Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

fm JWPR067-Mench December 13, 2007 20:22 Char Count=
Acknowledgments
First and foremost, I owe whatever accomplishments I have in my life to my God. I also
want to thank my wife, Laurel, and my children, Elizabeth Adeline and Michael, for their
willingness to let me vaporize from existence to finish this book. I am in great debt also to
all of my graduate students, past and present, who helped me to learn and teach what I know
and who continue to make life enjoyable and exciting in the process. Dr. Rama Ramasamy
and Dr. Emin Caglan Kumbur have been especially helpful in reviewing sections of the
text and providing valuable suggestions. I am greatly appreciative of Ms. Elise Corbin, who
provided a vast majority of the sketches in the text. I also want to thank my publisher and
his editorial assistants, Robert L. Argentieri, Bob Hilbert, Evan Jones, and Daniel Magers,
respectively, for the faith and patience they have showed in me. I am in debt to the hundreds
of students in my classes who have provided immensely valuable feedback that I have
tried to incorporate in all facets of the textbook. I am also in debt to my colleagues in the
profession and research sponsors who have given me insights and challenging problems to
study that have ultimately led me down this path. Professor C. Y. Wang gave me my initial
opportunity in the field. Professor Sukkee Um of Hanyang University in South Korea has
been a close friend and fuel cell expert t o whom I owe a lot. I also which to thank Professor
Peiwen Li of the University of Arizona, whose feedback has enhanced the quality of the
book. I wish to thank my academic advisor Keneneth Kuo, who taught me that, many
times, progress and insight comes from unrelenting tenacity and determination and who
encouraged me to write this book. Finally, I am grateful to my parents, J. Larry and Noreen
Mench, and in-laws, Larry and Arlene Tepke, who have taught me that true progress is
much more than what you do at work.
xi

c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
1
Introduction to Fuel Cells
The Stone Age didn’t end because they ran out of stones—but as
a result of competition from the bronze tools, which better met
people’s needs. I feel there’s something in the air—people are
ready to say that this is something we should do.
—Jeroen van der Veer, Chairman of Royal Dutch/Shell Group
2000
1.1 PRELIMINARY REMARKS
The science and technology of fuel cell engines are both fascinating and continually evolv-
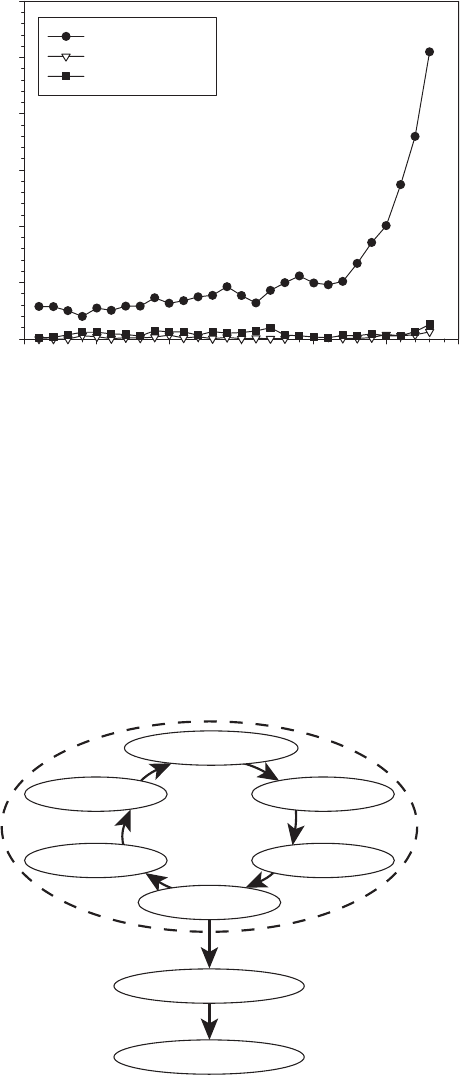
ing. This point is emphasized by Figure 1.1, which shows the registered fuel-cell-related
patents in the United States, Canada, and the United Kingdom from 1975 through 2003.
A similar acceleration of the patents granted in Japan and South Korea is also well un-
derway, led by automotive manufacturers. The rapid acceleration in fuel cell develop-
ment is not likely to wane in the near future, as the desire for decreased dependence on
petroleum supplies, lower pollution, and potential for high efficiency are driving this trend
toward an alternative power generation technology. Any attempt to bring the reader the
state-of-the art of the applied technology of fuel cell engines in a texbook would be hope-
lessly antiquated by the time it was published. The designs, materials, and components of
fuel cell systems are constantly being improved for increased efficiency, durability, and
lower cost.
At the heart of the ever-changing fuel cell technology, however, is an equally fascinating
and rich multidisciplinary fundamental science drawn from various engineering disciplines.
The fundamentals of fuel cell science, emphasized in this textbook, are shown schematically
in Figure 1.2. It is obvious that fuel cell science is not solely the domain of the electrochemist
and can encompass nearly all engineering disciplines. Electrochemistry, thermodynamics,
reaction kinetics, heat and mass transfer, fluid mechanics, and material science all play
integral roles in basic fuel cell design. Outside the basic science of an individual fuel cell lie
system and component issues that include manufacturing, sensing and control, vibration,
and a plethora of other technologies. The goal of this text is to provide a fundamental
background on fuel cell science shown in Figure 1.2 to serve as an introduction to this
captivating and rapidly expanding field.
As discussed in Section 1.6, there have been several waves of concentrated fuel cell
research and development, each driven by a somewhat different impetus. Throughout the
1
Fuel Cell Engines
Copyright © 2008 by John Wiley & Sons, Inc.
Matthew M. Mench
c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
2 Introduction to Fuel Cells
United States
600
400
200
0
800
1000
1200
United Kingdom
Canada
1975 1980
1985
1990 1995
Year of issue
Number of patents issued
2000 2005
Figure 1.1 Timeline of worldwide patents in fuel cells for select countries based on data from U.S.,
U.K., and Canadian patent offices.
history of development, however, the fundamental advantages common to all fuel cell
systems have included the following:
1. A potential for a relatively high operating efficiency, scalable to all size power
plants.
2. If hydrogen is used as fuel, pollution emissions are strictly a result of the production
process of the hydrogen.
Electrochemistry
Materials Thermodynamics
Fluid mechanics
Reaction kinetics
Heat/mass transfer
Specific FC phenomena
FC system components
Figure 1.2 Major engineering disciplines involved in fundamental fuel cell science.
c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
1.2 Fuel Cells as Electrochemical Engines 3
3. No moving parts, with the significant exception of pumps, compressors, and blowers
to drive fuel and oxidizer.
4. Multiple choices of potential fuel feedstocks, from existing petroleum, natural gas,
or coal reserves to renewable ethanol or biomass hydrogen production.
5. A nearly instantaneous recharge capability compared to batteries.
It should be noted that fuel cells must not be seen as a panacea for every power-
generating application need in the world. There are many specific applications, however,
where fuel cell use has great potential to have a major impact on future power generation.
Before this conversion can occur, however, the following technical limitations common to
all fuel cell systems must be overcome:
1. Alternative materials and construction methods must be developed to reduce fuel cell
system cost to be competitive with the automotive combustion engine (∼$30/kW)
and stationary power generation systems (∼$1000/kW). The cost of the catalyst no
longer dominates the price of most fuel cell systems, although it is still significant.
Manufacturing and mass production technology are now also key components to
the commercial viability of fuel cell systems.
2. Suitable reliability and durability must be achieved. The performance of every fuel
cell gradually degrades with time due to a variety of phenomena. The automotive
fuel cell must withstand load cycling and freeze–thaw environmental swings with an
acceptable level of degradation from the beginning-of-lifetime (BOL) performance
over a lifetime of 5500 h (equivalent to 165,000 miles at 30 mph). A stationary
fuel cell must withstand over 40,000 h of steady operation under vastly changing
external temperature conditions.
3. Suitable system power density and specific power must be achieved. The U.S.
Department of Energy year 2010 targets for system power density and specific
power are 650 W/kg and 650 W/L for automotive (50-kW) applications, 150 W/kg
and 170 W/L for auxiliary (5–10-kW peak) applications, and 100 W/kg and
100 W/L for portable (milliwatt to 50-W) power systems [1].
4. Fuel storage, generation, and delivery technology must be advanced if pure hydrogen
is to be used. Hydrogen storage and generation are discussed in Chapter 8. The
hydrogen infrastructure and delivery are also addressed in ref. [2].
5. Desired performance and longevity of system ancillary components must be
achieved. New hardware (e.g., efficient transformers and high-volume blowers)
must be developed to suit the needs of fuel cell power systems.
6. Sensors and online control systems for fuel cell systems are needed, especially for
transient operation, where performance instability can become a major issue.
The advantages and disadvantages of particular fuel cell systems are discussed in
greater detail throughout this book.
1.2 FUEL CELLS AS ELECTROCHEMICAL ENGINES
Fuel Cells versus Heat Engines The first question many people ask is “why are these
systems called fuel cell engines?” An engine is a device that converts energy into useful
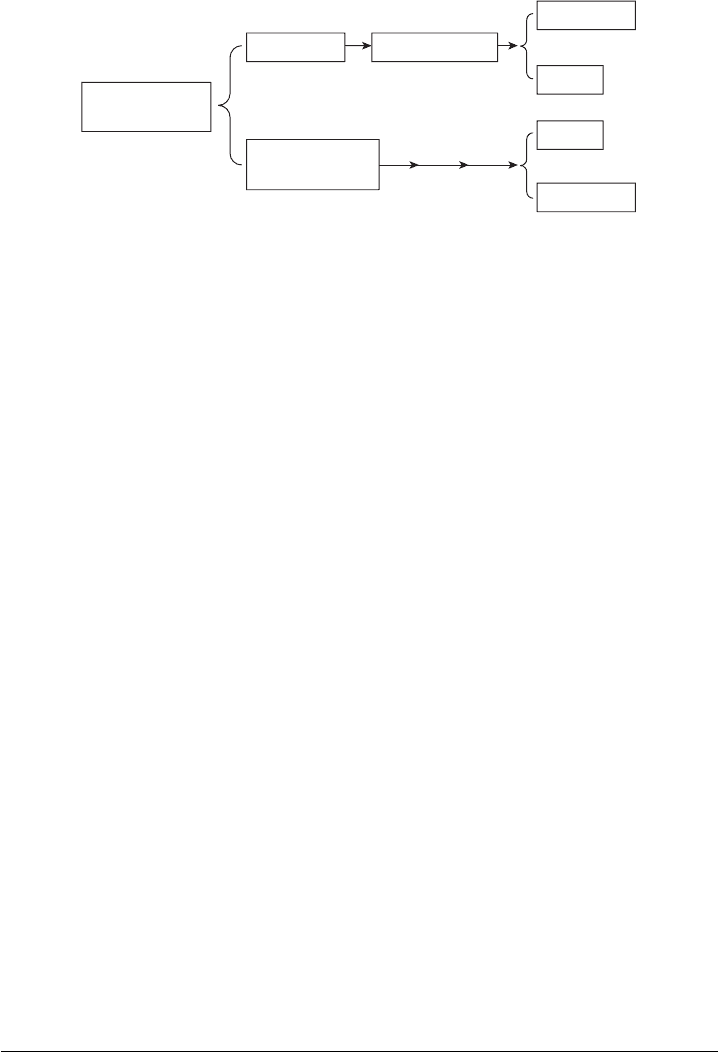
c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
4 Introduction to Fuel Cells
Same initial
chemical energy
Heat engine
Electrochemical
engine
Thermal energy
Waste heat
Power
Waste heat
Power
Figure 1.3 Conceptual comparison between heat engines and electrochemical engines.
work. While a combustion engine converts the chemical energy of the fuel and oxidizer into
mechanical work (i.e., it moves some mass through space), a fuel cell engine converts the
same initial chemical energy directly into electrical work (i.e., it moves electrons through a
resistance). Thus, fuel cells and batteries can both be considered electrochemical engines.
Figure 1.3 shows a conceptual comparison between a heat engine and an electrochemical
engine. Both systems utilize a fuel and an oxidizer as reactants. Both systems derive the
desired output of useful work from the chemical bond energy released via the oxidation
of the fuel. For the same fuel and oxidizer, the overall chemical reaction and the potential
energy released by the reaction are identical. At first glance, this fact may not seem
obvious. The difference between the heat and electrochemical engines lies in the process
of conversion of the enthalpy of reaction
1
to useful work.
In the heat engine, the fuel and oxidizer react via combustion to generate heat, which
is then converted to useful work via some mechanical process. An internal combustion
engine in a car is a good example. Combustion expands the gas in the combustion cham-
ber, which moves the pistons and is converted to rotational motion in the drive train.
This turns the wheels and propels the vehicle. Conversely, in an electrochemical engine,
the same enthalpy of reaction is directly converted into electrical current via an electro-
chemical oxidation process. The direct conversion of energy from chemical to electrical
energy has a profound impact on the maximum theoretical efficiency of electrochemical
devices, as we shall see in greater detail in Chapter 2. Before presenting the equations
to describe this, a simple thought experiment can be used to demonstrate the increased
potential efficiency of a fuel cell compared to a combustion engine. Consider a conven-
tional automobile and a hydrogen polymer electrolyte fuel cell stack, as shown in Figure
1.4. The combustion engine would be too hot to touch during operation without burning
one’s hand. The heat given off by the engine to the environment is not used to propel
the vehicle and is therefore a waste product of the chemical energy initially available
from the reaction. Now, consider a hydrogen fuel cell stack, which operates at around
70–80
◦
C, at the same useful power output. The fuel cell would be very warm to the touch
but much cooler than the combustion engine. Thus, the waste heat given off as an ineffi-
ciency in the fuel cell is less than the combustion engine. A fuel cell is not always more
1
If the reader is unfamiliar with enthalpy of reaction, a review of an undergraduate-level thermodynamics textbook
is suggested.
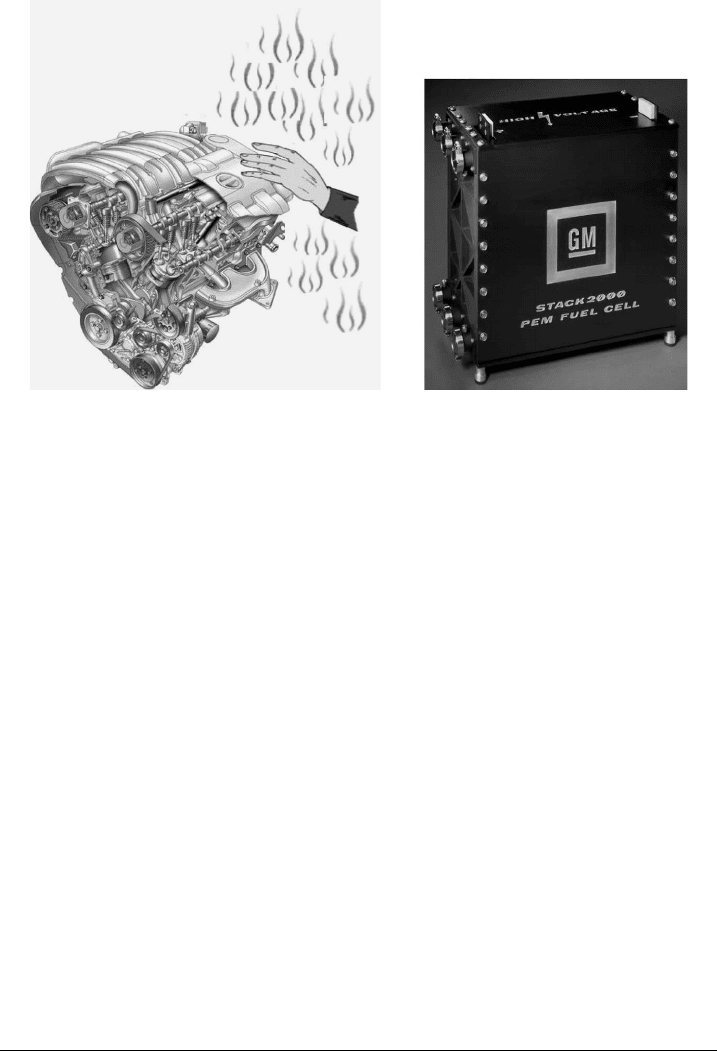
c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
1.2 Fuel Cells as Electrochemical Engines 5
(a) (b)
Figure 1.4 Conceptual comparison of efficiency of fuel cell versus combustion engine. (Fuel cell
stack image courtesy of General Motors Corporation.)
efficient than a combustion engine, but it is in many practical cases, as we shall discuss in
Chapter 2.
Fuel Cells versus Batteries Consider a common battery with stored fuel and oxidizer.
When used to power a particular application, the fuel and oxidizer react to generate current,
chemical products of the reaction and heat. This continuously depletes the reactants during
operation until performance becomes unacceptable. In the simplest analogy possible, a fuel
cell is similar to a battery, except with constant flow of oxidizer (commonly air) and fuel
(hydrogen, methanol, or other), as shown in Figure 1.5. Imagine creating a fuel cell by
drilling holes in a battery to allow a flux of oxidizer and fuel in and products of the reaction
out. Instead of having a sealed battery where stored fuel and oxidizer gradually deplete, a
fuel cell has constantly flowing reactants and products. In this way, a fuel cell can operate as
a true steady-state device. In fact, one can consider a fuel cell as an instantly rechargeable
battery. A battery, which derives energy from stored reactants, can never achieve a strict
steady-state operation. Unlike a fuel cell, a primary battery is nonrechargeable. A secondary
battery is rechargeable, but the process or recharging involves controlled reversal of the
electrochemical reactions and takes significantly longer than refilling the flow of oxidizer
and fuel in a fuel cell.
The difference between a battery and a fuel cell system can also be related to the
definitions of a system and control volume taken from basic thermodynamics.
2
Inather-
modynamic system, no mass flux is permitted to cross the system boundaries (battery),
2
See, for example, Fundamentals of Engineering Thermodynamics , M. S. Moran and H. N. Shapiro, John Wiley
and Sons, 1995.
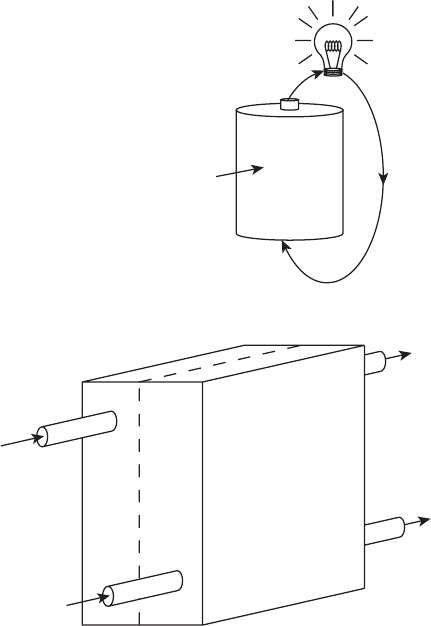
c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
6 Introduction to Fuel Cells
Depleting
fuel and
oxidizer
Battery
e
(a)
-
Fuel cell
(b)
Fuel in
Depleted
fuel and products out
Oxidizer in
Depleted
oxidizer and products out
Anode
Cathode
Figure 1.5 Basic comparison of batteries to fuel cells: (a) battery; (b) fuel cell.
while in a thermodynamic control volume, mass flux is permitted across the boundaries
(fuel cell).
1.3 GENERIC FUEL CELL AND STACK
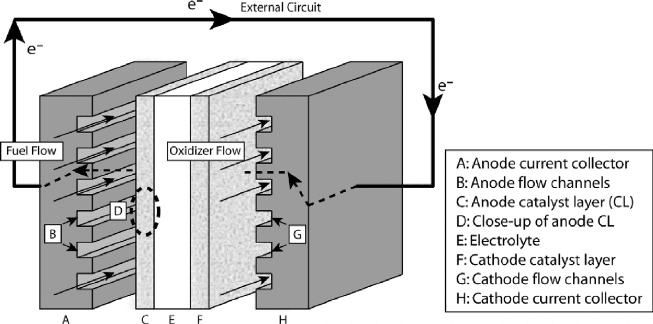
Basic Operating Principles Figure 1.6 shows a schematic of a generic fuel cell with
components common to most fuel cell types shown. Referring to Figure 1.6, separate
liquid- or gas-phase fuel and oxidizer streams enter through flow channels, separated by the
electrolyte/electrode assembly. Reactants are transported by diffusion and/or convection
to the catalyst layer (electrode), where electrochemical reactions take place to generate
current. Some fuel cells have a porous (typical porosity ∼0.6–0.8) contact layer between
the electrode and current collecting reactant flow channels that functions to transport
electrons and species to and from the electrode surface. In polymer electrolyte fuel cells
c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
1.3 Generic Fuel Cell and Stack 7
Figure 1.6 Schematic of a generic fuel cell.
(PEFCs) discussed in Chapter 6, an electrically conductive carbon paper or cloth diffusion
medium (DM) layer (also called gas diffusion layer, or GDL) serves this purpose, and a
DM covers the anode and cathode catalyst layer.
At the anode electrode, the electrochemical oxidation of the fuel produces electrons that
flow through the bipolar plate (also called cell interconnect) to the external circuit, while
the ions generated migrate through the electrolyte to complete the circuit. The electrons
in the external circuit drive the load (e.g., electric moter or other device) and return to the
cathode catalyst where they recombine with the oxidizer in the cathodic oxidizer reduction
reaction (ORR). The products of the fuel cell are thus threefold: (1) chemical products, (2)
waste heat, and (3) electrical power.
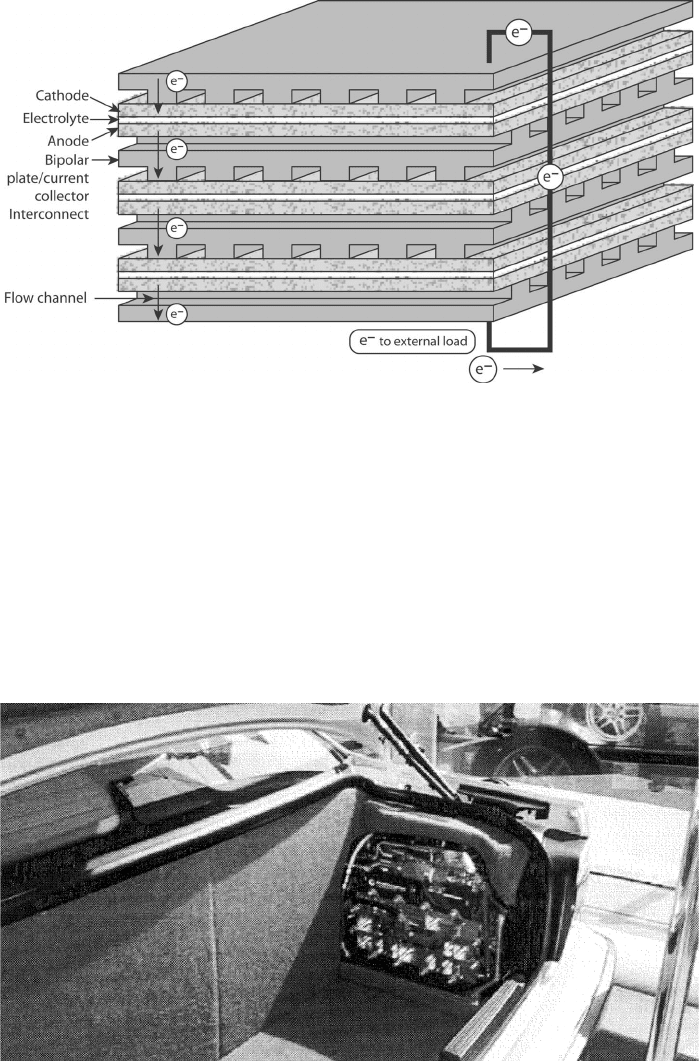
Description of a Fuel Cell Stack A single fuel cell can theoretically achieve whatever
current and power are required simply by increasing the size of the active electrode area
and reactant flow rates. However, the output voltage of a single fuel cell is limited by
the fundamental electrochemical potential of the reacting species involved and is always
less than 1 V for realistic operating conditions. Therefore, to achieve a higher voltage and
compact design, a fuel cell stack of several individual cells connected in series is utilized.
Series-parallel combinations are also utilized in some systems as well. Figure 1.7 is a
schematic of a generic planar fuel cell stack assembly without a flow manifold and shows
the flow of current through the system. For a stack in series, the total current is proportional
to the active electrode area of each cell in the stack and is the same through all cells in
series. The total stack voltage is simply the sum of the individual cell voltages. For fuel cells
in parallel, the current is additive and the voltage is the same in each cell. For applications
that benefit from higher voltage output, such as automotive stacks, over 200 fuel cells in a
single stack can be used.
Other components necessary for fuel cell system operation include subsystems
for fuel and oxidizer delivery, voltage regulation and electronic control, fuel and pos-
sibly oxidizer storage, fuel recirculation/consumption, stack temperature control, and
system sensing of control parameters. For the PEFC, separate humidification systems
are also needed to ensure optimal performance and stability. A battery is often used
c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
8 Introduction to Fuel Cells
Figure 1.7 Fuelcell stack in series. The total current is the same in each fuel cell; the voltage is
additive for each fuel cell plate in series. Not all stack arrangements are totally in series, however,
and a mixed series–parallel arrangement can be used.
to initiate reactant pumps/blowers during start-up. In many fuel cells operating at
high temperature, such as a solid oxide fuel cell (SOFC) or molten carbonate fuel
cell (MCFC), a preheating system is used to raise cell temperatures during start-up.
This can be accomplished with a combustion chamber that burns fuel and oxidizer
gases. Figure 1.8 shows a 5-kW hydrogen PEFC developed by United Technologies
Corporation (UTC) in the trunk compartment of a BMW 7 series car for use as an auxiliary
power unit for electronics and climate control.
Figure 1.8 UTC 5kW Hydrogen PEFC demonstrated in 1999 in the trunk compartment of a BMW
7 series car for use as an auxiliary power unit (APU) to control electronics and climate control. (Image
Courtesy of UTC Power Corporation.)

c01 JWPR067-Mench December 19, 2007 19:42 Char Count=
1.4 Classification of Fuel Cells 9
In all commercial fuel cells, provision must be made for residual fuel effluent recovery.
Fuel utilization is not 100% due to concentration polarization limitation on performance dis-
cussed in Chapters 3 and 4, so that unused fuel in the anode exhaust stream is always present
and must be actively recycled, utilized, or converted prior to exhaust to the environment.
Potential effluent management schemes include the use of recycling pumps, condensers
(for liquid fuel), secondary burners, catalytic converters, or dead-end anode designs.
1.4 CLASSIFICATION OF FUEL CELLS
A number of fuel cell varieties have been developed to differing degrees, and the most
basic nomenclature to describe them is according to the electrolyte material utilized. For
instance, a SOFC has a solid ceramic oxide electrolyte and a PEFC has a flexible polymer
electrolyte.
3
Additional subclassification of fuel cells beyond the basic nomenclature can
be assigned in terms of fuel used (e.g., hydrogen PEFC or direct methanol PEFC) or
the operating temperature range. Table 1.1 gives the operating temperatures, electrolyte
material, and likely applications for the most common types of fuel cells.
Each fuel cell variant has certain advantages that engender use for particular appli-
cations. Low-temperature fuel cells include alkaline fuel cells (AFCs) and PEFCs. The
primary advantages of operating under low temperature include more rapid start-up and
higher efficiency.
4
However, low-temperature systems generally require more expensive
catalysts and much larger heat exchangers to eliminate waste heat due to the low temper-
ature difference with the environment. High-temperature fuel cells (e.g., SOFC, MCFC)
have an advantage in raw material (catalyst) cost and the quality and ease of rejection of
waste heat. Medium-temperature fuel cells [e.g., phosphoric acid fuel cell (PAFC)] have
some of the advantages of both high- and low-temperature classifications.
Classification of fuel cells by temperature is becoming more blurred, however, since a
current SOFC research focus is lower temperature (<600
◦
C) operation to improve start-up
time, cost and durability, while a focus of PEFC research has been to increase operation
temperature to >120
◦
C to improve waste heat rejection and water management. The ideal
temperature seems to be around 150–200
◦
C which is where the PAFC typically operates.
However, the PAFC has its own historical limitations which have hampered enthusiasm for
its continued development.
Hydrogen PEFC The hydrogen polymer electrolyte fuel cell (H
2
PEFC) operates at
20–100
◦
C and is envisioned by many as the most viable alternative to heat engines and for
battery replacement in automotive, stationary, and portable power applications. It should
be noted that in the past, PEFCs have also been referred to as solid polymer electrolyte
(SPE) fuel cells and proton exchange or polymer electrolyte membrane (PEM) fuel cells.
Following the accepted nomenclature that fuel cell systems are named according to the
electrolyte used, the term polymer electrolyte fuel cell (PEFC) is most concise and cor-
rect, although the moniker “PEM fuel cell” retains popularity because it has been histor-
ically more prevalent and easier to say. Currently, the majority of fuel cell research and
development for automotive and stationary applications are on the H
2
PEFC. The H
2
PEFC
3
An exception to this nomenclature is biological process based fuel cells, which are identified as biological fuel
cells, or microbial fuel cells, regardless to the electrolyte used.
4
This is opposite to the heat engine, where higher operating temperatures bring increased efficiency. More on this
interesting trend in Chapter 2.
