiele, S.M., and Reinhart, M.A., 1989. Upper-regime
parallel lamination as the result of turbulent sediment transport
and low-amplitude bedforms. Sedimentotogy, 36: 47-60.
Reineck, H.E., and Singh, I.B., 1973. Depositional Sedimentary Envir-
onments. Springer.
Smith, D.G., Reinson, G.E., Zaitlin, B.A,, and Rahmani, R.A. (eds.),
1991,
ClasticTidalSeditnentology. Canadian Society of Petroleum
Geologists Memoir, 16: pp. 29-39.
Smith, N.D., 1971. Pseudo-planar stratification produced by very
low amplitude sand waves. Journal of Sedimentary Petrology, 41:
624-634.
Cross-references
Bedding and Internal Structures
Bedset and Laminaset
Biogenic Sedimentary Structures
Microbially Induced Sedimentary Structures
Parting Lineations and Current Crescents
Stromatolites
Surface Forms
Swash and Backwash, Swash Marks
Tidal Flats
Tides and Tidal Rhythmites
Varves
POREWATERS IN SEDIMENTS
Approximately 20 percent by volume of the sedimentary
portion of the earth's crust consists of water that occupies
most of the matrix and fracture porosity of sediments and
sedimentary rocks. These porewaters are variously called
groundwater in freshwater aquifer systems, interstitial
water
in
marine sediments, and formationwater or basinal brines in deep
sedimentary basins, Porewaters as a group range widely in
chemical and isotopic composition and in their physical
properties. There has been considerable interest in the origin
and migration of porewaters because of the role these fluids
play in the geological, geochemical, and economic resource
evolution of sediments and sedimentary basins. The presence
of porewaters under pressure facilitates or may even induce
tectonic deformation of sedimentary basins and continental
margins, Porewaters serve as transport agents for heat and
reactive solutes in sediments, and variations in the composition
of porewaters in space and time yield important information
on mechanisms and rates of solute transport and diagenetic
reaction. The study of porewaters thus complements the study
of diagenesis obtained by the sedimentological, mineralogical,
and geochemical study of the solid phases present in a
sediment.
Historical development and outline
The origin of waters in the earth's crust has been speculated
upon since ancient times (Hanor, 1988), Prior to the 1600s,
there was a general belief that fresh groundwaters were formed
by the subsurface distillation of seawater which had infiltrated
deep into the earth. These freshwaters get discharged at the
surface to form rivers and streams, Seawater salt was left
behind in the form of subsurface brines. Beginning with the
publication of Perrault's mass balance studies in 1674, it was
eventually recognized that rivers, streams, and fresh ground-
waters are meteoric in origin. Debate on the origin of
subsurface brines in sediments, however, has continued up
until the present time. With the advent of modern geochemical
techniques in the last half of the past century, much has been
learned about the chemical and isotopic composition of
porewaters in sediments and their origin. Chief sources of
information have been the analysis of produced waters from
oil and gas fields, the analysis of potable groundwaters, and
the extensive body of analyzes of waters in deep sea sediments
generated as part of the JOIDES and ODP drilling projects.
This article reviews the types of solutes found in sedimen-
tary porewaters and the general types of processes which
control or influence porewater compositions. This background
information is then applied to three major types of sedimen-
tary pore waters: (1) shallow continental waters; (2) marine
interstitial waters in continental shelf and deep sea sediments;
and (3) waters in deep sedimentary basins. The focus is
primarily on the geochemistry of porefluids, but it should be
recognized that many important physical properties of pore-
waters and bulk sediments, such as density, viscosity, thermal
and electrical conductivity, and sonic transit time, are directly
dependent on fiuid temperature, pressure, and chemical
composition.
General composition of porewaters
Concentration units
A wide variety of units have been used to describe the
concentration of solutes in sedimentary porewaters. Concen-
trations are typically expressed in terms of mass, moles, or
equivalents of solute per liter of solution, kilogram of solution,
or kilogram of H2O, Many groundwater analyzes are
presented in mg/L or g/L, units which are convenient because
of the volumetric methods used in most analytical techniques.
Many in the marine geochemistry community, however, use

538
POREWATERS IN SEDIMENTS
mass or moles per kg of solution, which are pressure and
temperature independent concentration units. The concentra-
tion unit molality, mol/kg H2O, is used in thermodynamic
calculations of saturation state. One final unit is the equivalent
(eq),
where the number of equivalents of a solute is equal to the
number of moles of solute times absolute charge of the solute.
The units eq/L or meq/L are widely used in making charge
balance calculations from water analyzes and in defining
hydrochemieal facies, as described below.
Salinity and major solutes
The polar nature of the H2O molecule makes water an
excellent solvent for charged ions and other polar molecules.
The salinity or total dissolved solute (TDS) concentrations of
porewaters varies greatly, even within individual sedimentary
units.
Salinities of a few hundred mg/L or less are typical of
sediments fiushed by meteoric waters. The average salinity of
seawater is approximately 35,000 mg/L, and the porewaters in
many marine sediments have similar salinities. The salinities of
waters in the deeper parts of sedimentary basins, however, can
reach levels of 350,000 mg/L or more.
Although a wide range of dissolved species exist in natural
waters, the greatest proportion of TDS is contributed by a
relatively few species, typically Na+, K+, Mg^+, Ca^+, Cl",
HCOs", and SO4 , Several factors determine the major solute
concentration of a porewater. These include relative abun-
dance of the potential solute, the ease of accommodating the
solute into liquid water, and reaction rates and solubility
constraints imposed by coexisting mineral phases and reactive
mineral and organic surfaces. Cation accommodation in water
is determined in part by ionic potential, the ratio of cation
charge to ionic radius in Angstroms, Cations of small ionic
potential, roughly 3 or less, are readily accommodated in
aqueous solution as hydrated cations. These include most of
the group IA and IIA cations, including Li"*", Na"*", and K"*",
and Mg^+, Ca^+, Sr^+, and Ba^*, Small, highly charged
cations having an ionic radius of 12 or greater form soluble
charged anionic complexes with oxygen. Common examples
include the anionic complexes ofthe oxidized forms of C, N, P,
and S, which include HCO3" and CO3", NO3", PO4" , and
SO4",
Cations of intermediate ionic potential, however, are
accommodated in aqueous solution with much greater
difficulty. These include the abundant rock-forming cations
Al''"'",
Fe^"*", and Mn'*"'", which tend to react with water to form
insoluble hydroxides or to remain bound in silicate or oxide
mineral lattices. Silica forms the sparingly soluble complex,
silicic acid, H4SiO4, Valence state plays a key role in the
relative solubility and mobility of a cation. The reduced forms
of iron and manganese, Fe^"*" and Mn^""", are far more soluble
than the oxidized forms Fe^^ and Mn''"'", Halogen elements
form soluble, hydrated anions: F~, CP, Br~, I~,
Minor inorganic species
Sedimentary porewaters as a group contain measurable
concentrations of virtually every naturally occurring element
in the periodic chart. Some elements, such as Pb, Zn, and Cu,
sometimes occur in sufficient concentration to make the
porewater in question, generally a basinal brine of high
salinity, a potential ore-forming fluid. Others, such as Br, are
useful in tracing the origin of high salinity in porewaters. Some
minor and trace elements, such as Ba, Ra, and As, pose
potential health problems when they are present in drinking-
water supplies.
Gases and dissolved organics
Most gases and nonpolar organic molecules are only sparingly
soluble in aqueous solution, but their presence can be of
importance in driving diagenetic reactions and in deducing the
origin of porewater. The microbial consumption of dissolved
oxygen and production of dissolved CO2 are two important
subsurface reactions which influence the dissolved gas compo-
sition of natural porewaters (Chapelle, 1993), Groundwaters
derived from rainwater typically contain atmospherically
derived noble gases, whose concentration can be used to
estimate the temperature of the water at the time of recharge.
Waters in the deeper parts of sedimentary basins can contain
primordial helium, '^He, thought to be derived from the mantle
or deep crust. Very shallow groundwaters typically contain
minor concentrations of fulvic and humic acids derived from
the breakdown of organic matter (Drever, 1997), and basinal
waters often contain a variety of low molecular weight organic
compounds produced by the thermal maturation of organic
matter,
pH and redox state
The pH is defined as minus the log of the activity of the
hydrogen ion, —log a(H''') and is routinely determined on
porewater samples as a measure of acid-base conditions. The
pH of natural porewaters varies from over 9 for some shallow
groundwaters to less than 4 for some subsurface brines.
Several conventions are in use to describe the redox state of
natural waters: Eh, or electrode potential; pe, minus the log of
the activity of the electron; and foe, the fugacity of oxygen.
Sediments and fiuids in close proximity to the atmosphere or
to oxygenated seawater are typically oxidized because of the
availability of dissolved O2, In organic-rich sediments, how-
ever, conditions generally become progressively reducing with
depth or distance from recharge area,
Isotopic composition
Several major isotopic systems have been utilized to deduce the
origin of solutes and of H2O in different types of porewater.
Hydrogen-oxygen systematics have been used to distinguish
between H2O derived from meteoric precipitation and H2O left
behind in residual brines during the evaporation of seawater
(Hanor, 1988), Chlorine and t isotopic systematics have been
used to help constrain the apparent age of groundwaters.
Carbon and Sr, and more recently Li and B, isotopic
systematics have been used to deduce the sources of these
solutes and type and extent of diagenetic reaction between
fluids and mineral phases.
Graphical representation of porewater compositions
and hydrochemieal facies
The composition of shallow fresh groundwaters varies
considerably in terms of the relative proportion of major
cations and anions, more so than either marine-derived
interstitial waters or waters in deep sedimentary basins. It
has been a challenge to develop simple graphic approaches
to portray the spatial and temporal variations in typical

POREWATERS IN SEDIMENTS
539
groundwater composition (Domenieo and Schwartz, 1990), A
useful convention is the concept of hydrochemieal facies
(Back, 1961), where the composition of porewater is described
in terms of those few cations and anions which contribute the
bulk of the ionic charge in equivalents (eq/L) or milliequiva-
lents (meq/L), A Ca-HC03 porewater, for example, is one
dominated in terms of equivalents of charge by Ca^^ and
HCO3",
although lesser amounts of many other solutes may be
present. Prefixes such a F", B", S", and HS", representing
i"resh, brackish, saline, and hypersaline, may be added so that
some qualitative information on TDS is conveyed as well.
General physical and chemical controls on the
composition of porewaters
The general types of chemical reactions and physical processes
which control porewater compositions are general to each of
the three end-member types of pore waters discussed here. The
physical processes include advection, which is the bulk
transport of solutes through the pore spaces of a sediment
by the fluid flow, and fluid mixing which occurs as a result of
molecular diffusion and hydrodynamic dispersion around
mineral grains, Advection can be induced by sediment
compaction, by differences in topographic elevation, by
differences in fluid density, and by fluid pressures in excess
of hydrostatic (Domenieo and Schwartz, 1990),
The types of chemical reactions which influence porewater
composition include; hydrolysis or dissolution-precipitation
reactions involving porewater and bulk mineral phases, many
of which involve hydrogen ion and are thus acid-base
reactions; redox reactions in which there is a transfer of
electrons, usually between C, N, Fe, Mn, and S-bearing solutes
and solids; and mineral and organic surface reactions involving
adsorption and ion exchange. Many reactions involving
porewaters and ambient mineral phases occur nearly simulta-
neously. For example, the production of
H"*"
by the oxidation
of a sulfide mineral may induce the hydrolysis or dissolution of
a silicate mineral. Some of the cations released in the process
may be adsorbed onto the surface of newly formed clay
minerals produced by the destruction of the silicate precursor.
The combined effects of advection, dispersion, and chemical
reaction on the changes in spatial and temporal concentration
of a solute with time (dC/7d/) along a one-dimensional flow
path can be described by the following mass balance equation:
1-1)
where / is time,
v
is advective fluid velocity,
(/>
is porosity, and .v
is distance, O, is the coefficient of hydrodynamic dispersion for
solute /, and Rij is the net source-sink term for solute / for
chemical reaction y (Liehtner
e;a/,,
1996),
The first term on the right accounts for the net increase or
decrease in C, as the result of the advection or bulk flow of
fluid of varying C, through the sediment. The second term
describes the mass balance resulting from mixing due to
molecular or Fickian diffusion and dispersion. The final term
is the net sum of the rate of the chemical reactions involving
other phases and solute /, Variants on this mass balance
equation in one, two, and three dimensions are at the heart of
mathematical computations designed to couple the physical
and chemical processes which control porewater compositions.
These calculations are also used to quantify the mass transfer
which occurs between mineral and fiuid phases during
diagenesis. In one-dimensional systems, it is sometimes
possible to solve the equation analytically (Berner, 1980), but
in complex heterogeneous systems numerical techniques are
required (Liehtner etal., 1996),
Continental groundwaters
Topographically-driven meteoric groundwater systems
The sources of porewaters found in shallow freshwater
aquifers and interbedded aquicludes are meteoric precipitation
and recharge by lakes and streams during high surface water
levels.
That portion of rain or snow melt which does not form
runoff infiltrates downward into the unsaturated zone, where it
may be returned to the atmosphere via evapotransporation
and/or migrate further downward to recharge an underlying
aquifer. The groundwater within an aquifer will then typically
migrate both downward and laterally along preferred flow
paths and eventually discharge into either another continental
surface environment, such as a spring, lake, or stream, or into
the offshore submarine environment. Most meteoric ground-
water flow is driven by differences in topographic elevation
between areas of recharge and discharge (Domenieo and
Schwartz, 1990),
Rainwater typically contains several mg/L or more of
dissolved salts, Diagenetic alteration of meteoric water
compositions, however, occurs immediately within the soil
environment (Drever, 1997), The carbonic acid and organic
acids produced by soil bacteria are important weathering
agents, and the consequent dissolution of carbonate and
silicate minerals results in the leaching of the more soluble
cations, such as Na"*",
K."*",
Mg^"*", and Ca^""^, from the soil.
Although there is some complexing of Al and Fe by organics,
the less soluble cations, such as AP"^, Si"*"^, and Fe "*" are
preferentially left behind in residual mineral phases. Oxidation
of sulfide minerals produces hydrogen ions, which can induce
further weathering, and dissolved sulfate. Cation exchange on
newly formed clay minerals favors retention of the divalent
cations Mg^""" and Ca^"*" in freshwater environments. High
rates of evapotranspiration relative to rainfall in arid climates
can serve to increase the TDS of soil waters.
Mineral-fluid and organic reactions continue as ground-
water migrates along flow paths within the saturated zone,
flow paths which are determined by spatial variations in
permeability and hydraulic head. The local presence of
evaporite minerals such as halite, anhydrite, and gypsum can
add Na, CI, Ca, and SO4 to the groundwater. As the
groundwater flows down gradient from recharge areas it may
encounter progressively reducing conditions as dissolved
oxygen is depleted by aerobic respiration. Nitrate, manganese,
amino acids, iron, and organic carbon are the typical
succession of electron acceptors microbes then use to oxidize
organic carbon as subsurface conditions pass progressively
from oxidizing to reducing (Chapelle, 1993), When conditions
become sufficiently reducing, the reduction and dissolution of
Fe and Mn minerals commonly gives rise to significant
concentrations of these metals in shallow groundwaters. With
a further decrease in redox state, however, sulfate is reduced to
sulfide, and Fe may be precipitated out as an Fe-sulfide,
In carbonate aquifers, it is not surprising to find ground-
waters dominated by Ca-HCO3 or Ca-Mg-HCO3 as the
principal solutes, A well-studied example is the Floridian

540
POREWATERS IN SEDIMENTS
Aquifer in central Florida (Sprinkle, 1989), tn silieielastic
aquifers, however, there is typically a progression from Ca-Na-
HCO3 waters in shallow recharge areas to Na-Ca-HCO3 and
then Na-HCO3 waters further down dip. Examples include the
US,
Atlantic and Gulf coastal plain aquifers, where bicarbo-
nate concentrations increase down flow paths from initial
values of less than 50 mg/L to over 500 mg/L (Chapelle, 1993),
The probable source of CO2 is bacterially oxidized organic
material present in the aquifers and/or the interbedded
aquicludes. The origin of high Na-HC03 groundwaters in
silieielastic aquifers has been a matter of some debate. One
school of thought holds that the removal of Ca is predomi-
nantly by ion exchange, the other that Ca is removed as
CaCO3 by the introduction of HCO3,
Continental brines
In arid continental climates where potential evapotranspira-
tion far exceeds rainfall, circumstances can exist which give rise
to the formation of brine lakes. Well-known examples include
Lake Magadi, Kenya; Lake Tyrrell, Murray Basin, Australia;
and Great Salt Lake, Utah, The composition of the brines
produced depends critically on the relative proportions of Ca,
HCO3,
SO4, and Mg in the fluid being evaporated. Documen-
ted brine types include Na-Ca-Mg-Cl, Na-Mg-SO4-Cl, and
Na-CO3-SO4-Cl saline waters (Eugster and Hardie, 1978),
These brines recharge into underlying sediments as a result of
density-driven flow, where dissolved Mg is removed by the
formation of Mg-rich smectite, sepiolite, Mg-calcite, and/or
dolomite, and sulfate can be reduced by reduction to sulfide. In
contrast to freshwater conditions where ion exchange favors
the adsorption of divalent cations, monovalent cations,
particularly K, are preferentially adsorbed on clays in saline
conditions and removed from solution.
Porewaters in marine sediments
Fluid flow and solute transport in many marine sediments,
particularly in deep sea pelagic and hemipelagic sediments are
dominated by compaction-driven advection and molecular
diffusion (Berner, 1980), More dynamic fluid environments are
found on continental shelves, where topographically driven
meteoric fluids can mix with marine porewaters; in accre-
tionary prisms, where there is well-documented seaward
expulsion of fluids; and along mid-ocean rises and ridges and
other submarine volcanic settings, where thermal convection
operates (Hesse, 1990),
Seawater is a Na-Ci dominated fluid with SO4, Mg, Ca, and
K as the other major species. The relative proportion of these
solutes is nearly constant throughout the world's oceans, with
the exception of S04-depleted, anoxic bottom waters in closed
submarine basins. Much of the water trapped in pelagic and
hemipelagic sediments at the time of deposition is seawater.
This water, however, undergoes continuous chemical diagen-
esis and a change in composition with time and depth of burial
(Hesse, 1990; Schuiz and Zabel, 2000), There is typically a
systematic increase in Ca and systematic decreases in Mg and
K with depth. The variations in Ca and Mg are generally
thought to be the result of hydrolysis of silicate minerals in
underlying oceanic basement basalts. The downward decrease
in dissolved K may be related to the formation of the
potassium-bearing zeolite, phillipsite, and adsorption by ion
exchange on clays. There is often a distinct downward decrease
in 5'*0 values of marine interstitial waters, commonly
interpreted as resulting from preferential removal of heavy
oxygen by the formation of phyllosilicates in altered igneous
rocks and volcaniclastic sediments. Increases in 5'^0 increases,
however, have been noted in gas-hydrate bearing sections of
DSDP cores. Elevated temperatures and enhanced reaction
rates associated with submarine hydrothermal activity, such as
in the Guyamas Basin, methase release of Ca and uptake of
Mg by hydrothermally altered basalts and volcaniclastic
sediments. There are also documented increases in dissolved
Li,
K, and Rb in these areas.
Silica concentrations are typically highly variable with depth
and are dependent on lithology. Elevated silica concentrations,
for example, are found in siliceous oozes, Recrystallization of"
biogenic pelagic carbonates releases substantial amounts of Sr
but does not seem to have a significant effect on Ca
concentration gradients.
With the exception of dissolution of evaporite minerals,
chloride does not participate in mineral-fluid reactions in the
subsurface. Its behavior is thus said to be conservative. As a
conservative species chloride often does not display vertical
concentration gradients in marine sediments. The ciianges in
chloride which have been observed in evaporite-free sections
result from mixing with freshwater released from gas hydrates,
loss of water in hydration reactions, and possibly slight changes
in seawater chlorinity during glacial-interglacial cycles. There
is typically a reduction in porewater chloride and salinity in
decoilments and faults in accretionary wedges as a result of
dehydration reactions and large-scale fluid expulsion.
In more organic-rich hemipelagic sediments redox condi-
tions become progressively reducing with depth. As a result of
a progression of redox reactions similar to those which
characterize continental groundwaters, dissolved ammonia,
phosphate, and alkalinity typically increase in the upper part
of the sediment column, and dissolved sulfate is reduced
(Berner, 1980),
Saline waters in deep sedimentary basins
The hydrogeology of deep sedimentary basins is less well-known
quantitatively than that of shallow groundwater systems, but
deep basin circulation plays a key role in controlling the salinity
and composition of basinal fluids through advection and
dispersive mixing of fluids of varying salinity. The deep pore
fluids in many sedimentary basins are overpressured, i,e,, they
exhibit fluid pressures in excess of hydrostatic pressures. There
has been considerable debate on the origin of overpressuring
(Hanor, 1988), but rapid deposition of fine-grained sediment of
low permeability is one likely mechanism. There is evidence
from variations in salinity and fluid composition that over-
pressured fluids can be episodically expelled upward into
overlying hydropressured sediments (Roberts and Nunn,
1995),
The presence of bedded and diapiric evaporites provide
other mechanisms for inducing fluid flow. The dissolution of
halite produces dense brines which can eonvect downward.
Salt, however, is also an excellent conductor of heat, and there
have been documented examples of upward fluid flow along the
flanks of warm salt domes (Hanor, 1994),
Sources of chloride and controls on salinity
Pore waters in the deeper parts of sedimentary basins,
including basins now part of the continental crust, and basins

POREWATERS IN SEDIMENTS
541
containing tectonically deformed sedimentary sequences, are
typically saline. The salinities are commonly higher than that
of seawater (35,000 mg/L) and can range from 100,000 mg/L to
150,000 mg/L in basins with salt domes to over 350,000 mg/L
in basins with bedded basal evaporites. In some sedimentary
basins, such as the Illinois, Alberta, and Michigan basins, there
is a progressive increase in salinity with depth. In other
sedimentary basins, such as the Gulf of Mexico basins there
can be reversals in salinity with depth (Hanor, 1988), In the
mid-20th century, much discussion was given to the origin of
high salinity by the process of membrane filtration or
reverse osmosis. In this process overpressuring forces un-
charged water molecules through clay membranes, but the
negative charge on the clay particles repels charged ions and
produces high concentrations of solutes on the influent side of
the membranes. Most workers today, however, consider the
chloride in basinal brines to have been introduced by some
combination of the subsurface dissolution of halite (Land,
1997) and the infiltration of subaerially evaporated marine
waters (Carpenter, 1978), During the evaporation of seawater,
Br preferentially remains in solution while CI is precipitated
out, first as halite and then as K and Mg salts (McCaffrey
et al., 1987), Brines formed by dissolution of halite thus
typically have low Br/Cl ratios, which reflect the low Br/Cl
ratio of the parent halite. Brines formed by the subaerial
evaporation of seawater, in contrast, typically have elevated
Br/Cl ratios.
Major solutes
Most basinal brines of moderate salinity are dominated by
dissolved Na and CI. As salinity increases, however, there is a
progression from Na-Cl to Na-Ca-Cl to Ca-Cl brines. The
controls on the composition of basinal brines have been
discussed and debated for a century and a half (Hanor, 1988),
In the mid-1800s it was proposed that the high Ca concentra-
tions were connate or syngenetic in origin and reflected high
Ca/Na ratios of ancient seawater, a hypothesis which is no
longer accepted. Although most of the chloride in basinal
brines is derived from halite dissolution or from marine and
evaporated marine waters, basinal brines have neither the
composition of a NaCI solution or of evaporated marine
waters. Instead, there are systematic increases in Na, K, Mg,
Ca, and Sr with increasing salinity. The 1:1 slope of the
monovalent cations and 2:1 slope of the divalent cations on
log-log plots is consistent with the buffering of brine
compositions by multi-phase silicate and carbonate mineral
assemblages (Hanor, 1994), The phases involved most likely
include minerals such as quartz, K-feldspar, albite, illite,
smectite, chlorite, calcite, and dolomite. The general decrease
in pH and carbonate alkalinity with increasing salinity is also
consistent with rock-buffering of fluid compositions, Sulfate
concentrations, in contrast to the other major solutes, do not
show any systematic variations with salinity, Sulfate con-
centrations instead are controlled by anhydrite dissolution and
the reduction of sulfate to sulfide, Basinal brines and gases
in iron-poor sediments can contain significant concentrations
of H2S,
Organic and tieavy metals
Volatile fatty acids (VFAs), such as acetate, propionate, and
butyrate, are found in concentrations exceeding 100 or even
1000 mg/L in some basinal waters. These waters tend to be of
low salinity and may reflect water released from hydrocarbon
source rocks during maturation of organic matter. Under
conditions of high fluid pressure, concentrations of dissolved
methane exceeding several thousand mg/L can be forced into
solution,
Basinal brines have long been considered the fluids involved
in the formation of some sediment-hosted ore deposits,
including Mississippi Valley Type (MVT) and sedimentary-
exhalative (SEDEX) deposits. Although much consideration
has been given to the role of organic complexing agents, such
as acetate, in solubilizing heavy metals such as Pb and Zn,
more recent work has shown that chloride is the key
complexing agent (Hanor, 1997), At chloride levels in excess
of
100
g/L, the PbCll" and ZnCl^" complexes become effective
solubilizing agents for Pb and Zn, Barite (BaSO4) is also a
common mineral phase in MVT and SEDEX deposits. High
concentrations of Ba are found in those basinal brines that
have low sulfate concentrations.
Isotopic composition
The isotopic composition of basinal brines is significantly
influenced by diagenetic reactions. Exchange of oxygen with
silicate minerals can increase the <5'*0 values of basinal
waters and the introduction of H from maturation of organic
matter can lower 5^H values. Other changes include the
introduction of radiogenic *'Sr and changes in the Li and B
isotopic compositions from the diagenesis of silieielastic
sediments.
Summary
Even though the salinity and composition of porewaters in
sediments and sedimentary rocks varies widely, many of these
variations are systematic and can be explained in terms of
basic principles of solute transport and chemical reaction.
In terms of major solutes, redox reactions involving organic
matter are important in influencing the concentrations of SO4
and HCO3 in all porewaters. Reactions involving sili-
cates,
carbonates, and evaporites, however, are the ultimate
controls on the addition and removal of Na, K, Mg, Ca,
and CI,
As a group, meteoric groundwaters have the highest
variation in proportions of major solutes, which reflect highly
dynamic flow systems and local lithologic control on water
compositions. Many interstitial marine waters still retain a
strong seawater signature despite on-going organic and
mineral diagenesis, Diffusional exchange with overlying sea-
water and within the sediment column is one factor contribut-
ing to the buffering of fluid compositions in these sediments.
Saline waters in deep sedimentary basins show systematic
changes in the concentrations and relative proportions of most
major solutes, reflecting rock buffering involving multiple
sediment lithologies.
Much work remains to be done in quantifying our under-
standing of the controls on the properties of pore fluids,
including establishing the driving forces and rates of solute
transport and the kinetics of the diagenetic reactions which
cause mass transfer to occur between porefluids and their
sedimentary matrix,
Jeffrey S, Hanor

542
PRESSURE SOLUTION
Bibliography
Appelo, C,A,J,, and Postma, D,, 1993,
Geochemistry,
Groundwater,
and
Pollution. Rotterdam: A,A, Balkema,
Back, W,, 1961, Techniques for mapping hydrochemieal facies, US
Geological
Survey Professional
Paper,
424 (D): 380-382,
Berner, R,A,,
\9%Q.
Early
Diagenesis:
ATheoretical Approach. Princeton
University Press,
Carpenter, A,B,, 1978, Origin and chemical evolution of sedimentary
brines in sedimentary basins. Oklahoma Geological Survey Circular,
79:
60-77,
Chapelle, F,H,, 1993, Ground-Water Mierohiology and Geochemistry.
Wiley,
Domenieo, P,A,, and Schwartz, F,W,, 1990, Physical and Chemical
Hydrogeology. New York: Wiley,
Drever, J,I,, 1997, The Geochemistry of Natural Waters, Surface and
Groundwater Environments, 3rd edn, Prentiee-Hall,
Eugster, H,P,, and Hardie, L,A,, 1978, Saline Lakes, In Lerman, A,
(ed.).
Lakes—Geochemistry,
Geology,
Physics. New York: Springer-
Verlag, pp. 237-293,
Hanor, J,S,, 1988, Origin and Migration of Suhsurface Sedimentary
Brines, SEPM Short Course No. 21, Tulsa: Society of Economic
Paleontologist and Mineralogists.
Hanor, J.S,, 1994. Physical and chemieal eontrols on the composition
of waters in sedimentary basins. Marine and
Petroleum
Geology,
11:
31-45,
Hanor, J,S,, 1997. Controls on the solubilization of dissolved lead and
zinc in basinal brines. In Sangster, D,F, (ed.), Carhonate-Hosted
Lead-Zinc Deposits, Littleton: Society of Economic Geologists,
Special Publication, 4, pp. 483-500.
Hesse, R,, 1990, Early diagenetic porewater/sediment interaction:
modern offshore basins. In Mcllreath, LA., and Morrow, D.W.,
(eds.),
Diagenesis, Geoscience Canada Reprint Series
4,
pp, 277-316.
Land, L,S,, 1997. Mass transfer during burial diagenesis in the Gulf of
Mexico Sedimentary Basin: an overview. In Montanez, L, Gregg,
J,M,, and Shelton, K,S, (eds,), Basinwide Fluid Elow and Assoeiated
Diagenetic Patterns, Tulsa: (SEPM) Society for Sedimentary
Geology, Special Publication, 56, pp, 29-40.
Liehtner, P.C., Steefel, C.L, and Oelkers, E,H, (eds.), 1996, Reactive
Transport
in Porous Media, Reviews in Mineraiogy 34. Mineralogieal
Society of America,
McCaffrey, M,A., Lazar, B,, and Holland, H.D,, 1987, The evapora-
tion path of seawater and the eopreeipitation of Br~ and K+ with
halite, Journai of Sedimentary
Petrology,
57: 928-937,
Roberts, S,T,, and Nunn, J,A,, 1995. Episodic fluid expulsion from geo-
pressured sediments. Marine and
Petroleum
Geology, 12: 195-204.
Schuiz, H,D,, and Zabel, M, (eds,), 2000, Marine Geoehemistry.
Springer-Verlag,
Sprinkle, C.L., 1989, Geoehemistry ofthe Floridian aquifer system in
Florida and in parts of Georgia, South Carolina, and Alabama,
U.S.
Geological
Survey Professional
Paper,
1403-1,
Cross-references
Diagenesis
Diffusion, Chemical
Evaporites
Fabric, Porosity, and Permeability
Hydrocarbons in Sediments
Isotopic Methods in Sedimentology
Oceanic Sediments
Weathering, Soils, and Paleosols
PRESSURE SOLUTION
A grain-scale mechanism of ductile and
water-assisted deformation
The transition from loose sediments to hard rocks arises
through physico-chemical processes at the grain scale. Pressure
solution, in addition to cataclasis, grain sliding, and plastic
deformation, is one of these processes. Pressure solution takes
place when some aqueous fluid coating is present around the
grains. It is a water-assisted diffusional mass transfer normally
occurring in the top few kilometers of sedimentary basins and
in other geological environments such as fault gouges and low-
grade metamorphie rocks. This slow mechanism of deforma-
tion can induce large amounts of ductile strain over geological
times when stress and temperature are not high enough to
promote brittle or plastic deformation.
The classical pressure solution structures have been de-
scribed since the last century, for example by Sorby in 1863,
They include grain and pebble indentations, stylolites, partly
dissolved voids, dissolution seams, and crenulation cleavage.
Since, extensive field evidence has accumulated which confirms
the prevalence of this mechanism in nature.
Following Sorby's pioneering observations, Weyl (1959)
performed the first quantitative interpretation of pressure
solution and proposed that the driving force for the deforma-
tion be related to stress concentration at grain or pebble
contacts. From natural observations such as those of
Figure PI2, he deduced that deformation by pressure solution
involves several successive steps: dissolution at the grain
contacts, transport of the dissolved solutes toward the open
pore,
precipitation in the pore or transport to other pores. This
results in an indentation of the grains into each other and a
decrease of rock porosity resulting in a ductile compaction,
Mechano-chemical processes
A crucial parameter for pressure solution is the presence of
water in the pores, which acts as a medium of reaction and
transport with the minerals. If a porous medium is not
saturated with respect to water, pressure solution will be
localized in the pores where water is present. This effect can
create compacted regions in the sediment whereas the porosity
of other regions will not be modified.
At the grain scale, pressure solution deformation occurs
through three serial steps, the whole process being driven by
stress gradients along the grain surface. First, minerals dissolve
at grain contacts because of a concentration of stress. The
stress variation between the grain contact and the pore surface
can be related to the cliemical potential of the dissolving
mineral (Paterson, 1973), The result is that the coriceritratipn
in aqueous species is greater in the contact film and induces a
gradient of concentration between the contact film and the
pore.
The second step involves solutes in the contact film
diffusing along the grain boundaries. The nature of the
interface between two grains is crucial because it is a medium
of dissolution and diffusion of matter. It is assumed that some
water is trapped inside this interface. And the third and last
step is precipitation on the free face of the grains. Note that
solutes can be transported by diffusion in the pore fluid and
precipitate at some distance from the dissolution area. This
provides an explanation of mass transfer at centimeter scale in
sedimentary environments under local gradients of stress.
If one of these three steps is slower than the two others, it
will control the kinetics of the overall deformation. For
example, diffusion within grain contact films controls the
compaction of salt aggregates at room temperature. In
sedimentary basins, compaction of quartz-rich sediments is
limited by the step of quartz precipitation between 3 km and
5 km and by the diffusion at the grain contact below
5
km,
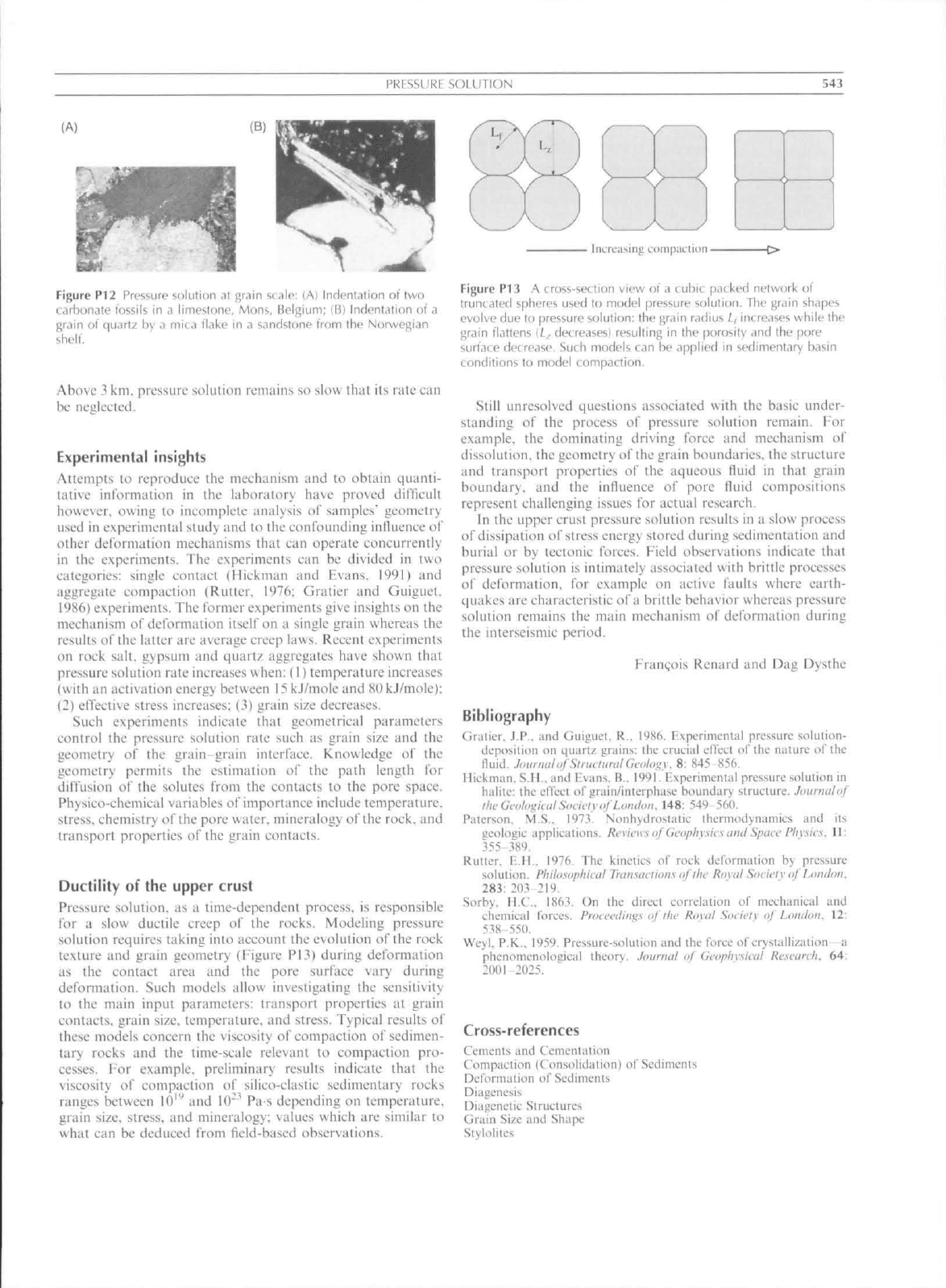
PRESSURE SOLUTION
543
Figure
P12
Pressure soluliun
,Tt
grain scale:
(A)
Indentation
ol two
L.irbon.ile
lossiU
in a
limestone, Mons, Belgium;
(B)
Indonlation
of a
grain
of
quartz
by a
mica flake
in a
sandstone trom
ihe
Norwegian
shell.
Above
3
km, pressure soltition remains so slow that its rate can
be neuleeted.
Experimental insights
Attempts to reproduce the mechanism and to obtain quanti-
tative infortnation in the laboratory have proved diftieult
however, owing to incomplete analysis of samples" geometry
used in L'\perimenta! study and to the confounding influence of
other deformation meehanisms that ean operate concurrently
in the experiments. The experiments can be divided in two
categories: single contact (Hickman and Evans. 1991) and
aggregate compaetion (Rutter. 1976; Gratier and Guiguet.
19S6) experiments. The fortner experiments give insights on the
meehanism of deformation itself on a single grain whereas the
results ofthe latter are average creep laws. Recent experiments
on rock salt, gypsum and quartz aggregates have shown that
pressure solution rate increases
when;
(1) temperature inereases
(with an activation energy between
15
kJ/mole and
SO
kJ/mole);
(2) effective stress inereases; (3) grain size deereases.
Such experiments indicate that geometrical parameters
control the pressure solution rate sueh as grain size and the
geometry of the grain-grain interface. Knowledge of the
geometry permits the estimation of the path length for
diffusion of the solutes from the eontacts to the pore space.
Physico-chemical variables of importance inelude temperature,
stress, chemistry ofthe pore water, mineralogy ol the rock, and
transport properties of the grain contacts.
Ductility of the upper crust
Pressure solution, as a time-dependent process, is responsible
for a slow ductile creep of the rocks. Modeling pressure
solution requires taking into account the evolution of Ihe roek
texture and grain geometry (Figure P13) during deformation
as the contact area and the pore surface vary during
deformation. Sueh models allow investigating the sensitivity
to the main input parameters: transport properties at grain
contacts, grain size, temperature, and stress, Typieal results of
these models eoneern the viscosity of compaetion of sedimen-
tary rocks and the time-scale relevant to eompaetion pro-
cesses, }-\ir example, preliminary results indieate that the
viscosity of eompaetion of silico-clastic sedimentary rocks
ranges between lO''* and lO'"' Pa
s
depending on temperature,
grain size, stress, and mineralogy; values which are similar to
what can be deduced from field-based observations.
g compaction-
Figure
PI
3
A
cross-section view
ot a
cubic pocked network
of
truncated spheres used
to
model pressure solulion.
The
grain shapes
evolve
due to
pressure solution:
the
grain radius
i,
increases while
ihe
grain flattens
it^
decreases! resulting
in the
porosity
and Ihe
pore
surface decrease. Such models
can he
applied
in
sedimentary basin
conditions
to
model compaction.
Still unresolved questions associated with the basie under-
standing of the process of pressure solution remain. For
example, the dominating driving foree and meehanism of
dissolution, the geometry ofthe grain boundaries, the structure
and transport properties of the aqueous fluid in thai grain
boundary, and the infiuenee of pore fluid compositions
represent challenging issues for aetual research.
In the upper erust pressure solution results in a slow proeess
of dissipation of stress energy stored during sedimentation and
burial or by teetonic forces. Field observations indieate that
pressure solution is intimately assoeiated with brittle processes
of deformation, for example on active faults where earth-
quakes are eharaeteristic of
a
brittle behavior whereas pressure
solution retiiains the main mechanism of deformation during
the interseismic period.
Frant;ois Renard and Dag Dysthe
Bibliography
Gralier,
J.P., and
Giiigtiet,
R,,
I98(S, ExpcrimetUal pressure solulion-
depositioti
on
qaariz grains:
the
crucial etlect
of the
naltirc
of ihe
fluid,
.fournalof Struetund
Geology.
8: S45 S56,
Hiekman,
S,H,,
and
Hvans,
R,,
19'JI,
F_\perimem;il presstirc solulion
in
halite:
the
effect
of
grain/interphasc boundary siructure, .foarnalof
ilic
Geological .Society
of London,
148: 549 560.
Paterson,
M,S,,
197."^, Nonhydrostatic thermodynamies
and its
geologic appliciUions,
Reviens
of
Geophysics
ami
.Space
Physics.
11:
355-.189,
Rutier,
E,H., 1976. The
kinelics
of
rock dclormaiion
by
pressure
solulion.
Phihi.sophical
Trim,sad ions
of
the Royal Society
of
London.
283:
203 219.
Sorby,
H,C.. 1863, On the
direct correlation
of
mechanical
and
chemical forces.
Proeeedim^s
of
the
Royal Society
of
London.
12:
53S-.S.SO.
Weyl.
P.K., 1959,
Prcssure-solulion
and the
force
of
crystallization
a
phenomenologiciil theory. Journal
of
Geophrxieal Researeh.
64:
2001-2025,
Cross-references
Cements iind Cementaiion
Compaction (Consolidation)
of
Sediments
Delormalion
oi'
Sediments
Diagenesis
Diagenetic Struetures
Grain Size
and
Shape
Stylolites

544
PROVENANCF
PROVENANCE
Introduction
On Eiirtli. sedimentation is diivcn principally by tectonic
forces, subordinately by climatic iind eustatic forces, and to a
minor extent in response to impact processes. Detrital
sediments retain a record of eroded crustal material. Knowl-
edge of crusts ofthe past is gleaned from provenance studies.
Tracking the immediate and ultimate sources and origins of
sediments, principally of silieielastic sedimeiils. is the domain
of" provenance studies. In addition, estimating amounts,
proportions, and rates of supply of sediments from multiple
sources is also within the purview of provenance studies.
Contemporary investigations, therefore, focus not only on
types,
identification, and tectonic settings of source rocks, or
the climatic conditions in source areas, or paleogeographie
localities of source areas, but also on quantitative distributions
of source material and the kinematics of source area tectonics.
Approaches, i.e,, methodologies of investigations, depend
generally on the purpose ofthe investigators and (he scientific
questions address by them, but are largely multidisciplinary
and diverse (Zuffa, 19S5; Morton etal.. 1991; Johnsson and
Basu, 1993; Bahlbourg and Floyd, 1999; DeCelles elal.. 19yS).
No parent rock or parent roek-assemblage is ever fully
preserved in any sediment, Beeause post-derivation weath-
ering, transport, and depositional, and espeeially diagenetic
processes determine the preservation potential of original
detrital signatures and lead to disproportionate representation
of parent material in sediment, methodologies have to be
appropriately adapted in specific cases. We discuss some of the
methods before deseribing the results and the kinds of
inferences made in a few recent studies.
The detective work to infer provenance is thus equivalent lo
restoring a jigsaw puzzle with many missing and damaged
pieces.
Historical
Provenance studies have prompted and progressed with newer
instrumentation to determine sediment properties. More than
50 years ago color and shapes of heavy minerals were guides to
parent rocks; some 25 years ago detrital zircons were separated
on the basis of color and shape to obtain U-Pb age-dates to
identify sources in the igneous basement of the Potsdam
Sandstone; currently, age-dating of and oxygen isotope
systematics in single crystals of detrital zircon provides
iiifonnation on the genesis and recycling of crustal roeks.
Similarly, optical microscopic observations are now supple-
mented by backseattercd eleetron image analyzes; ehemical
compositions of sediments extend beyond major element to
trace and rare earth element distribution; and, chemical and
isotopic compositions of single detrital grains are dclermined
by using electron and ion mieroprobes.
Approaches
Methodologies of provenance determination are broadly
divisible into three types: petrographie, ehemical, and isotopic.
Ail three types are increasingly used in provenance interpreta-
tion. Except for the uncommon occurrence of some index
mineral (e.g., glaucophane to indicate a blueschist-type souree
rock) or an index fossil (e.g.. coeval taxa from a disintegrating
bank into a deep sea turbidite). most eontemporary data are
quantitative in nature. Statistical principles of point-counting
methods are applied in most measurements.
Petrographic
Mineralogic compositions and elassification of sandstones
have been linked to source rocks for a long time. For example,
arkosic sandstones and conglomerates, many with granitic
rock-fragments, have been linked to continental shields, and,
lithic graywacke sandstones, many with volcanic and schistose
rock fragments, have been linked to orogenic sources. Relative
concentrations of detrital quartz (Q), feldspar (F), and rock
fragments (R) define sandstone classification. Because the
proportion of roek fragments increases with the increasing
grain size of sediments, and. because quartz and feldspars also
oeeur in rock fragments, estimating the proportions of QFR is
difficult. Results of QFR determination differ depending on
the methodology used especially in treating the grain-size
effect. Some investigators restrict the grain size range of
samples, for example, to medium sand si/e (0.25 0.50mm) for
sandstones. Others point-count quartz and feldspars in coarse-
grained roek fragments as mineral fragments, and restrict
the eounts of rock fragments, designated as lithics (L), to very
fine-grained sedimentary, schistose, and voleanie grains.
Composition fields in QFL space (triangular diagrams) are
sensitive to detritus from different tectonic regimes (Dickinson,
1985),
This is especially true if very fine-grained aggregates of
quartz ( polycrystalline quartz; chert) are assigned to the L-
polc leaving the Q-pole reserved for monocrystalline quartz
only. This converts the QFL diagram to a Q,,,FL, diagram.
Although the fields in OFL/Q,,,I-X, diagrams are intended to
be guides, many sandstone petrologists tend to use a
classificatory approach lo infer tectonic provenance. The
diagram was modified later to erase definitive eompositional
fields emphasizing the essence of the relationship between
sandstone composition and teetonic provenance (Dickinson,
1988;
Figure PI4).
The QFL approach has two limitations. First, errors
associated with the boundaries of compositional fields are
large. Seeond, it ignores the information contained in rock
fragments; especially that carbonate grains may be inlrabasinal
and coeval with the hosl sediment or extrabasinal and
oldcj-
detritus. Both are useful as provenance indiealors. Never-
theless, point counting by the Gazzi Dickinson method, using
QFL/Q||,FL,, is the preferred mode for inferring tectonic
provenance from petrographie analysis of bulk sediments.
Climate profoundly affects mineralogical and ehemical
compositions of sediments. Under wann and wet conditions
relative to cold and dry eonditions. rock-fragments break
down to their constituent minerals more rapidly; they become
depleted in a sediment while quartz, being the most durable
mineral at Earth's stirtace, is enriehed relative to other
minerals. Duration of weathering, be it at roek-outerops or
while a body of sediments is parked temporarily (e,g,, river
valleys, deltas, coastal plains, beaches), contribute to the
degree of quartz enrichment. Common upper erustal meta-
morphie roeks are generally enriched in quartz and are finer
grained than eommon igneous roeks (feldspar-rich and eoarser
grained). Therefore, il is possible to differentiate between
sediments derived principally from metamorphie and igneous
rocks under similar climatic conditions: and, to assess relative
