Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
60 Basic Electrochemical Principles
where methanol density is 700 kg/m
3
and methanol molec-
ular weight is 32 g/mol.
(a) Calculate the minimum volume (in cubic centime-
ters) of a pure methanol fuel tank required to run
a soldier’s uniform equipment for three days. The
nominal power is 20 W. There are 10 cells in the
stack in series and the total stack voltage is 5 V.
(b) What is the molar rate of water consumption at the
anode?
(c) What is the molar rate of water production at the
cathode?
(d) Is there a water flow rate required at the anode?
(e) What is the net molar rate of water production per
mole of methanol for the cell?
(f) What is the minimum molar rate of air required
for reaction?
2.14 Consider a dimethylether [DME, (CH
3
)
2
O] fuel cell
stack running at a DME stoichiometry (λ
DME
)of2.4anda
cathode oxygen Faradic efficiency (ε
f
) of 0.3. There are 15
cells (connected in series) in the stack, all operating at 0.4 V.
The active area of all cells in the stack is 25 cm
2
, and the
current density of each cell is 0.1 A/cm
2
.
The anode electrochemical reaction is
(CH
3
)
2
O + 3H
2
O → 12H
+
+ 12e
−
+ 2CO
2
The cathode electrochemical reaction is
O
2
+ 4e
−
+ 4H
+
→ 2H
2
O
with the following molecular weights:
DME 44 g/mol
H
2
O 18 g/mol
O
2
32 g/mol
Air 28.85 g/mol
(a) How many moles of water are created at the cath-
ode per mole of DME?
(b) What is the theoretical consumption rate of DME
at the anode in grams per second? What is the
actual supply rate in grams per second?
(c) What is the theoretical consumption rate of H
2
O
at the anode in grams per second?
(d) What is the actual supply rate of air at the cathode
in grams per hour?
(e) Would a DME fuel cell theoretically need a water
storage tank? Explain your answer.
2.15 Consider the following reactions typical of many fuel
cells:
H
2
→ 2H
+
+ 2e
−
4H
+
+ 4e
−
+ O
2
→ 2H
2
O
(a) Which reaction occurs at the anode of the fuel cell
and which reaction occurs at the cathode?
(b) Is this a galvanic or electrolytic cell?
(c) Which is the positive electrode?
2.16 Consider a 25-cell hydrogen/air PEM fuel cell stack
producing a total of 2.0 kW at 10 V.
(a) What is the total stack mass flow rate of hydrogen
if the anode stoichiometry is 1.3?
(b) What is the total stack rate of generation of water
at the cathodes in grams per hour?
(c) If the theoretical maximum voltage of a single cell
is 1.23 V, what is the voltaic efficiency of a single
cell. Assume all cells have the same voltage.
2.17 Consider an ideal fuel cell to be run on pure oxygen
and hydrogen for a given length of time. Determine the ra-
tio of the minimum size of fuel to oxidizer storage tanks,
V
fuel
/V
oxidizer
, assuming they are stored as gases at the same
pressure and the anode and cathode stoichiometries (λ
a
and
λ
c
) are 1.5 and 2.0, respectively.
2.18 Considering the concepts discussed for the generic
fuel cell, list three reasons why the original fuel cells in-
vented by Grove worked so poorly relative to modern fuel
cells? The Grove fuel cell operated with the use of flat-
plate platinum electrodes in an aqueous dilute sulfuric acid
electrolyte solution, as shown below:
O
2
H
2
e
-
e
-
e
-
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
References 61
Open-Ended Problems
2.19 Consider the generic fuel cell of Figure 2.9.
(a) List three to five qualities that each of the com-
ponents shown should have. Note: For repeating
units (e.g., cathode and anode catalyst layers) you
can just say “same as the other.”
(b) Using your intuition about the function of these
components, describe a loss or limitation that can
occur with (a) the electrodes and (b) the bipo-
lar plates and how you would solve or improve
it. Then discuss what limitations or other off-
shoot advantages you might face with your so-
lution/improvement.
As an example the bipolar plate needs to be noncorrosive.
So we could use some kind of plastic composite or coated
metal. The limitations encountered could be that the plat-
ing is expensive or will not last or the plastic composite has
high electrical resistance compared to the metal it replaced.
Other advantages would be that it is potentially cheaper to
produce, faster to machine, and much lighter.
2.20 Estimate how much weight savings in terms of fuel
and oxidizer would be realized by replacing a 100 W, 20 A
fuel cell stack designed for 4000 h service with a reversible
fuel cell recharged by a solar panel for a space application.
Because fuel and oxidizer are recycled, you can assume an
effective stoichiometry of 1.0 for the anode and cathode in
both cases.
REFERENCES
1. G. J. Binczewski, “The Point of a Monument: A History of the Aluminum Cap of the Washington
Monument,” J. Met.,Vol.47, No. 11, pp. 20–25, 1995.
2. Battery and EV Industry Review, Business Communications Co, Formington, CT Distributed by
Global Information, 2005.
3. J. R. Hofmann, Andr
´
e-Marie Amp
`
ere: Enlightenment and Electrodynamics, Cambridge Univer-
sity Press, New York, 1996.
4. G. Pancaldi, Volta: Science and Culture in the Age of Enlightenment, Princeton University Press,
Princeton, NJ, 2005.
5. T. L. Brown and H. E. LeMay, Chemistry, 4th ed., Prentice-Hall, Englewood Cliffs, NJ, 1988.
6. A. J. Bard and L. R. Falkner, Electrochemical Methods, Fundamentals and Applications, 2nd
ed., Wiley, New York, 2001.
7. W. Feldenkirchen, Werner Von Siemens: Inventor and International Entrepreneur,OhioState
University Press, Columbus, OH, 1994.
8. J. S. Newman, Electrochemical Systems, 2nd ed., Prentice-Hall, Englewood Cliffs, NJ, 1991.
9. G. Prentice, Electrochemical Engineering Principles , Prentice-Hall, Englewood Cliffs, NJ, 1991.
10. D. Thompsett, “Pt Alloys as Oxygen Reduction Catalysts,” in Handbook of Fuel Cells—
Fundamentals, Technology and Applications,Vol.3, W. Vielstich, A. Lamm, and H. A. Gasteiger,
Eds., Wiley, New York, 2003, pp. 467–480.

c03 JWPR067-Mench December 18, 2007 1:59 Char Count=
3
Thermodynamics of Fuel
Cell Systems
I believe fuel cell vehicles will finally end the hundred-year reign of
the internal combustion engine as the dominant source of power for
personal transportation. It’s going to be a winning situation all the
way around—consumers will get an efficient power source, com-
munities will get zero emissions, and automakers will get another
major business opportunity—a growth opportunity.
—William C. Ford, Jr., Ford Chairman, International Auto
Show, January 2000
3.1 PHYSICAL NATURE OF THERMODYNAMIC
VARIABLES
Thermodynamics is the study of equilibrium at a macroscopic level. When a system is
in mechanical equilibrium, there is no net force imbalance that causes motion. Complete
thermodynamic equilibrium is more extensive and requires not only mechanical equilibrium
but also thermal, phase, and chemical equilibrium. We can use classical thermodynamics
to analyze chemically reacting and nonequilibrium flows, such as those in fuel cells, but
are restricted to only the quasi-equilibrium beginning and end states of the process, with
no details of the reaction itself. Thermodynamics can tell us the potential for reaction
and direction of spontaneous reaction, but not how fast the reaction will occur. Classical
thermodynamics also assumes a continuous fluid, meaning that there are enough molecules
of a substance to yield accurate values of thermodynamic variables like pressure and
temperature. As such, classical thermodynamics is generally inappropriate for use with
microscopic-level molecular charge transfer processes and electrochemical reactions.
In this chapter, the fundamentals of classical thermodynamics as it applies to the study
of fuel cells is introduced. Although the reader is assumed to have a background in basic
thermodynamics, this chapter includes a review of the physical meaning of several param-
eters used frequently in electrochemistry and how calculations of their values can be made.
This chapter concludes by applying the thermodynamic concepts presented to determine
the maximum expected thermodynamic efficiency and open-circuit voltage expected for a
fuel cell at a given condition.
62
Fuel Cell Engines
Copyright © 2008 by John Wiley & Sons, Inc.
Matthew M. Mench
c03 JWPR067-Mench December 18, 2007 1:59 Char Count=
3.1 Physical Nature of Thermodynamic Variables 63
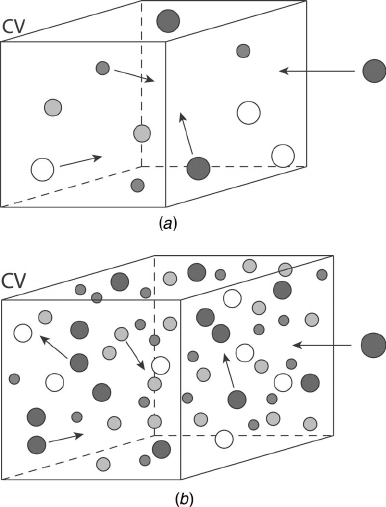
Figure 3.1 Concept of temperature and a continuum: (a) continuum assumption not valid;
(b) continuum assumption valid. CV = control volume.
3.1.1 Physical Meaning of Parameters
Temperature (T) Temperature is a thermodynamic parameter that everyone has common
experience with, yet many people have no concept of what temperature physically repre-
sents. Temperature is a measure of the mean kinetic energy of the continuum of molecules
being measured. For an individual molecule, temperature has little physical meaning in a
macroscopic sense (imagine trying to measure the temperature of an individual molecule).
Consider a very small box representing the space of interest, as in Figure 3.1. When the
box is very small (Figure 3.1a), only a few molecules travel in and out of it, and any mea-
sure of temperature would be erratic and unsteady, as the number of molecules in the box
changes with time. Now consider that the box is large enough so that the average number
of molecules in the box remains constant over time, which is the continuum assumption,
illustrated in Figure 3.1b. In this case, a measurement of the average kinetic energy of the
molecules is a meaningful quantity represented by the temperature.
Pressure (P) Pressure is similar to temperature in that, from a macroscopic perspective,
there is no physical meaning for pressure of an individual molecule. For a continuous
mixture, pressure is a measure of the molecular momentum transfer from collision on the
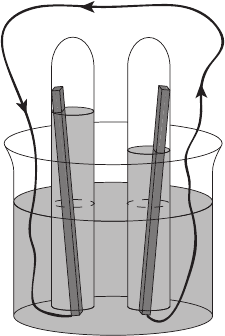
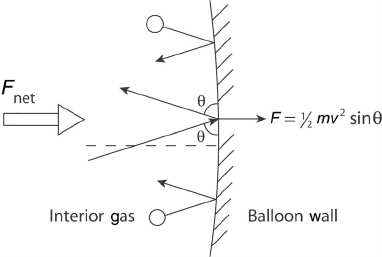
plane of measurement. Consider a balloon filled with helium, as in Figure 3.2. To expand
the balloon against the restraining force of the elastic balloon material, there must be an
internal pressure greater than the atmospheric pressure. At the balloon’s internal surface,
the molecules are colliding and reflecting off of the wall. Since the internal pressure must
c03 JWPR067-Mench December 18, 2007 1:59 Char Count=
64 Thermodynamics of Fuel Cell Systems
Figure 3.2 Molecular collision and concept of pressure at a balloon wall.
be greater (or the balloon would not inflate), either there is a greater density of molecules
colliding on the inside of the balloon than the outside, or the average momentum of the
molecules inside is larger, which corresponds to a higher temperature. This also explains
why pressure is linearly related to temperature and the number of moles in the ideal gas
equation of state (EOS):
PV = nR
u
T (3.1)
where n is the number of moles of the mixture, P is the absolute pressure, R
u
is the universal
gas constant (8.314 kJ/kmol·K), V is the system volume, and T is the absolute temperature.
Nonideal behavior Although much of the thermodynamic state behavior for the gas-
phase species in fuel cells can be well approximated with the ideal gas law, it is important
to realize that Eq. (3.1) is not a perfect representation of the true physics, and there are
some applications pertinent to fuel cells where the assumption of ideal gas behavior is not
accurate. The ideal gas law assumes that (1) there are no net intermolecular interaction
forces and (2) the volume of the molecules is very small relative to the volume of the
containment. While these assumptions are accurate at low pressure and high temperature
(where density is low), they are not accurate at low temperature and high pressure, or
near the critical point of a substance. Obviously, ideal gas behavior is not appropriate in a
two-phase regime or to describe condensing or vaporizing water in a low-temperature fuel
cell. Thermodynamic parameters for the water vapor in mixtures should generally be taken
from thermodynamic steam tables, except at very low vapor pressure with no phase change
where ideal gas behavior can sometimes be used. In fact, tabulated thermodynamic data are
always preferred to empirical or semiempirical correlations or generalized charts. Another
application where the assumption of the ideal gas law is not perfectly accurate is at the high
pressures used for fuel or oxidizer storage, where the intermolecular interactions and finite
molecular volume can become significant.
There are many methods to correct for nonideal gas behavior, including use of empiri-
cally or semiempirically modified EOSs. Actually, hundreds of EOSs have been developed
to describe the pressure–density–temperature relation for a wide variety of gas-, liquid-, and
solid-phase substances. For additional background, the reader is referred to a fundamental
thermodynamics textbook [e.g., 1]. An early attempt to improve the ideal gas EOS was
c03 JWPR067-Mench December 18, 2007 1:59 Char Count=
3.1 Physical Nature of Thermodynamic Variables 65
proposed by Johannes Diderik van der Waals in 1873. Van der Waals was awarded the 1910
Nobel Prize in Physics for his work, which can also be applied to compressible fluids with
modification. For gases, the van der Waals EOS is shown as
P =
R
u
T
v − b
−
a
v
2
(3.2)
where v is the molar specific volume, V/n, and n is the number of moles. The van der Waals
EOS achieves a higher accuracy than the ideal gas law because the two constants a and
b correct for the main assumptions implicit in the ideal gas law. The positive constant a
represents the net intermolecular attractive forces and therefore acts to reduce the effective
pressure in Eq. (3.2). Theoretically, this constant can be shown to be [2]
a =
27
64
R
2
u
T
2
c
P
c
(3.3)
where T
c
and P
c
are the critical temperature and pressure, respectively. The constant b
accounts for the finite volume of the molecules; thus it is subtracted from the molar specific
volume of the system. Theoretically, this can be shown to be [2]
b =
R
u
8
T
c
P
c
(3.4)
Table 3.1 provides some theoretical correction factors for the van der Waals EOS, calculated
based on the critical-point data. Although inconvenient to use, improved accuracy can be
achieved by using empirically derived correction factors, rather than the theoretical values
determined from Eqs. (3.3) and (3.4). Such data are available for many species but are
rarely, if ever, needed in the study of fuel cells.
While the van der Waals EOS has improved accuracy compared to the ideal gas law and
is historically quite important, it is not frequently utilized because more accurate approxi-
mations are now available, especially for behavior near the critical point. Other approaches
include two or more parameters that are empirically defined by fitting experimental data,
or the so-called virial EOSs, which have a series expansion form with coefficients based on
molecular theory, statistical mechanics, or experimental data.
Table 3.1 Van der Waals EOS Coefficients for Various Species Calculated from Critical-Point Data
Force Parameter, a Volume Parameter, b
Species Formula [kPa · (m
3
/kmol)
2
](m
3
/kmol)
Hydrogen H
2
24.73 0.02654
Oxygen O
2
136.95 0.03169
Water vapor H
2
O
g
553.12 0.03045
Carbon dioxide CO
2
364.68 0.04275
Nitrogen N
2
136.57 0.03863
Air Mixture property 136.83 0.03666
Methanol CH
3
OH 965.32 0.06706
Methane CH
4
229.27 0.04278
c03 JWPR067-Mench December 18, 2007 1:59 Char Count=
66 Thermodynamics of Fuel Cell Systems
Perhaps the simplest approach to EOS modification is that of a compressibility factor
Z. Here, the ideal gas EOS is modified with a compressibility factor:
Pv
nR
u
T
= Z (3.5)
Obviously, for a true ideal gas, Z = 1. Analytical expressions for Z can be derived as a func-
tion of various parameters, based on the van der Waals correction factors or those in various
other EOS formulations. Compressibility charts for many species have been experimentally
generated and can be used to estimate the compressibility factor. For a wide variety of gas-
phase species such as air and water vapor, the compressibility factor follows a consistent
behavior when correlated by the reduced pressure (P
r
) and reduced temperature (T
r
):
P
r
=
P
P
c
T
r
=
T
T
c
(3.6)
where P
c
and T
c
are the critical pressure and temperature, respectively. This behavior is
known as the law of corresponding states, although it is not really a law of nature. Many
species follow this relationship, and the ideal gas correction factor can be represented on a
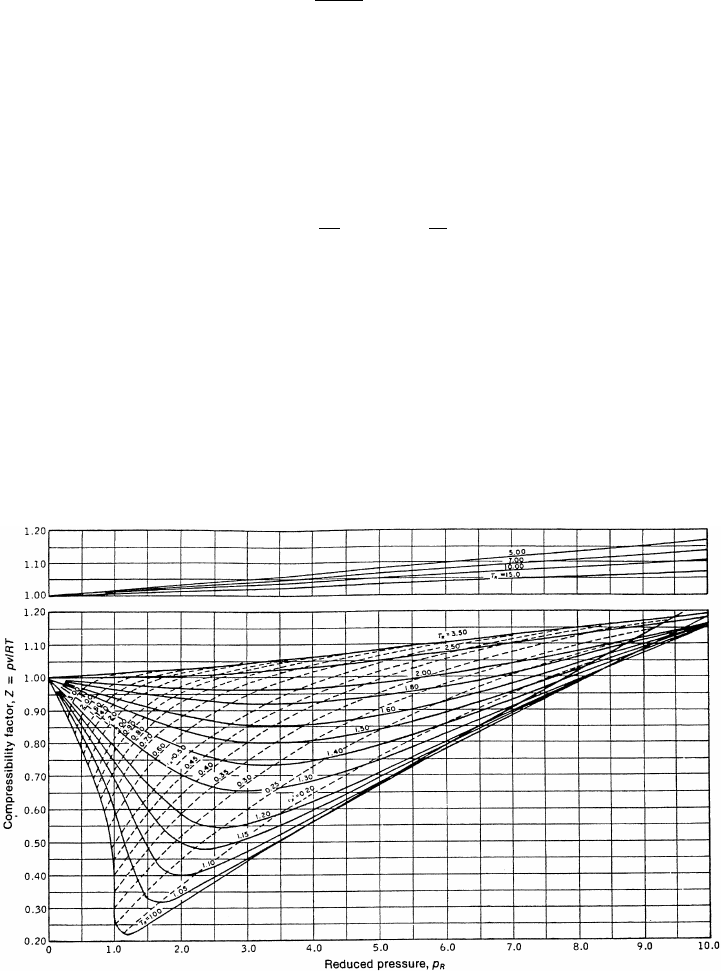
generalized compressibility chart. Figure 3.3 is a generalized compressibility chart that can
be used in lieu of species-specific data and provides a good estimate of the compressibility
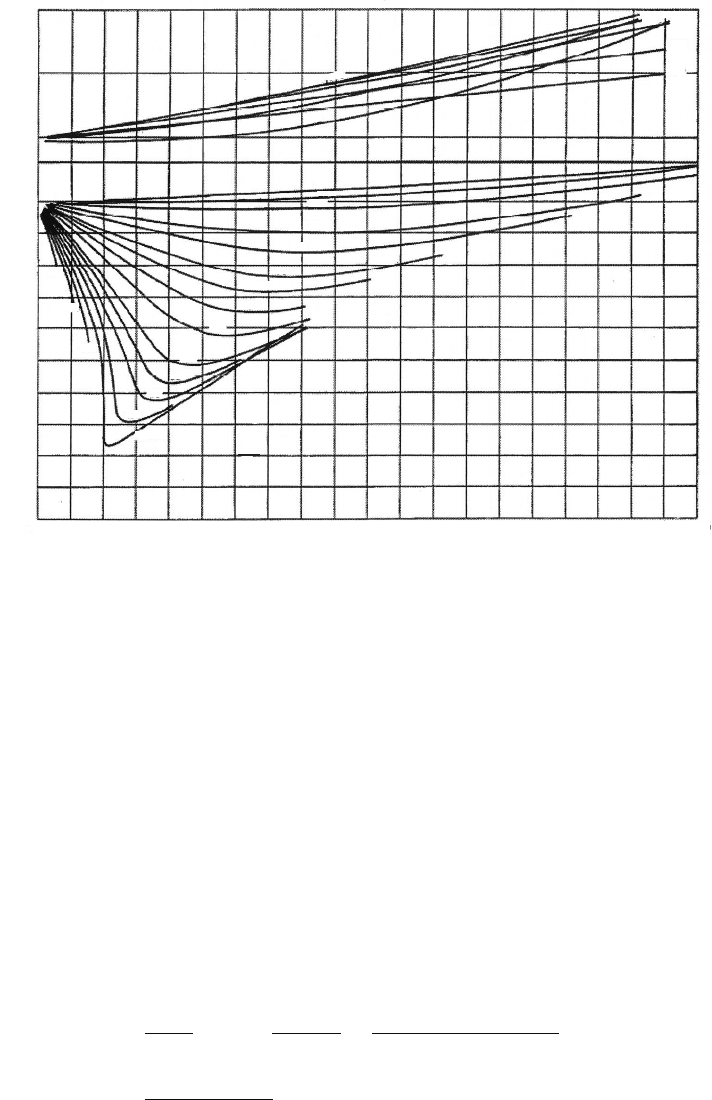
factor. For hydrogen and some noble gases like helium, which do not follow the generalized
compressibility chart trends well, a specific chart based on measured data should be used.
Figure 3.4 is a hydrogen-specific compressibility chart. If a specific chart is not available,
hydrogen and other noble gases can be approximated with a generalized compressibility
Figure 3.3 Generalized compressibility chart. (Reproduced from E. F. Obert, Concepts of Ther-
modynamics, McGraw-Hill, New York, 1960.)
c03 JWPR067-Mench December 18, 2007 1:59 Char Count=
3.1 Physical Nature of Thermodynamic Variables 67
Reduced Pressure, P
r
4.0
3.5
3.02.5
2.01.5
1.0
0.50
0
0.1
0.3
0.4
0.5
0.6
0.7
0.50
0.90
0.95
T
R
=100
105
110
115
120
130
140
150
160
180
200
250
300
300
400
600
500
300
700
1000
500
350
400
500
0.8
0.9
1.0
1.00
Compressibility Factor, Z
1.05
1.10
4.5 5.0 5.5 6.0
6.5 7.0
7.5
8.58.0 9.5 10.09.0
Figure 3.4 Experimentally measured hydrogen compressibility chart. (Reproduced with permis-
sion from [3]. Copyright American Chemical Society.)
chart if 8 K and 8 atm are added to the critical temperature and pressure used in Eq. (3.6)
respectively. Although more accurate, this is a rather arbitrary empirical correction and
only appropriate over a limited range [3].
Example 3.1 Hydrogen Storage Volume Consider a hydrogen tank storage system for
a fuel cell automobile. Seven kilograms of hydrogen gas compressed to 68 MPa (approxi-
mately 10,000 psig) and stored at 20
◦
C is required to provide a driving range of about 480 km
(approximately 300 miles). Using the ideal gas law, the van der Waals EOS, and the gen-
eralized compressibility chart, determine the interior volume required for the hydrogen
storage tanks.
SOLUTION (a) Ideal gas law EOS:
V =
nR
u
T
P
⇒ V =
mR
u
T
MW
H
2
P
=
kg · N · m/kmol · K · K
kg/kmol · N/m
2
= m
3
=
7 × 8314 × 293
2 × 68,000,000
= 0.1254 m
3
c03 JWPR067-Mench December 18, 2007 1:59 Char Count=
68 Thermodynamics of Fuel Cell Systems
(b) Van der Waals EOS: We first need to rearrange Eq (3.2) to suit the problem, which
becomes a cubic expression in V:
P
n
3
V
3
+
−
Pb
n
2
−
R
u
T
n
2
V
2
+
a
n
V − ab = 0
where n is the number of moles, which in this case is 3.5 kmol.
For hydrogen, we look up T
c
= 33.2 K from the Appendix and P
c
= 1.3 MPa. From
Eq. (3.3)
a =
27
64
R
2
u
T
2
c
P
c
(N · m/kmol · K) · K
N/m
2
=
27
64
8,314
2
33.2
2
1,300,000
= 24,725 N · m
4
/kmol
2
The constant b is defined in Eq. (3.4):
b =
R
u
8
T
c
P
c
=
8314 × 33.2
8 × 1,300,000
= 0.02654 m
3
/kmol
Solving the cubic expression we find V = 0.2064 m
3
.
This result is substantially higher than using the ideal gas assumption due to the
intermolecular attractive forces and finite molecular volume effects.
(c) Generalized compressibility chart: Here, we use the generalized compressibility
chart with the 8 K and 8 atm correction for hydrogen along with some unit conversion
(1 MPa = 9.869 atm):
P
r
=
671.1atm
12.83 atm + 8atm
= 32.21 T
r
=
T
T
c
=
293 K
33.2K+ 8K
= 7.111
From the generalized compressibility chart, we see that Z ∼ 1.42. Then
V = Z
nR
u
T
P
⇒ V = Z
mR
u
T
MW
H
2
P
=
kg · N · m/kmol · K · K
kg/kmol · N/m
2
= 1.42 ×
7 × 8314 × 293
2 × 68,000,000
= 0.178 m
3
If we had not used the correction, we would have found that
P
r
=
671.1atm
12.83 atm
= 52.31 T
r
=
T
T
c
=
293 K
33.2K
= 8.83
and we could extrapolate Z to be ∼1.58 from the generalized compressibility chart:
V = 1.58 ×
(7 kg)(8314 N · m/kmol · K)(293 K)
(2 kg/kmol)(68,000,000 N/m
2
)
= 0.198 m
3
The most accurate result would be from the hydrogen-specific compressibility chart in
Figure 3.4. We would have found that
P
r
=
671.1atm
12.83 atm
= 52.31 T
r
=
T
T
c
=
293 K
33.2K
= 8.83

c03 JWPR067-Mench December 18, 2007 1:59 Char Count=
3.1 Physical Nature of Thermodynamic Variables 69
Z ∼ 1.4
V = 1.4 ×
(7 kg)(8314 N · m/kmol · K)(293 K)
(2 kg/kmol)(68,000,000 N/m
2
)
= 0.176 m
3
COMMENTS: Note that there is significant deviation for each approach. Of them, the
hydrogen-specific compressibility chart should be the most accurate since it is based on
directly measured experimental data. The corrected generalized compressibility chart values
were also quite close. Considering the vehicle range is directly related to the mass of fuel
that can be stored, use of the ideal gas law alone would greatly underestimate the storage
volume required, by almost 30% in this case.
If the hydrogen were stored in a liquid form, the tank volume could be decreased, but
insulation would be needed to prevent excessive boil-off. The ideal gas law would definitely
not be appropriate for liquid H
2
storage, and direct saturated and liquid thermodynamic
data tables for hydrogen would be appropriate.
Internal Energy (U) We should first distinguish the nomenclature used in this text for the
intensive (mass-related) variables, including internal energy. For these variables, a capital
letter refers to the absolute value of the parameter of interest. For example, U, the internal
energy, is taken to be in units of energy, or joules. A lowercase parameter represents a
mass-intensive quantity. For example, u represents the internal energy per unit mass in
kilojoules per kilogram. An overscored lowercase letter is representative of a molar specific
quantity. Therefore
¯
u represents the internal energy in kilojoules per kilomole.
Internal energy U is a macroscopic measure of the total thermal energy stored in a
thermodynamic system. Considering a closed container with gas as our system, we need to
understand the way thermal energy can be stored in the gas-phase constituents to understand
the concept of total internal energy. From the first law of thermodynamics for the system,
the internal energy can be converted into potential energy or kinetic energy or transferred
out of the system as heat or as work:
dE = dKE +dPE + dU = δ Q − δW (3.7)
Where dE is the change in energy of the system and dPE and dKE represent the change in
potential and kinetic energy of the system, respectively. The internal energy U is a measure
of the system total thermal energy stored in the individual, nonreacting species at the given
temperature and pressure condition.
In individual molecules, the internal energy can be stored in the following ways:
Ĺ Translational Kinetic Energy This is different from the kinetic energy of the system
in Eq. (3.7), which is a measure of the change in kinetic energy of the system as a
whole. As an example, consider a stationary container of gas. There is no change in
the kinetic energy of the system (it is zero); however, the individual molecules can
store energy in the form of their translational motion. The thermodynamic measure
of this form of energy is the temperature.
Ĺ Vibrational Motion A molecule can store energy via the oscillation of the bond
distance between atoms. The more bonds in a molecule, the greater the vibrational
motion contribution to the total stored energy. A monatomic species such as argon
has no bonds and therefore cannot store energy in this form.
