Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
210 Transport in Fuel Cell Systems
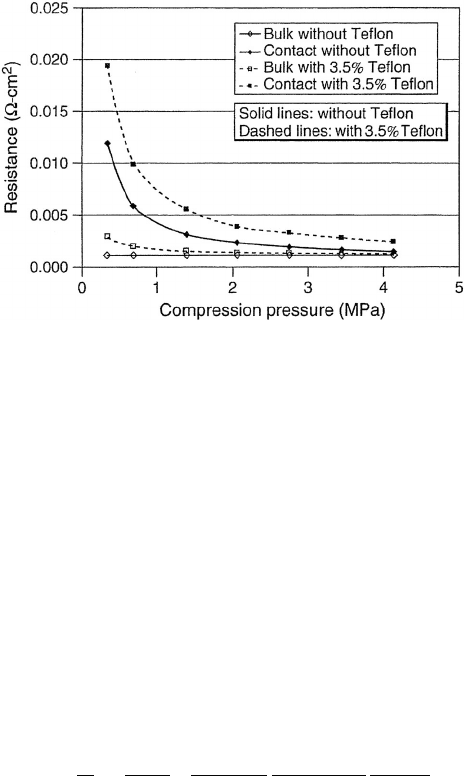
Figure 5.11 Through plane electrical resistance as function of compression pressure for common
carbon fiber paper used as gas diffusion layer in PEFCs. The gas diffusion layers typically have some
fraction of PTFE added to promote liquid water removal, which increases electrical bulk and contact
resistance. (Reproduced from Ref. [18].)
In general, the electrical contact resistance between the current collector and the diffusion
media (PEFC and AFC) or catalyst layer is the largest electrical conductivity loss. In PEFCs,
a common through-plane resistivity is around 0.07 ·cm, with a contact resistance of 0.002
· cm
2
that depends on compression pressure from the lands and Teflon content in the
diffusion media, as shown in Figure 5.11.
Electrical conductivity is much more facile than ionic conductivity, with typical metal
bulk conductivity on the order of
σ
e
≈ 10
4
− 10
6
(
S/cm
)
This compares to a typical ionic conductivity of 0.1–1 S/cm for a polymer electrolyte and
0.1–1 S/cm for a ceramic electrolyte and liquid electrolyte. For a 1-mm-thick metal, the
voltage loss from 1 A/cm
2
current would be very low:
V = iAR =
il
σ
i
=
1 × 1
10
5
m
1000 mm
10,000 cm
2
m
2
m
100 cm
= 1 µV
Because of the low bulk conductivity losses, in practical low-temperature fuel cell applica-
tions, a passivation oxide layer on the metal can dominate the bulk electron transfer losses.
Metal fuel cell bipolar plates are highly robust and can be less than 0.5 mm in total thick-
ness. Because the current collectors and flow fields are often used for mechanical support,
they must have higher electrical conductivity to assure low losses. Remember, each fuel
cell has only 1 V to work with, so even millivolts are important.
5.3 GAS-PHASE MASS TRANSPORT
5.3.1 General Diffusion
Similarly to mass diffusion in electrolytes, gas-phase mass diffusion is driven by concen-
tration gradients. If a spray of perfume is released at the front of a classroom, the students

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.3 Gas-Phase Mass Transport 211
at the front of the room will smell the perfume first. Progressively, students at locations
farther back in the room will be able to smell the perfume. In time, the perfume will disperse
evenly throughout the room. The process by which random molecular motion acts to mix
and eliminate concentration gradients is called diffusion. The fundamental principle behind
bulk diffusion is that of intermolecular collisions, as illustrated in Figure 5.1. Imagine a
room full of moving basketballs representing molecules, initially with red basketballs at one
end of the room and white basketballs at the other end. As the molecules move, they will
occasionally collide with one another. The collisions change the trajectory of the basket-
balls, eventually resulting in a completely homogenous mix of white and red. The average
speed of red basketballs from one end to the other end of the court is representative of the
diffusion coefficient for the red balls into white balls. The average diffusion coefficient of
a given species is a function of the following:
1. The other species present. The other molecules will collide with the diffusing species
and affect the net rate of motion.
2. The number of molecules, which corresponds to pressure. The greater the number of
molecules, the greater the number of collisions, which reduces the average diffusion
rate.
3. The velocity of the molecules, which is proportional to temperature.
4. The size and mass of the molecules (e.g., molecular collision diameter and molecular
weight).
We shall see that the diffusion coefficient for bulk diffusion is indeed a function of pressure,
temperature, molecular size, and weight. Diffusion is a spontaneous process that is a result
of the second law of thermodynamics. The second law of thermodynamics requires that
thermodynamic processes proceed in a way that maximizes entropy. This ultimately requires
uniform mixing of everything in the universe. When there is nonuniform mixing, diffusion
occurs to eliminate concentration gradients and can be written as
˙
n
j,i
=−D
j,i
A
∂C
j
∂x
i
(5.27)
which is identical to the ion diffusion rate equation (5.2) except that it is expressed as a
total rate, by multiplying the flux by the corss-sectional area, A. In general, this is known
as Fick’s law of diffusion, where D
j,i
is the diffusion coefficient of species j with units
of cubic meters per second, A is the area through which diffusion occurs, C
j
is the molar
concentration of j, x
i
is the direction of transport (x, y,orz direction), and n
j,i
is the molar
rate of transport of j in the i direction. In principle, the diffusion coefficient can be a function
of concentration, temperature, pressure, other species, and other molecular interactions. For
one-dimensional mass flux, Fick’s law of diffusion can be written as
˙
n
j
=−D
j
A
dC
j
dx
(5.28)
Diffusion coefficients tend to be around 0.1 cm
2
/s for gases and 10
−5
cm
2
/s for liquids.
Solid-state diffusion coefficients are strong functions of temperature and are generally
less than 10
−10
cm
2
/s but can vary by as much as 15 orders of magnitude. Diffusion
of gases into solid polymers is generally around 10
−8
cm
2
/s. Some typical values for
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
212 Transport in Fuel Cell Systems
Table 5.4 Some Experimental Gas-Phase Diffusion Coefficients at 1 atm
Gasl Pair Temperature (K) D (cm
2
/s)
Air–O
2
273 0.176
Air–H
2
O 298 0.260
Air–He 282 0.658
O
2
–H
2
O 308 0.282
N
2
–O
2
293 0.220
O
2
–He 317 0.822
Air–H
2
282 0.710
H
2
–H
2
O 307 0.915
H
2
–He 317 1.706
H
2
–CO
2
298 0.646
CO–H
2
296 0.743
Source: From [19].
gas-phase, liquid-phase, and solid-phase diffusion coefficients from [19] are given in Tables
5.4–5.6, respectively.
The diffusion coefficient in a mixture is obviously affected by the other species present,
and to account for this a binary diffusion coefficient D
12
is specifically related to the
diffusivity of species 1 into 2 can be used. Since diffusion of species 1 into 2 is identical to
species 2 into 1, D
12
= D
21
. In cases where the density is low and diffusion flux is primarily
a result of self-interaction, the species self-diffusion coefficient (D
jj
= D) is used, and the
effect of interaction with other species is not included. Later in this chapter methods for
calculation of diffusion coefficients are given.
Transient diffusion problems can be solved with Fick’s second law. If the diffusion
coefficient is assumed to be independent of concentration:
∂C
j
∂t
= D
j
∂
2
C
j
∂x
2
i
(5.29)
where D
j
represents the proper diffusion coefficient of j in its surrounding environment.
Table 5.5 Diffusion Coefficients in Water at 298 K and Infinite Dilution
Gas into Water Chemical Formula D (cm
2
/s)
Air (N
2
)
0.79
(O
2
)
0.2l
2.00 × 10
−5
Carbon monoxide CO 2.03 × 10
−5
Carbon dioxide CO
2
1.92 × 10
−5
Hydrogen H
2
4.50 × 10
−5
Methane CH
4
1.49 × 10
−5
Oxygen O
2
2.10 × 10
−5
Methanol CH
3
OH 0.84 × 10
−5
Ethanol C
2
H
6
O 0.84 × 10
−5
Formic acid CH
2
O
2
1.50 × 10
−5
1-Propanol CH
3
CH
2
CH
2
OH 0.87 × 10
−5
Source: From [19].
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.3 Gas-Phase Mass Transport 213
Table 5.6 Some Representative Solid-Phase Diffusion Coefficients
System Temperature (K) D (cm
2
/s)
Hydrogen in SiO
2
293 4.0 × 10
−10
Cerium in tungsten 2000 95 × 10
−11
Hydrogen in nickel 358 1.16 × 10
−8
Sliver in aluminum 323 1.2 × 10
−9
Aluminum in copper 293 1.3 × 10
−30
Source: From [19].
A useful result can be found from this equation that allows us to determine the diffu-
sion penetration distance with time [19]. We can calculate the characteristic time scale of
diffusion τ
d
, which is the time for significant diffusion to reach a given distance δ:
τ
d
=
δ
2
D
(5.30)
For example, in 1 h, oxygen initially released into water vapor at 308 K (Table 5.1) will
travel a penetration distance of
τ
d
D = δ =
(3600 s)(0.282 cm
2
/s) = 31.86 cm
Example 5.6 Characteristic Times for Gas-, Liquid-, and Solid-State Diffusion Estimate
typical characteristic times for a gas into gas, a gas into a liquid, a gas into a solid, and a
gas into a polymer to diffuse a distance of 1 mm.
SOLUTION We assume a typical diffusion coefficient of 0.1 cm
2
/s for gases, 10
−5
cm
2
/s
for liquids, 10
−8
cm
2
/s for polymers, and 10
−10
cm
2
/s for solids.
Solving Eq. (5.30)
τ
d
=
δ
2
D
=
(
0.1cm
)
2
D
Mode of Diffusion τ
d
for 1 mm Diffusion
Gas phase 0.1 s
Liquid phase 16.7 min
Gas in polymer 278 days
Solid state 76.1 years
COMMENTS: The second law of thermodynamics provides a driving force for everything
to become homogeneously mixed in time, so that any material differences (and in a larger
sense, every gradient) have some natural driving for mixing. The vast difference between
gas- and liquid-phase transport times is an important factor in the stability and control of
fuel cells.

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
214 Transport in Fuel Cell Systems
Table 5.7 Critical Temperature and Pressure Data for Fuel Cell Gases
Gas Formula T
c
(K) P
c
(atm)
Water H
2
O 647.3 218.0
Hydrogen H
2
33.2 12.8
Oxygen O
2
154 49.8
Nitrogen N
2
126 33.5
Air Mix 133 37.2
Carbon dioxide CO
2
304 72.9
Carbon monoxide CO 133 34.5
Methanol CH
3
OH 513 78.5
Source: From [20].
Calculation of Binary Gas-Phase Diffusion Coefficients There are many different meth-
ods to estimate gas-phase diffusion transport parameters. Based solely on molecular dy-
namic theory, we can derive
D
j
∝
T
3/2
P · MW
1/2
σ
2
(5.31)
This is a simple expression of self-diffusion of species j into a mixture of j, independent
of other species, where σ is the collision diameter of the molecule.
4
In practice, these
qualitative dependencies are generally observed, with some deviation, especially for polar
molecules. Many other theories of different levels of complexity and accuracy have been
developed but are not discussed here for brevity.
For fuel cells with gas-phase reactants, an effective estimation of binary (two-species)
diffusion coefficients can be taken from [21], developed from kinetic theory and the law of
corresponding states [22]:
D
12
cm
2
s
=
a
P
T
√
T
c1
T
c2
b
(
P
c1
P
c2
)
1/3
(
T
c1
T
c2
)
5/12
1
MW
1
+
1
MW
2
1/2
(5.32)
where D
12
represents diffusivity of species 1 into species 2, temperature T is in Kelvin and
pressure P is in atmospheres, and T
c
and P
c
refer to the thermodynamic critical temperature
and pressure, respectively (some are listed in Table 5.7). For a nonpolar gas pair, a and b are
2.745 × 10
−4
and 1.823, respectively. For a nonpolar gas–H
2
O pair, a and b are 3.640 ×
10
−4
and 2.334, respectively. For commonly used gases, this simple equation can be used,
since everything but temperature and pressure are constants.
For example, for oxygen in water vapor, we can show that
D
H
2
O–O
2
cm
2
s
=
4.19836 × 10
−7
P
(T )
2.334
(5.33)
where pressure P is in atmospheres and temperature T is in Kelvin. This theory matches
trends predicted by molecular collision theory as well as experimental data fairly well and
4
The reader is referred to advanced texts on transport theory, including Diffusion, by E. L. Cussler, Cambridge
University Press, 1997, and Transport Phenomena, 2nd ed., by R. B. Bird, W. E. Stewart, and E. N. Lightfoot,
Wiley, 2002.
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.3 Gas-Phase Mass Transport 215
Table 5.8 Diffusion Volumes for Eq. (5.34)
Gas Formula
V
ij
Water vapor H
2
O 12.7
Hydrogen H
2
7.07
Oxygen O
2
16.6
Nitrogen N
2
17.9
Air Mix 20.1
Carbon dioxide CO
2
26.9
Carbon monoxide CO 18.9
Source: From [19].
includes the capability to more accurately model polar interacting gases, such as H
2
O. It
should be noted that there is additional error for hydrogen and helium because these gases
do not exactly follow the law of corresponding states, as discussed in Chapter 3. Because
the hydrogen is usually not the source of diffusion limitations in a fuel cell, however, the
additional error is generally tolerable in light of the convenience of the model. If greater
accuracy for hydrogen is required, another method developed using molecular volumes can
be used [23]:
D
12
(T , P) = 10
−3
×
T
1.75
P
1/MW
1
+ 1/MW
2
i
(V
i1
)
1/3
+
i
(V
i2
)
1/3
2
(5.34)
where T and P are in Kelvin and atmospheres, respectively, and some of the molecular
volumes V are given in Table 5.8. This method can also be used for other gases besides
hydrogen if desired.
Another way to quickly obtain approximate data is to assume the functional dependence
of pressure and temperature in Eq. (5.31) and extrapolate from known experimental data
such as shown in Table 5.4. That is,
D
12
(T , P) = D
12,known
(T
ref
, P
ref
)
T
T
ref
3/2
P
ref
P
(5.35)
This will provide fairly accurate results as well, especially for nonpolar molecules. In
summary, there are several approaches of varying complexity and precision available to
estimate the gas-phase diffusivity. The choice taken depends on the level of precision
required. Direct experimental data for all modes of transport are always preferred over a
generalized correlation.
Multicomponent Diffusion Approaches In most cases, more than two species will be
mixed, and calculation of the diffusion rate of a given species into a mixture will be
required. The approach for these calculations is as follows:
1. Mixture Property Approach If there are more than two gases involved, such as
in a cathode with nitrogen, oxygen, and water, a mole-fraction-based averaging
approach can be taken as an estimation. In fact, the critical properties of air are

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
216 Transport in Fuel Cell Systems
already tabulated this way (prove it to yourself by making the calculation), although
it is really a mixture property of nitrogen, oxygen, and other minor species. For
example, to calculate the diffusivity of oxygen into humidified air:
Ĺ Determine the mole fractions of the constituents using the saturation pressure and
psychrometric analysis tools of Chapter 3.
Ĺ Evaluate the diffusion coefficient based on one of the many methods discussed.
Ĺ Evaluate the mixture diffusivity using molar averaging.
2. Ignore Inert Species Approach In this approach, we simply ignore the contribu-
tions of the inert species. In the cathode, that means we treat the gas as a binary
mixture of oxygen diffusing into water vapor. This is frequently employed in the
literature for simplicity.
3. Stefan–Maxwell Approach A more rigorous approach to multispecies diffusion
effects is known as the Stefan–Maxwell diffusion model and should be used for
higher order models seeking the greatest accuracy. The Stefan–Maxwell equation
for multicomponent diffusion flux in the x direction is shown as
dy
i
dx
=
1
C
j=i
y
i
n
j
− y
j
n
i
D
ij
(5.36)
where C is the total molar concentration of the gas mixture and the y
i
’s are the mole
fractions, which can be determined by the pressure and temperature based on the
ideal gas law,
C =
P
R
u
T
(5.37)
The n terms are the molar flux of the components in the x direction. The effective
binary diffusion coefficient of the various gas species combinations, D
ij
, is cal-
culated under specified temperature and pressure according to one of the methods
already described. As an example, consider a mixture of oxygen, water vapor, and
nitrogen. From Eq. (5.36), we get following equations for the gradients for oxygen,
nitrogen, and water vapor mole fractions in the cathode:
dy
O
2
dx
=
R
u
T
P
y
O
2
n
H
2
O
− y
H
2
O
n
O
2
D
O
2
−H
2
O
+
y
O
2
n
N
2
− y
N
2
n
O
2
D
O
2
−N
2
(5.38)
dy
N
2
dx
=
R
u
T
P
y
N
2
n
H
2
O
− y
H
2
O
n
N
2
D
N
2
−H
2
O
+
y
N
2
n
O
2
− y
N
2
n
O
2
D
N
2
−O
2
(5.39)
dy
H
2
O
dx
=
R
u
T
P
y
H
2
O
n
N
2
− y
N
2
n
H
2
O
D
H
2
O−N
2
+
y
H
2
O
n
O
2
− y
O
2
n
H
2
O
D
H
2
O−O
2
(5.40)
Example 5.7 Estimation of Diffusivity of Hydrogen and Oxygen in Water Vapor Es-
timate the diffusivity of hydrogen in water vapor and oxygen in water vapor at 1 atm and
307 K and compare with experimental data in Table 5.4.

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.3 Gas-Phase Mass Transport 217
SOLUTION From Eq. (5.32), for the water–nonpolar gas pair, a and b are 3.640 × 10
−4
and 2.334, respectively:
D
12
cm
2
s
=
a
P
T
√
T
c1
T
c2
b
(
P
c1
P
c2
)
1/3
(
T
c1
T
c2
)
5/12
1
MW
1
+
1
MW
2
1/2
Plugging in the numbers yields
D
H
2
O−H
2
cm
2
s
=
3.64 × 10
−4
1
307
√
647.4 × 33.3
2.334
×
(
217.5 × 12.8
)
1/3
(
647.4 × 33.3
)
5/12
1
18
+
1
2
1/2
= 1.36 cm
2
/s
Due to hydrogen self-interaction, this value is substantially different (49% higher) from
the experimentally determined value of 0.915 cm
2
/s. We expect some error for hydrogen,
though, as discussed.
For the oxygen, we have
D
H
2
O−O
2
cm
2
s
=
3.64 × 10
−4
1
307
√
647.4 × 154.4
2.334
×
(
217.5 × 49.7
)
1/3
(
647.4 × 154.4
)
5/12
1
18
+
1
32
1/2
= 0.268 cm
2
/s
which is much closer to the 0.282 cm
2
/s found from experimental data in Table 5.4.
If we desire greater accuracy for hydrogen, we can use Eq. (5.34):
D
H
2
O−H
2
(T , P) = 10
−3
×
T
1.75
P
1/MW
1
+ 1/MW
2
i
(
V
i1
)
1/3
+
i
(
V
i2
)
1/3
2
= 10
−3
×
307
1.75
1
1/2 + 1/18
12.7
1/3
+ 7.07
1/3
2
= 0.69 cm
2
/s
This estimation is closer but still has significant (24%) error, in this case underestimating
the measured value.
COMMENTS: As discussed, the error in using hydrogen is usually tolerable in light of
the fact that it is not typically hydrogen that limits reaction. The molecular volume approach
also can be used for oxygen and other fuel cell species with high accuracy, although the
need for the tabulated molecular diffusion volumes is an inconvenience and therefore the
approaches shown in Eqs. (5.34) and (5.35) are generally preferred. Also note for later use
that the diffusivity of water vapor into hydrogen is 3–4 times greater than that of water
vapor into oxygen (and air). This fact is important in PEFCs, which will be discussed in
Chapter 6.
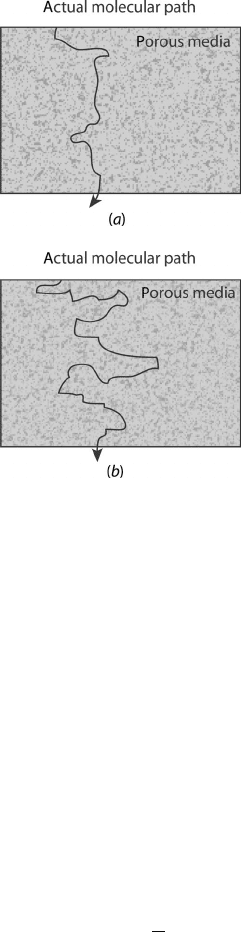
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
218 Transport in Fuel Cell Systems
Figure 5.12 Illustration of porous media with (a)lowand(b) high tortuosity.
5.3.2 Gas-Phase Flow in Porous Media
For gas-phase flow in porous media, such as with the electrodes or gas diffusion media
of a PEFC or AFC, the porous nature inhibits the diffusion rate through the media. The
inhibition must be related to two factors:
1. Porosity At zero porosity (i.e., a complete solid), the effective gas-phase diffusivity
must be zero. At a porosity of 1 (i.e., an open volume with no solid), the effective
diffusivity must equal the bulk value.
2. Tortuosity Tortuosity is a measure of the effective average path length though the
porous media compared to the linear path length across the media in the direction of
transport. The more tortuous the path, the longer the effective path length through
the media, and the greater reduction in the effective diffusivity, as illustrated in
Figure 5.12.
The effective diffusivity for gas-phase flow in porous media can be written as
D
eff
= D
φ
τ
(5.41)
where D
eff
is the effective bulk gas-phase diffusivity in the porous media φ is the porosity
(void volume fraction), D is the diffusivity in gas, and τ is the tortuosity. Since tortuosity is a
difficult parameter to estimate except through direct experiment, a Bruggeman correlation
is often used for fuel cell studies. This relationship assumes τ is proportional to φ
−0.5
,
resulting in the simpler expression
D
eff
= Dφ
1.5
(5.42)
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.3 Gas-Phase Mass Transport 219
Physically, the porosity correction is to adjust for the longer effective path length through
the porous media. Using another approach, Salem and Chilingarian estimated tortuosity to
be related to porosity for high-porosity material (φ = 0.62–0.88) [24], which is appropriate
for fuel cell media:
τ =−2.1472 +5.2438φ (5.43)
This relationship yields a value of τ ∼ 1.5φ for a typical PEFC diffusion layer (φ = 0.8),
or
D
eff
=
D
1.5
(5.44)
Comparing Eq. (5.44) to (5.42), there is not much difference between the result of using
either approximation at high porosities typical of fuel cell media, especially given the
inherent uncertainty of many other parameters involved.
Gas-Phase Limiting Current Density and Diffusion Resistance In Chapter 4, we derived
an expression for concentration polarization based on the concept of a mass-transport-
limited reaction rate at a given electrode. Now, we can derive an analytical expression for
this value, assuming one-dimensional transport to the catalyst surface. First, we equate the
rate of reactant consumption to the diffusive and adjective transport to the reaction surface:
i
l
A
nF
Consumption
=−D
j
A
dC
j
dx
Diffusion
transport
+ C
j
Av
x
Advective
transport
(5.45)
where i
l
is the mass transfer limiting current density. In some fuel cell designs, flow is
intentionally forced to the surface by convection, which is the reason for the inclusion of
the advective transport in Eq. (5.45). Additionally, the suction of reactant to the surface
will naturally cause some bulk motion and convection near the electrode. However, this
blowing/suction effect is typically small relative to the diffusion.
The diffusion rate is limited by flow through the porous media diffusion layer (PEFC
and AFC) and electrode. If we assume one-dimensional flux to the electrode surface in the
x direction with no bulk flow velocity (see Figure 5.13), we can write this as
C
s
= 0ati
l
i
l
=− nFD
eff
C − C
s
=− nFD
eff
C
=− nFD
eff
y
i
P/ R
u
T
(5.46)
where C
s
, the surface concentration, is reduced to zero at the limiting condition and C
∞
is
the concentration of the reactant at the boundary with the flow channel and is calculated
with the ideal gas law. Here, δ is the distance to the electrode surface from the flow channel
boundary. For high-temperature fuel cells, the diffusivity is usually high enough that the
diffusion limiting current is not a factor. For the PEFC and AFC, the diffusion coefficient
through the diffusion media is typically modified by the Bruggeman relationship to account
