Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
200 Transport in Fuel Cell Systems
critical importance, since the abrupt change in water content results in a similarly abrupt
variance in ionic conductivity, swelling, and other important transport parameters. The
physicochemical reason for this phenomenon is still subject of debate. A recent attempt to
explain the phenomena based on physical considerations is given by Weber and Newman
in [5].
If the membrane itself is partially in contact with liquid and vapor, as can commonly
be the case in an operating fuel cell, the water content and uptake in the membrane can
vary with location, although in equilibrium the water content in the membrane will become
homogeneous with uptake depending on the overall water availability. Transport in this case
can be modeled as occurring in parallel between gas and liquid equilibrated modes, with a
suitable fraction denoting the liquid and gas phase fractions of contact with the membrane.
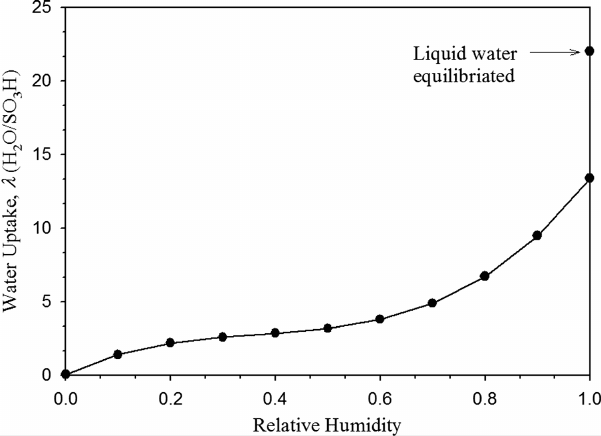
Example 5.1 Water Uptake in Nafion Plot the expected water uptake in Nafion as a
function of RH and include Schroeder’s paradox uptake value of 22 at 80
◦
C and a water
activity of one for liquid water.
SOLUTION Using Eq. (5.18), we can plot:
The uptake of Nafion when equilibrated with liquid water illustrates Schroeder’s paradox,
whereby a water content of up to λ = 22 has been measured in elevated temperature
environments [7].
COMMENTS: There is a sharp change in the slope of the uptake curve beyond a RH
value of 0.6. This is believed to be a result of the changing structure of the water distributed
in the membrane. At low water activity, the water is strongly bound to the sulfonic acid
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.1 Ion Transport in an Electrolyte 201
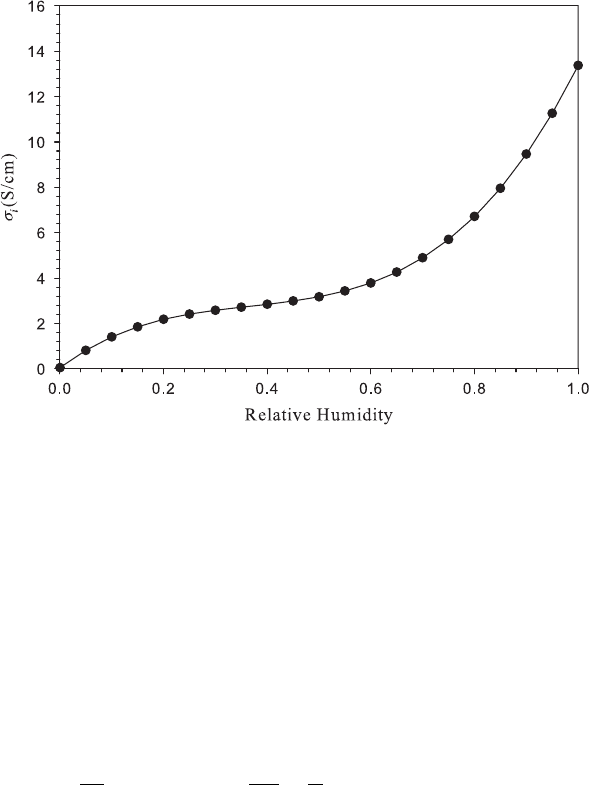
Figure 5.6 Calculated Nafion ionic conductivity (σ
i
) at different RH values at 80
◦
C.
side chains. With increasing water activity in the membrane, the bonding is weak and the
water behavior approaches unbounded water in a dilute solution.
Nafion Ionic Conductivity Because the conductivity of the membrane is related to the
uptake of water in the membrane, the ionic conductivity is strongly related to the hydration
level of the membrane. Fundamentally, the water provides the ion conduction pathways, so
that increased water content increases the ionic concentration, increasing the conductivity
for dilute solutions. Like other electrolytes, temperature also affects conductivity. The
ionic conductivity of Nafion 1100 EW ionomer was correlated based on measurements of
conductivity over 25–90
◦
C and a full humidity range [12], and is shown as:
σ
i
S
cm
= exp
1268
1
303
−
1
T
(
0.005193λ − 0.00326
)
(5.20)
σ
e
≈ 0 (5.21)
where T is in Kelvin. Schroeder’s paradox also effects the conductivity of the membrane, as
the jump in water uptake from contact with liquid water is included implicity in Eq. (5.20)
through λ. Plots of the ionic conductivity as a function of water uptake and temperature
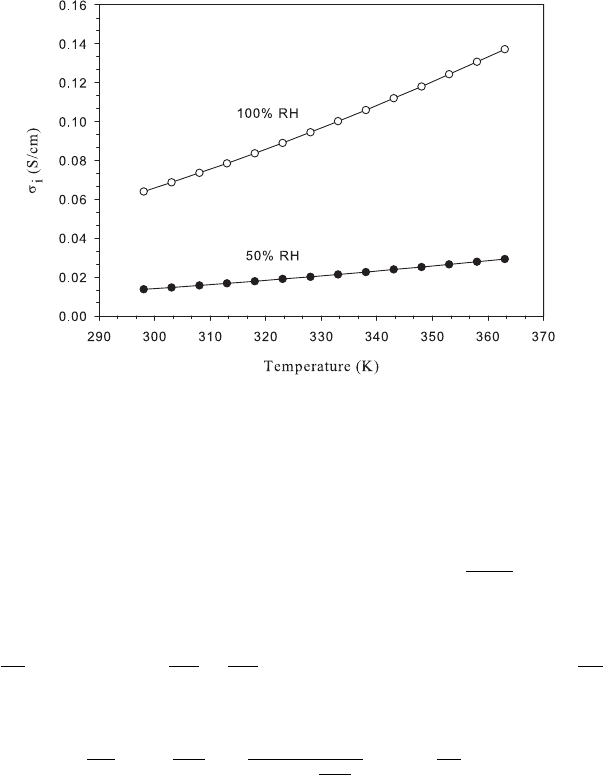
are shown in Figures 5.6 and 5.7. Note the high impact of humidity on the conductivity
especially above RH = 60%. One of the major limitations of perflourinated ionomers is
the high hydration level needed to sustain performance. At high temperatures, the water
vapor mole fraction becomes excessively large in the gas-phase which can limit reactant
transport. For a well-hydrated PEFC membrane, typical conductivities are around 10 S/cm.
Example 5.2 Determine the ohmic drop through a Nafion 112 electrolyte in a 50% RH
environment at 80
◦
C, 1A/cm
2
.
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
202 Transport in Fuel Cell Systems
Figure 5.7 Calculated Nafion ionic conductivity (σ
i
) at different temperature values at 50 and
100% RH.
SOLUTION Nafion 112 is 0.002
,or51µm thick in a dry state. In a wet state, from
Table 5.1, we estimate the thickness to be approximately 60 µm. Then from Eq. (5.18), we
calculate:
λ = 0.043 + 17.81a − 39.85a
2
+ 36.0a
3
= 3.49
H
2
O
SO
3
H
where a = RH = 0.5 from Eq. (5.19).
σ
i
S
cm
= exp
1268
1
303
−
1
353
(
0.005193λ − 0.00326
)
= 0.0268
S
cm
Then from Ohm’s law:
V = iA
l
σ A
= 1
A
cm
2
×
60 × 10
−6
(
m
)
0.0268
1
·cm
× 100
cm
m
= 0.224 (V)
COMMENTS: Notice the active area cancels out of the relationship. For this system, we
lose 224 mV at 1 A/cm
2
through the electrolyte. There are additional ohmic losses in the
contacts and through the electrolyte in the catalyst layers, as discussed in Chapter 4.
5.1.2 Ceramic Electrolytes
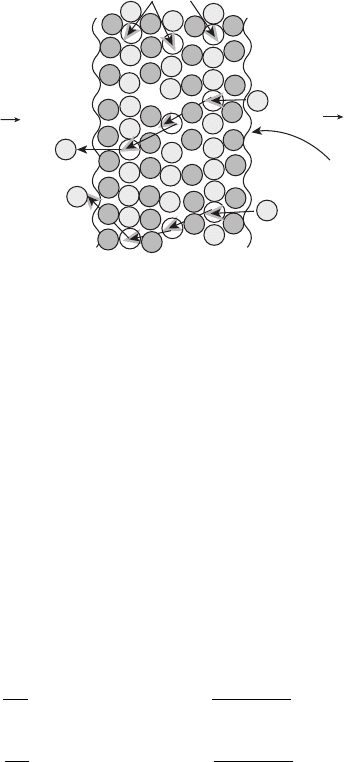
Solid Oxide Fuel Cell In contrast to the PEFC, in the SOFC O
2−
ions are passed from the
cathode to the anode via oxygen vacancies in the electrolyte molecular matrix (see Figure
5.8). In ceramic electrolytes, the ionic mobility is usually two to three orders of magnitude
lower than polymer or liquid electrolytes, at around 10
−11
m
2
/s. Due to acceptable per-
formance, availability and low cost, the most commonly used SOFC electrolyte is yttria-
(Y
2
O
3
-) stabilized zirconia (ZrO
2
), termed YSZ. Besides yttria, several other aliovalent
oxide materials have been used to dope YSZ, such as Yb
2
O
3
,Nd
2
O
3
, and Sc
2
O
3
[13].
Doped YSZ conducts negative O
2−
ions, which are transported through oxygen vacancies
in the zirconia structure. Yttria is typically added in 8–10 mol % to the ZrO
2
to stabilize
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.1 Ion Transport in an Electrolyte 203
Anode Cathode
Electrolyte
Oxygen Vacancies
H
2
+O
2-
H
2
O+2e
-
O
2
+4e
-
20
2-
O
2
Zr
4+
O
2-
O
2-
O
2-
O
2-
O
2-
O
2-
O
2-
O
2-
Y
3+
Y
3+
Y
3+
Y
3+
Zr
4+
Zr
4+
Zr
4+
Zr
4+
Zr
4+
O
2-
O
2-
Y
3+
Y
3+
Zr
4+
Zr
4+
O
2-
O
2-
Y
3+
Y
3+
Zr
4+
Zr
4+
Zr
4+
O
2-
O
2-
Zr
4+
Zr
4+
Zr
4+
Zr
4+
O
2-
O
2-
Zr
4+
Zr
4+
Zr
4+
O
2-
O
2-
Y
3+
Y
3+
Zr
4+
Zr
4+
O
2-
O
2-
Y
3+
Y
3+
Zr
4+
Zr
4+
O
2-
O
2-
O
2-
O
2-
O
2-
O
2-
Figure 5.8 Illustration of oxygen ion transport in ceramic SOFC electrolytes.
ZrO
2
in a cubic fluorite structure and maximize oxygen vacancies, as pure YSZ in not a
suitable ion conductor.
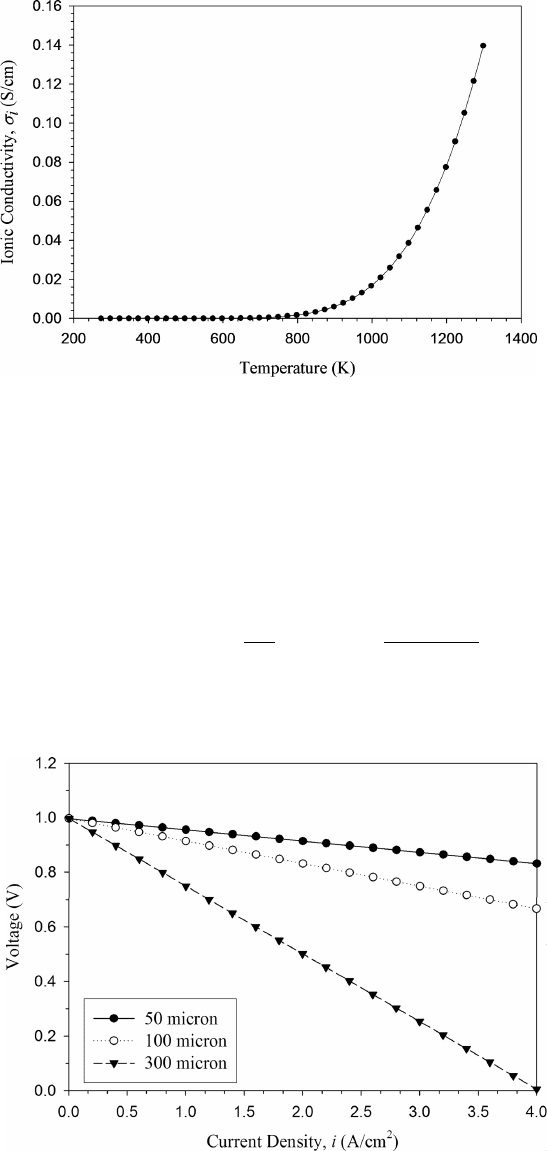
High electrolyte temperature is required to ensure adequate oxygen ion conductiv-
ity in the solid-state ceramic electrolyte, as oxygen ion mobility is nearly zero below a
critical light-off temperature in the electrolyte. As a result, conventional SOFCs will not
produce any significant current until being externally heated to the light-off temperature.
Commonly used electrolyte conductivity is nearly zero until around 650
◦
C [14], although
low-temperature SOFC operation at 500
◦
C using doped ceria (CeO
2
) ceramic and other
electrolytes has shown feasibility [13].
The YSZ has a mixed (electrical and ionic) conductivity, but the electrical conductivity
is nearly negligible for typical operating conditions in the SOFC. The ionic and electrical
conductivity of 8% mole fraction yttria YSZ, (ZrO
2
)
0.92
(Y
2
O
3
)
0.08
, has been well studied
[14]:
σ
i
S
cm
= 1.63 × 10
2
exp
−0.79 eV
k
B
T
(5.22)
σ
e
S
cm
= 1.31 × 10
7
exp
−3.88 eV
k
B
T
P
−0.25
O
2
(5.23)
where k
B
is the Boltzmann constant (8.617339 × 10
−5
eV/K = 1.3807 × 10
−23
J/K).
A plot of the ionic conductivity of (ZrO
2
)
0.92
(Y
2
O
3
)
0.08
as a function of temperature is
given in Figure 5.9. The electronic conductivity from Eq. (5.23) is very low for all normal
values of oxygen partial pressure, resulting in an ionic transference number (see Chapter 4)
of approximately unity. The boundary where electrical conductivity becomes nonzero is
evident around 600
◦
C. As mentioned, other electrolyte materials do show better ionic
conductivities at lower temperature operation but have other limitations such as intrinsic
cost, stability, or finite electrical conductivity.
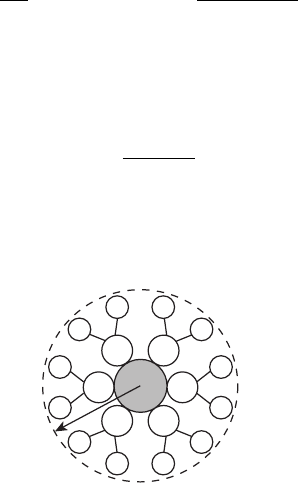
Example 5.3 Ohmic Losses in SOFC as a Function of Electrolyte Thickness Plot an
ohmic-only polarization curve for a SOFC with (ZrO
2
)
0.92
(Y
2
O
3
)
0.08
electrolyte at 1000
◦
C
for a 50-, 100-, and 300-µm-thick electrolyte and an OCV of 0.997 V. That is, ignore kinetic
and concentration polarization losses. Assume neat hydrogen and air at 1 atm back pressure
is used.
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
204 Transport in Fuel Cell Systems
Figure 5.9 Ionic conductivity of (ZrO
2
)
0.92
(Y
2
O
3
)
0.08
as function of temperature.
SOLUTION In this case, our fuel cell model reduces to the Nernst potential and ohmic
polarization:
E
cell
= E
◦
(T , P) − η
r
Plugging in the numbers, where σ
i
is determined from Eq. (5.22) at 1000
◦
C, yields
E(T, P) = 0.997 − iA
l
σ
i
A
= 0.997 − i
l
0.121 S/cm
Plotting this for several different l values representing the electrolyte thicknesses results in
the following:
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.1 Ion Transport in an Electrolyte 205
COMMENT: Quick calculation of the ion transference number yields a value of unity, so
that deviation from the Nernst potential from internal electrical shorting is not significant.
The SOFC designs typically rely on mechanical support from one of the electrode assembly
components. Designs are typically anode, cathode, or electrolyte supported, although an
inert support structure is also used in some designs. The thicker range shown (300 µm)
is representative of an electrolyte-supported design. Because the ohmic resistance is dom-
inating in these high-temperature systems, an electrode-supported design is a common
choice in planar SOFC designs. This topic is covered in Chapter 7.
5.1.3 Liquid Electrolytes (AFC, MCFC, PAFC)
In liquid electrolytes, like other electrolytes, ionic conductivity ultimately depends on the
mobility and charge of the ionic species. In liquid electrolyte solutions, the ionic species
is solvated by the strong dipoles of the water molecule which surround the ion (Figure
5.10). Consider a charged particle in a potential vector field of strength dφ
i
/dx = E. It will
experience a motive force F = z
j
eE, where e is the fundamental unit of charge, 1.602 ×
10
−19
C, and z
j
is the charge on the ion. In the steady state, a spherical ion moving with low
velocity and high viscosity (Re < 1) in viscous fluid has a frictional retarding force of [16]:
F = 6πνr
j
z
j
V (5.24)
where ν, r
j
, and V represent the electrolyte solution viscosity, ion radius, and ion velocity
vector, respectively.
The maximum velocity of an ion under the effect of an electric field force is determined
by equating the electric field and frictional forces:
z
j
e
dφ
dx
i
= 6πνr
j
V ⇒ V =
z
j
e(dφ/dx
i
)
6πνr
j
(5.25)
In addition to the relationship shown in Eq. (5.11), the ionic mobility u
j
of an ion in an
electrolyte in solution is really a measure of the maximum velocity of the ion for a given
potential field:
u
j
=
V
|
dφ/dx
i
|
(5.26)
A high mobility physically represents a high ion velocity for a given potential field and is
a function of ionic radii, solution viscosity, and ion charge number through Eq. (5.25).
O
-
H
+
O
-
O
-
O
-
O
-
O
-
H
H
H
H
H
H
H
H
H
H
H
H
r
eff
Figure 5.10 Illustration of effective ionic radius of solvated ion.

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
206 Transport in Fuel Cell Systems
Table 5.3 Some Typical Electrolyte Conductivities Under Operation for Various Liquid Electrolyte
Fuel Cells
Type of Fuel Cell Electrolyte Temperature (
◦
C)
Ionic Conductivity
σ (S/cm)
Alkaline (AFC) KOH in 30–50% water 60–80 ∼0.4
Phosphoric acid (PAFC) Concentrated H
3
PO
4
200 ∼0.6
Molten Carbonate (MCFC) Li
2
CO
3
and K
2
CO
3
650 ∼0.3
Source: From [16].
The ionic conductivity in a liquid electrolyte is a function of the following:
1. Ion Concentration Theoretically, the more charge carriers that are available, the
more charge that can be carried. If the electrolyte is highly diluted with water,
conductivity will obviously decrease. Instead of a monotonic increase for increasing
ionic species concentration, however, an optimal ion concentration corresponding
to maximum electrolyte conductivity is observed. This relationship is due to ion–ion
interaction at high concentrations.
2. Ionic Mobility From Eqs. (5.11) and (5.26), ionic mobility is related to electrolyte
viscosity, atomic radii, and ion charge number.
3. Temperature This affects the ionic mobility through the solution viscosity.
4. Atomic Radii This theoretically is the radius of the ion but also includes the
effective ionic radius, including solvating water, which clusters around the ion (see
Figure 5.10).
5. Ion Charge According to Eq. (5.15), the higher the charge, the higher the con-
ductivity. Unfortunately, the higher charges also result in increased water solvation
and an increased effective radius. As a result, the mobility is decreased, and a clear
trend between differently charged ions cannot be simply derived.
As a result of the competing trends in conductivity, theoretical values often differ from those
experimentally measured. Some typical electrolyte ionic conductivity values are given in
Table 5.3. It should be noted that the values in Table 5.3 are under ideal conditions, and
actual values in fuel cells may not exactly match.
Example 5.4 Conductivity of a Dilute Electrolyte Solution Consider a solution of 0.05 M
aqueous solution of A
2
B, that completely dissolves into A
+
and B
2−
ions. The mobility
of A is 5 × 10
−4
(cm
2
/Vs), and the mobility of B is 3 × 10
−3
(cm
2
/Vs). Determine the
approximate ionic conductivity of the solution assuming no ion–ion interactions.
SOLUTION Since the 0.05 M solution completely dissociates, the result will be a final
electrolyte solution with 0.1 M A
+
and 0.05 M B
2−
ions. Both ions will contribute to the
charge transfer, thus the conductivity is the sum of the individual contributions.
From Eq. (5.14):
σ
i
= F|z
j
|u
j
C
j
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.1 Ion Transport in an Electrolyte 207
Thus
σ
A
= 96,485
C
eq
1
eq
mol
5 × 10
−4
cm
2
Vs
C
A
mol
cm
3
and
σ
B
= 96,485
C
eq
2
eq
mol
3 × 10
−3
cm
2
Vs
C
B
mol
cm
3
where the molar concentrations of the ions are found from simple unit conversions:
C
A
mol
cm
3
= 0.1
mol
L
1
1000
L
cm
3
= 1 × 10
−4
mol
cm
3
and
C
B
mol
cm
3
= 0.05
mol
L
1
1000
L
cm
3
= 5 × 10
−5
mol
cm
3
Therefore,
σ
A
= 96,485(5 ×10
−4
)(1 × 10
−4
) = 0.00482
cm
2
Vs
and
σ
B
= 96,485(2.3 ×10
−3
)(5 × 10
−5
) = 0.0289
cm
2
Vs
And the total ionic conductivity is therefore the sum of the two:
σ
i
= σ
A
+ σ
B
= 0.0338
cm
2
Vs
COMMENTS: The reader should verify the unit conversion in this example, as it is not
straight forward. As discussed, temperature, concentration, and other factors can drasti-
cally affect the ionic mobility of a species. For example, we would expect the solution
conductivity to increase with temperature by decreasing solution viscosity, and increase
with ionic concentration. The concentration effect will reach a peak value at relatively low
concentrations however, where ion–ion interactions begin to become important and reduce
concentrated solution conductivity.
Example 5.5 Ionic Conductivity of MCFC Electrolyte Shown below is a plot and table
with published performance data comparing the relative performance of MCFCs at 1 atm
pressure (adapted from [17]). Perform the following analysis:
1. Determine an order-of-magnitude estimation for ionic conductivity per electrolyte
thickness in the electrolyte in 1976, 1984, and 1999 based on the plot below.
2. Why do you think the ohmic slope was better in 1984 but overall performance was
worse than in 1992 and 1999?
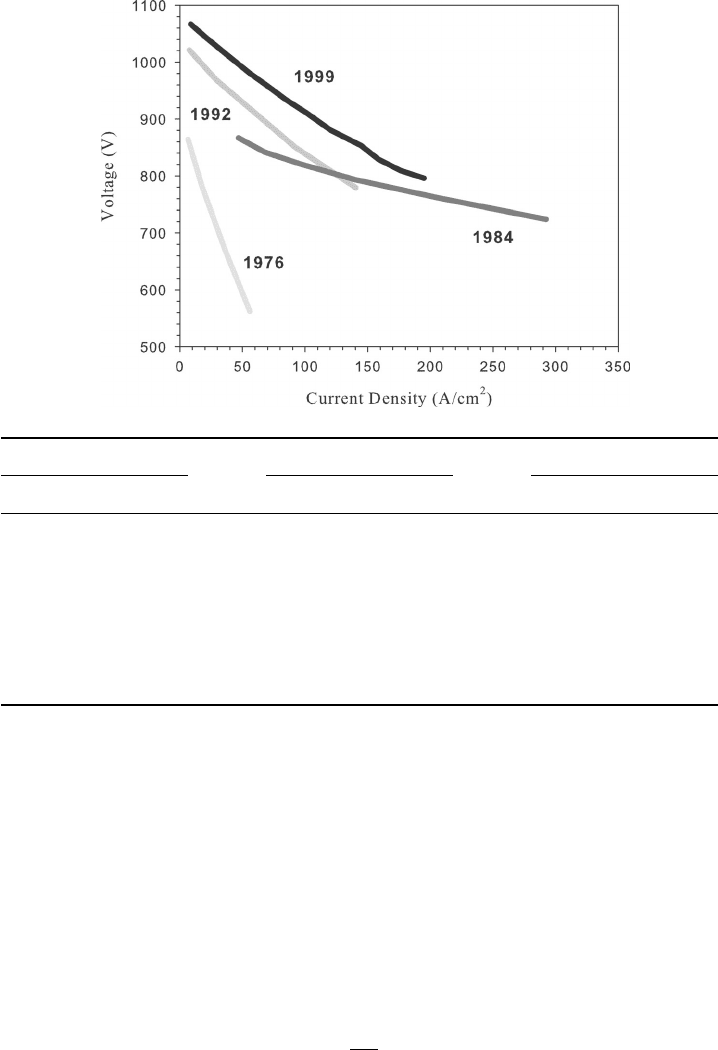
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
208 Transport in Fuel Cell Systems
3. How could you explain the trends in OCV over the years?
1976 1984 1999
Current Voltage Current Voltage Current Voltage
7 864 47 867 21.4 1042
18 781 68 842 37.52 1013
36 672 98 820 56.16 980
56 562 140 793 69.7 958
207 761 101 911
249 743 120.5 880
293 724 176.4 809
194.9 796
Note that there has been tremendous improvement in the MCFC performance through the
years, and not all can be attributed to ohmic loss improvements, as we will assume in this
example for simplicity. However, since this is a high temperature system, polarization is
typically dominated by ohmic losses.
SOLUTION
1. From the given data, we can determine the slope of the approximately linear curves,
which we can use to estimate the order of the electrolyte conductivity per unit
thickness of electrolyte.
From Ohm’s law
dV
di
= RA

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.2 Electron Transport 209
where A is the geometric active area of the cell. This combination of RA is known
as the area specific resistance (ASR) and is sometimes a figure of merit specified
for fuel cell ohmic losses to provide a scaling factor between differently sized cells.
To determine the conductivity per unit thickness of electrolyte, we do not need
to know the A of the fuel cells, however. From the definition of conductivity
σ
i
l
=
1
RA
=
1
dV/di
The absolute value is needed because the slope is negative. From the data shown, a
simple linearized curve-fit can be used to determine the slopes.
3
Year dV/di (V · cm
2
/A) σ
i
/l (Ω/cm
2
)
1976 1.81 1.81
1984 0.56 0.56
1999 1.48 1.48
2. The overall ohmic slope was better in 1984, but overall performance was worse
because the initial OCV was lower. Therefore, the benefit of the reduced ohmic
polarization was not realized until very high current densities where the efficiency
is already undesirably low. One possible explanation for this trend would be the use
of a thinner electrolyte bath. The thinner electrolyte would result in reduced ohmic
losses but increased crossover and hence reduced OCV.
3. The OCV has steadily increased over the years, indicating that crossover of reactants
through the electrolyte and electrical shorts has been significantly improved.
COMMENTS: This is an example of how one can use simple polarization data to estimate
certain parameter values and compare different cell designs without much information. In
this sense, you can diagnose fuel cell systems by a comparison of polarization data. One
must be very careful, however, not to overanalyze. Due to the complexity and interrelation
between many variables, most of the time, many factors are important. In high-temperature
systems, though, the situation is a little more simplified since ohmic losses tend to dominate
and mostly linear polarization curves result.
5.2 ELECTRON TRANSPORT
Electron transport in fuel cells is mostly ignored in analysis, because, relative to ionic
transport, electronic resistance contributes little to the overall fuel cell polarization. Certain
cases exist where this may not be true, including
1. a poorly assembled cell or
2. an aged cell with some oxidation on current collector contacts.
3
This can be done by hand or with a variety of computer programs. Being able to curve-fit data is an important
technique. If you are unfamiliar with this, see your instructor.
