Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
220 Transport in Fuel Cell Systems
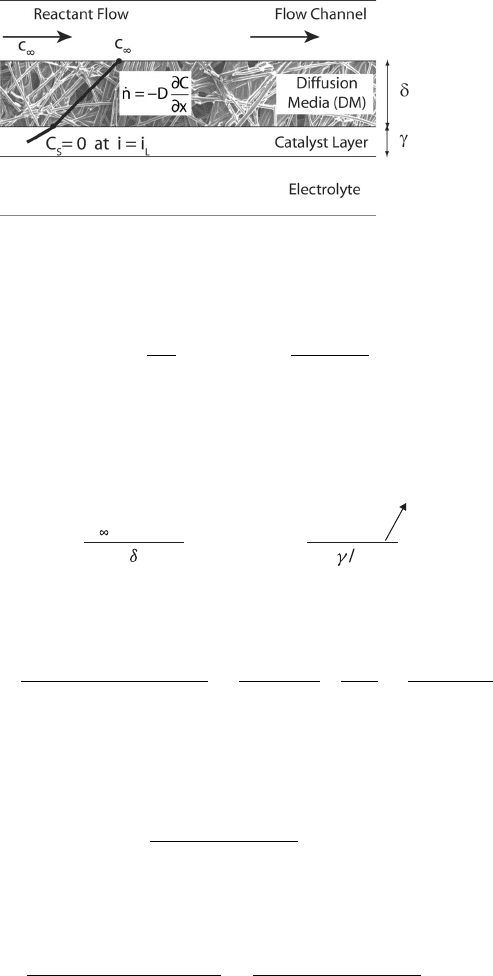
Figure 5.13 Schematic of gas-phase limiting current density at electrode.
for the porosity:
i
l
=−nFD
eff
C
∞
δ
=−nFDφ
1.5
y
i
P/R
u
T
δ
(5.47)
This approach can easily be extended from just the diffusion media to include the catalyst
layer as well. If we assume that the average reaction location is about midway through the
catalyst layer of thickness γ , then we can write
C
s
=0
i
l
=− nFD
eff,DM
C − C
DM
+
=− nFD
eff,CL
C
DM
+
− C
s
2
(5.48)
where C
DM
+
is the concentration of the reacting species at the diffusion media and catalyst
layer interface. Solving for C
DM
+
and plugging back in, we can show that
i
l
=−nFD
eff,DM
C
∞
− i
l
γ/(2nFD
eff,CL
)
δ
=
nFD
eff,DM
δ
y
i
P
R
u
T
−
i
l
γ
2nFD
eff,CL
(5.49)
If the porosities in the catalyst layer and diffusion media are the same so that the effective
diffusivities in the diffusion media and catalyst layer are identical, we can show that
i
l
=
nFD
eff
(
y
i
P/R
u
T
)
δ + γ/2
(5.50)
which is a result we could have expected. If the porosities are not the same (as is often the
case), then Eq. (5.48) can be solved for the limiting current as
i
l
=
nFD
eff,DM
(y
i
P/R
u
T )
δ + γ D
eff,DM
/(2D
eff,CL
)
=
nFDφ
1.5
DM
(y
i
P/R
u
T )
δ + (γ/2)(φ
1.5
DM
/φ
1.5
CL
)
(5.51)
Example 5.8 Calculation of Gas-Phase Transport Limited Current Density through
Diffusion Media Calculate the oxygen and hydrogen gas-phase transport limiting current
density in a hydrogen PEFC for the anode and cathode sides at 80
◦
C, 2 atm pressure
operation with fully humidified gas streams and high stoichiometry. Assume the anode
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.3 Gas-Phase Mass Transport 221
and cathode diffusion layers are 300 µm thick with 70% porosity. Ignore the catalyst layer
restriction in this problem.
SOLUTION We know the solution can be found with application of Eq. (5.47). However,
this problem also has several intermediate steps which integrate the previous chapters
nicely:
Step 1: Determine the mole fractions of reactants using mixture properties.From
Chapter 3, we know that
RH =
P
v
P
sat
(T )
=
y
v
P
P
sat
(T )
⇒ y
v
=
RH
P
P
sat
(T )
where
P
sat
(T ) =−2846.4 +411.24T (
◦
C) − 10.554T (
◦
C)
2
+ 0.16636T (
◦
C)
3
and
y
i
= 1
At 80
◦
C, P
sat
∼
=
31,287 Pa, and
y
v
=
1
202,650
× 31,287 = 0.154
on both the anode and cathode. On the anode
y
H
2
= 1 − 0.154 = 0.846
On the cathode we have two equations and two unknowns:
y
O
2
y
N
2
=
0.21
0.79
and y
O
2
+ y
N
2
+ 0.154 = 1
Solving, we find that y
O
2
= 0.178, y
N
2
= 0.668.
Step 2: Determine the diffusivities of the reactants. For the anode, this time we will use
the diffusion volume approach. For the cathode, from Eq. (5.34)
D
H
2
O−H
2
(T , P) = 10
−3
×
T
1.75
P
1/MW
1
+ 1/MW
2
i
(
V
i1
)
1/3
+
i
(
V
i2
)
1/3
2
= 10
−3
×
353
1.75
2
1/2 + 1/18
12.7
1/3
+ 7.07
1/3
2
= 0.44 cm
2
/s
For the cathode, from Eq. (5.33)
D
H
2
O−O
2
cm
2
s
=
4.19836 ×10
−7
P
(
atm
)
(T )
2.334
= 0.186 cm
2
/s
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
222 Transport in Fuel Cell Systems
Step 3: Determine the effective diffusivities of the reactants through the porous media.
From the Bruggeman relationship [we could also use Eq. (5.44) with little change],
D
eff
= Dφ
1.5
For the anode we have
D
H
2
O−H
2
,eff
= 0.44 × 0.7
1.5
= 0.258 cm
2
/s
which is lower than the open channel case, as expected. The porous media should
reduce the diffusivity. For the cathode:
D
H
2
O−O
2
,eff
= 0.186 × 0.7
1.5
= 0.109 cm
2
/s
Step 4: Evaluate Eq. (5.47) for the anode and cathode:
i
l
=−nFD
eff
C
∞
δ
=−nFD
eff
y
i
P/R
u
T
δ
For the cathode
i
l
=−(4 eq/mol)(96,485 C/eq)(0.109 cm
2
/s)
×
(
0.178 mol O
2
/mol mix
)
(202,650 N/m
2
)/(8.314 N · m/mol · K)(353 K)
(300 × 10
−4
cm)
×
1
1 × 10
6
m
3
cm
3
= 17.23 A/cm
2
For the anode
i
l
=−(2 eq/mol)(96,485 C/eq)(0.258 cm
2
/s)
×
(0.846 mol H
2
/mol mix)(202,650 N/m
2
)/(8.314 N · m/mol · K)(353 K)
300 × 10
−4
cm
×
1
1 × 10
6
m
3
cm
3
= 96.94 A/cm
2
The higher limiting current density on the anode compared to the cathode is typical
and a result of the relatively high diffusivity of hydrogen.
COMMENTS: In practical PEFCs, the limiting current density observed is much less,
around 2–4 A/cm
2
. The discrepancy is a result of factors not accounted for in this basic
gas-phase approach. Specifically, we have not included the diffusion resistances of liquid
water from flooding and the effect of an ionomer film on the catalyst. The assumption of
high stoichiometry is needed because at lower stoiciometry the consumption of reactant
means that the limiting current density near the inlet is different from the outlet because
of consumption of the reactant along the flow path. If we would have used the expression
including the catalyst layer resistance in Eq. (5.51), we would expect a slightly lower
limiting current density.

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.3 Gas-Phase Mass Transport 223
Figure 5.14 Diffusion in large channel. The molecular interactions with the channel wall are
negligible compared to the collisions with other molecules.
5.3.3 Knudsen Diffusion
Compared to the molecules themselves, the channels and pores through which the molecules
diffuse are typically large, and the main interaction for a particular molecule is collision
with other molecules, the driving force for bulk diffusion. In some cases for flow in
very small pores and channels, however, the molecular collisions with the solid walls
of the channel become a significant component of the overall number of collisions the
molecule experiences, and the effective diffusion will deviate from that predicted with
Fickian diffusion theory. Consider “normal” diffusion in a cylindrical pore (Figure 5.14).
The molecular interactions with the channel wall are negligible compared to the collisions
with other molecules.
For diffusion through very small pores (Figure 5.15), however, Knudsen diffusion be-
comes dominant at small pore sizes where wall interactions become significant. Physically,
the wall is acting as another interacting molecular species adding a “wall viscosity” to the
motion of the species, which acts to retard motion.
The key parameter in Knudsen diffusion is the average path length of the molecules
between collisions. If the path length becomes similar to the same length as the pore
diameter, then wall interaction (Knudsen diffusion) will be important. For liquids, the
density is so high that the path length is very small and Knudsen diffusion is unimportant.
For gases, however, the mean free path length, l, can be estimated based on molecular
dynamics [22]:
l =
k
B
T
√
2πσ
2
ii
P
(5.52)
The relationship is linear in temperature and inversely proportional to pressure. Here, σ
ii
is the collision diameter of the species, some of which are tabulated in Table 5.9, P is
the pressure in Pascals, T is the temperature in Kelvin, and k
B
is Boltzmann’s constant
Figure 5.15 Flow in a very small channel. The molecular interactions with the channel wall are no
longer negligible compared to the collisions with other molecules.
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
224 Transport in Fuel Cell Systems
Table 5.9 Collision Diameters σ
ii
for Various Fuel Cell Species From
Species Formula σ
ii
(
˚
A)
Carbon dioxide CO
2
3.941
Carbon monoxide CO 3.690
Hydrogen H
2
2.827
Oxygen O
2
3.467
Nitrogen N
2
3.798
Hydrogen peroxide H
2
O
2
4.196
Hydrogen sulfide H
2
S 3.623
Methanol CH
3
OH 3.626
Water H
2
O 2.641
Dimethyl ether CH
3
OCH
3
4.307
Air Mix 3.711
Source: From [19].
(1.3807 × 10
−23
J/K). For example, for oxygen at 1 atm pressure and 1000
◦
C,
l =
1.3807 × 10
−23
N · m/K
(1273 K)
√
2π
3.467 × 10
−10
m
2
(101,325 N/m
2
)
= 0.325 µm
For hydrogen, we can solve for a path length of 0.489 µm under the same conditions.
In order to determine if Knudsen diffusion is significant, the Knudsen number (Kn) must
be calculated according to the following criteria:
Kn =
l
d
=
k
B
T
√
2πσ
2
ii
Pd
(5.53)
Ĺ When Kn > 10, Knudsen flow dominates.
Ĺ When Kn < 0.01, bulk diffusion flow dominates.
Ĺ For 0.01 < Kn < 10, both Knudsen and bulk diffusion are important and a combi-
nation flow exists.
For Knudsen flow, the effective diffusion coefficient can be determined from the kinetic
theory of rigid spheres [19]:
D
Kn
cm
2
s
=
d
3
2k
B
T
m
i
1/2
(5.54)
where m
i
is the molecular mass of species i. Alternate theory yields a larger value [15]:
D
Kn
cm
2
s
=
4850d
√
T
MW
i
(5.55)
where MW
i
is the molecular weight of species i. The magnitude of Knudsen diffusion is a
direct function of pore size. It is around 3 × 10
−3
cm
2
/s for a 50-nm pore with hydrogen
diffusion at 353 K. As the molecular weight increases, Knudsen diffusivity decreases, and
as pore diameter increases, diffusivity increases (less collisions to interfere with motion), to
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.3 Gas-Phase Mass Transport 225
the limit of transition to bulk diffusion. Also notice there is no pressure or multicomponent
factor in Knudsen diffusion. This is because in the Knudsen limit intermolecular collisions
are rare. Also notice bulk diffusion varies with T
1.5
, where Knudsen diffusivity varies with
T
0.5
. Knudsen diffusion flux is modeled with the Fickian equation, with a modified Knudsen
diffusion coefficient replacing the binary diffusion coefficient:
˙
n
j
=−D
Kn
A
dC
j
dx
(5.56)
Also note that the model assumes a straight pore path. As in bulk media, any tortuosity
will increase the real path length, and this must be added to the result as in Eq. (5.41). In
the context of the length scales involved with Knudsen diffusion, however, it is unlikely
significant error will result without the use of a tortuosity correction.
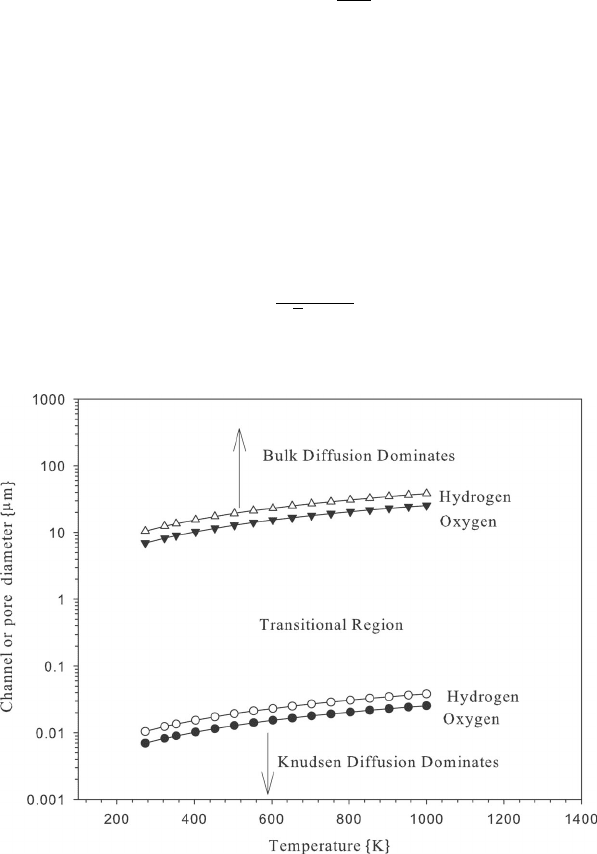
Example 5.9 Knudsen Flow Estimate the pore diameters where Knudsen flow will be
important as a function of temperature from 273 to 1000
◦
C for oxygen and hydrogen at
1atm.
SOLUTION We can easily solve for the mean molecular path length:
l =
k
B
T
√
2πσ
2
ii
P
From this we can plot the regions where the ratio of l/d falls within the three flow regimes.
COMMENTS: The range of pore diameters where Knudsen diffusion dominates is below
∼0.05 µm. Not many media in fuel cells have pore diameters less than 0.05 µm, so that
Knudsen diffusion does not likely dominate anywhere, besides possibly in very small pore
catalyst layers. However, for diameters less than around 10 µm, there should be some mixed
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
226 Transport in Fuel Cell Systems
bulk/Knudsen diffusion effect. For simple analysis, transition region flow can be modeled
in several ways: (1) as parallel flux of bulk and Knudsen flow if the media has a bimodal
(bulk- and Knudsen-dominated) pore distribution, as occurs in some PEFC diffusion media;
(2) as an effective diffusion resistance with a decrease in the bulk diffusivity to account for
the Knudsen effect; or (3) by using more advanced mathematical model multicomponent
gas mixtures specifically designed to predict flow behavior in the transitional flow regime,
as in ref. [25].
5.3.4 Liquid-Phase Diffusion
As shown in Table 5.4, diffusion coefficients of gases in liquids tend to fall in the 10
−5
-cm
2
/s
range. In fuel cells, diffusion of gases in liquids occurs between the gas phase and a liquid
electrolyte (although there can be additional interactions with the electrolyte besides pure
diffusion) and in low-temperature PEFCs under slightly flooded conditions or with a liquid
fuel solution feed such as a DMFC. Since diffusion in liquids is so slow relative to gases,
it can be limiting in these situations. The Stokes–Einstein equation is commonly used to
estimate liquid diffusion coefficients:
D =
k
B
T
6πµR
o
(5.57)
where k
B
is the Boltzmann constant (1.3807×10
−23
J/K), µ is the solvent viscosity, and R
o
is the solute radius, some of which are tabulated in Table 5.10. The accuracy of diffusion
correlations for liquid is less than that for gases but is typically within 20% in most
applications. Other empirical correlations exist and can be used if higher accuracy is
desired. (See, e.g., [19].) To determine the viscosity of the solvent, we can use Eq. (5.13).
Example 5.10 Liquid Layer Penetration Time Estimate the time required for oxygen to
penetrate and diffuse across a 10-µm liquid film on a catalyst in a partially flooded PEFC
at 80
◦
C.
Table 5.10 Estimated Solvent Radius
Gas Formula R
o
(
˚
A)
Hydrogen H
2
1.414
Oxygen O
2
1.734
Nitrogen N
2
1.899
Air Mix 1.856
Carbon dioxide CO
2
1.971
Carbon monoxide CO 1.845
Hydrogen peroxide H
2
O
2
2.098
Ethanol C
2
H
5
OH 2.265
Methanol CH
3
OH 1.813
Source: Adapted from data in [19].
c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.3 Gas-Phase Mass Transport 227
SOLUTION The diffusivity of oxygen in water can be estimated from Eq. (5.57):
D
cm
2
s
=
k
B
T
6πµR
o
=
(1.3807 × 10
−23
J/K)(353 K)
6πµ(1.734 × 10
−10
m)
(10,000 cm
2
/m
2
)
The viscosity of water can be estimated from a rearrangement of Eq. (5.13)
µ = exp
a + b
T
o
T
+ c
T
o
T
2
+ µ
o
= exp
−1.94 − 4.80
273.16
353
+ 6.74
273.16
353
2
(1.792 × 10
−3
kg/ms)
= (1.155 × 10
−3
kg/ms)
Now we can evaluate the diffusion coefficient:
D
cm
2
s
=
k
B
T
6πµR
o
=
(1.3807 × 10
−23
J/K)(353 K)
6π(1.155 × 10
−3
kg/ms)(1.734 × 10
−10
m)
× (10,000 cm
2
/m
2
) = 1.29 × 10
−5
cm
2
/s
and the characteristic time to penetrate this water layer is estimated from Eq. (5.30),
τ
d
=
δ
2
D
=
1 × 10
−4
cm
2
1.29 × 10
−5
cm
2
/s
= 7.75 s
COMMENTS: Note there is no pressure dependence for diffusion through the liquid.
However, there is a pressure dependence on the concentration of oxygen in the liquid, as
discussed later in this chapter.
5.3.5 Diffusion through a Polymer Electrolyte
As we have discussed, the diffusion into the polymer electrolyte is an important consider-
ation for PEFCs for two conflicting reasons:
1. The catalyst is typically covered in a thin electrolyte layer, so that high diffusivity
of reactants in the electrolyte is desired.
2. Reactant crossover through the electrolyte reduces OCV and efficiency, so that low
diffusivity of reactants in the electrolyte is desired.
Based on the competing needs of these factors, an electrolyte with some diffusivity of
reactants is needed. For the PEFC, the diffusivity has been correlated from experimental
data for 1100-EW Nafion, the most well-studied polymer electrolyte. Other electrolyte
polymers should have similar trends, but actual values change with EW values. In general,
low-EW polymers that absorb more water will have higher gas-phase species diffusion
coefficients than higher EW electrolytes. Transport properties in the polyflourosulfonic acid
(PFSA) based membranes such as Nafion are generally dependent on the water sorption
in the membrane. For dry membranes, ionic conductivity and water mobility are very low.
As water sorption is increased, the membrane swells, and particularly at over 60% RH,

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
228 Transport in Fuel Cell Systems
the water uptake increases dramatically, and transport properties approach that of a dilute
electrolyte solution.
The diffusivity of water vapor into PEFC electrolyte Nafion is a function of the ma-
terial’s water uptake, since the vapor must diffuse through the entire media. There is
significant discrepancy between various authors on the measured values of the water diffu-
sivity coefficient, since it is a difficult parameter to accurately measure, and the membrane
itself swells with water uptake. The diffusion coefficient of water in 1100-EW Nafion PFSA
polymer with λ>4 has been correlated as [12]:
D
w
cm
2
s
= 10
−6
exp
2416
1
303
−
1
T
(2.563 − 0.33λ + 0.0264λ
2
− 0.000671λ
3
)
(5.58)
where λ is given in Eq. (5.18). The diffusion coefficient of water in Nafion has been studied
by many authors, and a surprising degree of difference between results exists, Eq. (5.58)
is commonly used in modely however, and is considered a reasonable correlation. Oxygen
diffusivity into 1100-EW Nafion has been given empirically as [26]:
D
O
2
−Nafion
cm
2
s
= 2.88 × 10
−6
exp
2933
1
313
−
1
T
(5.59)
For 1200-EW polymer [27]:
D
O
2
−Nafion
cm
2
s
= 3.1 × 10
−3
exp
−
2768
T
(5.60)
Both approaches yield the same order of magnitude in results. Hydrogen diffusivity into
Nafion 1100-EW as a function of temperature (in Kelvin) for a fully moist Nafion 1100-EW
polymer has been correlated as [28]
D
H
2
−Nafion
cm
2
s
= 4.1 × 10
−3
exp
−2602
T
(5.61)
It should be cautioned that the availability of precise transport coefficients is incomplete
in the literature. There are significant differences between research results for much of
these data, and complete details under a full range of temperature and humidity, and for all
materials are not yet available. Nevertheless, the values presented here serve as a reasonable
approximation to the diffusion coefficients for calculation purposes.
5.3.6 Interfacial Flow between Phases and Film Resistance
From Example 5.7, we see that the pure gas-phase resistance is typically not the limiting
resistance to mass transport to the catalyst. The diffusion resistance model can be expanded
to make it more complete by adding in additional resistances, such as diffusion into the
electrolyte covering the catalyst or through a film of liquid water flooding the catalyst.
However, these film effects are localized around only a fraction of the catalysts, and by
including an effective film resistance, we are showing only qualitative aggregate effects.
However, in many instances in fuel cells, especially PEFCs, mass flux across different

c05 JWPR067-Mench January 23, 2008 18:58 Char Count=
5.3 Gas-Phase Mass Transport 229
y
O
2
, gas side
y
O
2
, liquid side
Figure 5.16 Schematic mass transfer across a gas/liquid-phase boundary. There is a sharp drop in
molar concentration from the gas phase to the liquid phase that is a strong function of temperature.
phases occurs. For example, reactant flux typically must penetrate a thin layer of electrolyte,
and possibly water, to reach the catalyst. In Example 5.7, we solved for the gas-phase
transport limited current density and found that, even when restricted by porous media, the
predicted mass transport limiting current density is much greater than actually observed for
PEFC systems. In this section, the film resistance caused by mass transport across a phase
boundary is examined. Unlike a no-slip condition in fluid mechanics or a no-temperature-
jump condition in heat transfer, there can be a significant concentration discontinuity across
a phase interface.
Mass Flux across Phase Boundary: Henry’s Law When there is species transport across
a phase boundary (e.g., gas into a liquid), there is a discontinuous change in the molar
concentration of the species across the phase interface, as shown in Figure 5.16. The
concentration discontinuity across the phase boundary between a liquid and gas on across
a membrane to gas interface is typically modeled with Henry’s law:
y
i,liquid/membrane side
=
y
i,gas side
P
gas side
H(T )
(5.62)
where H is Henry’s constant, known to be a function of temperature. Assuming a di-
lute solution, the liquid-phase mole fraction can be converted into concentration by the
following:
C
i,liquid/membrane side
mol
i
cm
3
= y
i,liquid/membrane side
mol
i
mol mix
×
ρ
liquid
kg/cm
3
MW
liquid
(
kg mix/mol mix
)
(5.63)
Sometimes the conversion to solid- or liquid-side concentration is already made part of
Henry’s constant and termed the solubility. In this text, we will use this convention. That
is, we will define Henry’s law as
C
i,liquid/membrane side
mol
cm
3
=
y
i,gas side
P
gas side
H(T )
(5.64)
Henry’s constant for a variety of common gas-phase species into liquid water is tabulated
in Table 5.11.
