Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

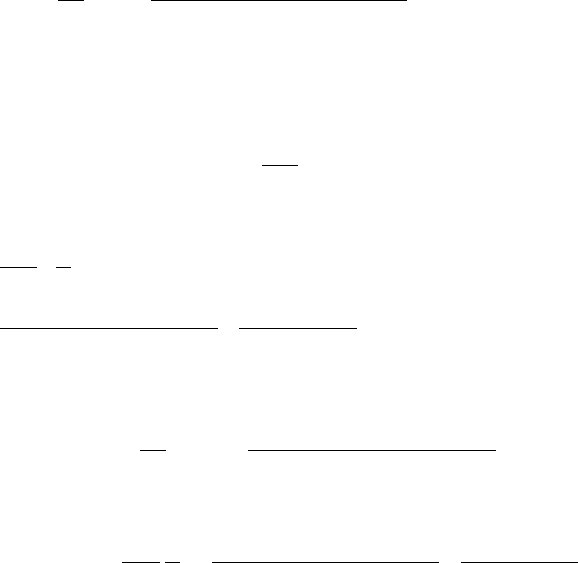
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
50 Basic Electrochemical Principles
SOLUTION (a) First we need to solve for the total hydrogen required to provide three
days of power: From Faraday’s law for the anode on pure hydrogen
˙
n
H
2
= λ
a
iA
nF
= 2 ×
(0.6A/cm
2
)(20 cm
2
)
(2 e
−
eq)/mol H
2
)(96,485 C/eq)
×(3600 s/h)(24 h/day)(3 days) = 32.25 mol H
2
consumed
(32.25 mol)(2 g mol) = 64.5gH
2
From the ideal gas law
PV = m
R
u
MW
T
For the hydrogen
V =
mR
u
MW
·
T
P
=
(64.5g)[8.314 J/(mol ·K)]
2g/mol
298 K
20,260,000 Pa
= 0.0039 m
3
= 3.9L
(b) Following along the same methodology for the cathode side,
˙
n
O
2
= λ
c
iA
nF
= 2.3 ×
(0.6A/cm
2
)(20 cm
2
)
(4 e
−
eq/mol O
2
)(96,485 C/eq)
×(3600 s/h)(24 h/day)(3 day) = 18.51 mol O
2
consumed
(18.51 mol)(32 g/mol) = 592.2gO
2
V =
mR
u
MW
T
P
=
(592.2 g)[8.314 J/(mol · K)]
32 g/mol
298 K
20,260,000 Pa
= 0.002265 m
3
= 2.27 L
COMMENTS: Note these results are for a very low power (9-W) system. Around 10–20
W is needed for many portable applications, so the storage volume would greatly increase.
Three days of power without a recharge in a portable device is quite difficult to achieve.
Storage volume could be improved with increased performance, higher pressure storage
fuel recirculation, or alternate storage techniques, such as use of liquid fuel.
2.6 THE GENERIC FUEL CELL
Now that the basics of electrochemical reactions are known, we can begin discussion of
basic fuel cell operation. A generic fuel cell is shown in Figure 2.9 with regions labeled A
to H. Each variety of fuel cell has unique materials, structure, and design features, but at a
basic level, all can be reduced to this generic design. Note that the basic components are the
same as shown in Figure 2.9. To reduce ionic and electronic resistive losses and increase
power density, the components in a fuel cell are designed to have the smallest possible path
length for ions and electrons.
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
2.6 The Generic Fuel Cell 51
Figure 2.9 Generic fuel cell.
For a single cell, component A in Figure 2.9 is known as the anodic current collector
when the flow is a fuel. In a normal stack arrangement, where the anode current collector is
also the cathode current collector on the opposing side, this is also known as a bipolar plate
or cell interconnect, since it connects the anode and cathode of adjacent cells in series. The
current collector functions as follows:
1. Conducts electrons from anode B to the external circuit or to the adjacent cathode
in a stack.
2. Delivers fuel (liquid or gas) flow through the flow channels labeled “B”. The fuel
diffuses or convects
4
to the anode electrode C, where fuel oxidation occurs.
3. Provides structural integrity of stack (in most, but not all, designs).
4. Dissipate waste heat generated by inefficiencies of the reaction to constant, often
with a coolant flow through the current collector.
Current collector materials for fuel cell stacks of all varieties must satisfy the following
requirements:
1. Lightweight, compact and highly robust.
2. Low-cost raw material and manufacturing process.
3. High electrical conductivity over the expected lifetime of operation.
4. High corrosion resistance in oxidizing and reducing environments.
5. Impermeability to fuel and oxidant flow.
6. No disintegration of material or electrical degradation over lifetime of operation.
7. Suitable thermal expansion properties, which is more of a concern for higher tem-
perature fuel cells.
8. Capable of proper sealing of reactant flow to prevent leakage. This is often accom-
plished with gaskets around the periphery to the cell plate.
4
Diffusion and convection are specific modes of mass transport. If the reader is unfamiliar with these, a quick
survey of Chapter 5 or an undergraduate mass transfer textbook is recommended for review.
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
52 Basic Electrochemical Principles
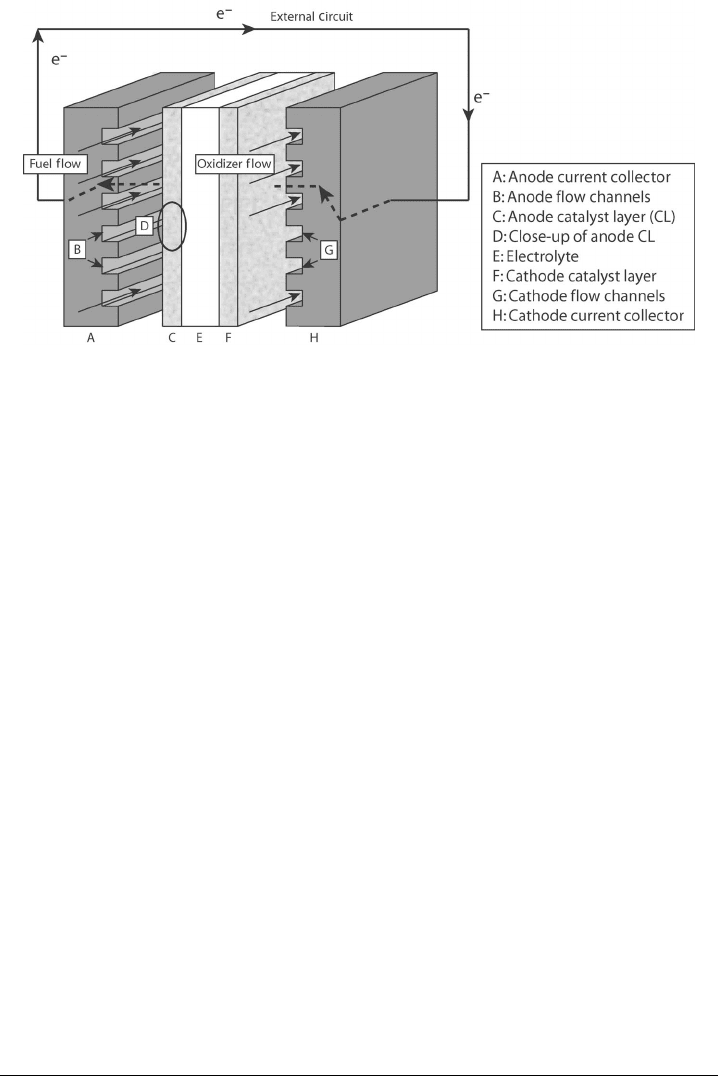
Figure 2.10 Examples of various flow field designs. (Images courtesy of Soowhan Kim.)
Current collector material and manufacturing engineering are active areas of research and
development. Presently, a variety of materials are used for bipolar plates in fuel cell stacks.
Specially coated metals, graphite, and doped polymers have been used for low-temperature
fuel cells. High-temperature fuel cells have primarily utilized ceramics for this purpose.
Component B in Figure 2.9 is known as the anodic flow field, or fuel flow field. It is
typically machined or formed directly in the current collector plate, although it can also be
a discrete part. The anode flow field main functions are to
1. facilitate transport of fuel to the anode and
2. facilitate removal of products of reaction.
For each variety of fuel cell, many different configurations for the flow fields have been
used to optimize heat and mass transfer, current collection, and so on. Because of the
highly coupled interaction between heat, mass, and electrochemical phenomena involved,
flow field design is not a straightforward matter. Basic flow field patterns include a simple
serpentine arrangement, a parallel arrangement, a parallel serpentine combination, and
others, as shown in Figure 2.10. A great deal of the engineering at the individual cell
level is based on obtaining the best possible flow field design to balance reactant and heat
transport, product removal, pressure drop, and machinability. Each design has particular
advantages and limitations that will be discussed in detail in later portions of this text.
Component C in Figure 2.9 is the anode electrode. The anode contains a thin region
of catalyst that greatly facilitates the electrochemical reaction. This region is often referred
to as the catalyst layer, although the region is much more complex than this name implies.
The anode is the location of the fuel oxidation reaction. The basic functions of the anode
catalyst layer are to
1. Enable the fuel oxidation reaction via a catalyst.
2. Conduct ions from the reaction site to the electrolyte region D.
3. Conduct electrons from the reaction site to the anode current collector A.
4. Facilitate reactant transport and product removal to and from the catalyst locations.

c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
2.6 The Generic Fuel Cell 53
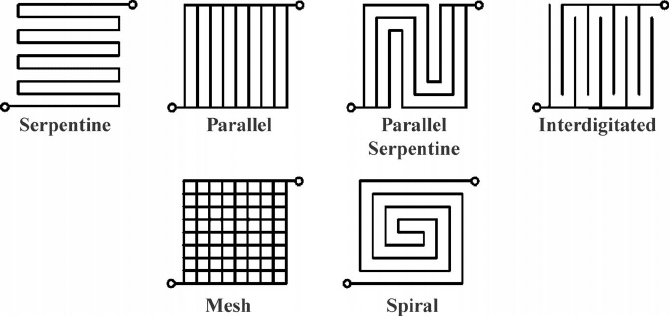
Figure 2.11 Transmission electron micrograph of PEFC catalyst layer [10].
Notice that the catalyst layer must have high electrical and ionic conductivity. The catalyst
enables the electrochemical reaction by providing a facilitated reaction site, and the fuel is
said to be “galvanically burned” at the anode. The catalyst layer is typically quite thin but
is porous and three-dimensional in nature, and is the only interface with reactant, catalyst,
and ion and electron conductors that enables a reaction.
Consider a microscopic view of the anode catalyst layer, labeled in Figure 2.9 as D
and shown in Figure 2.11. As discussed, both the anode and the cathode (labeled F) must
have a high degree of mixed ionic and electronic conductivity and porosity. To achieve
this, the catalyst layers are a highly three-dimensional porous structure consisting of the
catalyst, electrolyte, electron conductor, and voids for reactant transport. Note the high
relative porosity of this layer, typically around 40 to 70% for most fuel cells. The reaction
in this highly porous structure depends on the simultaneous presence of reactant, catalyst, an
ionic conductor with a continuous path to the main electrolyte, and an electronic conductor
with a continuous path to the current collector. This is shown schematically in Figure 2.12.
Although we conveniently use the geometric, or planform,
5
area of the electrode as the
superficial active area of the electrode for calculation of current density, the true active area
of the porous electrode available for reaction can be orders of magnitude larger due to the
three dimensional nature of the surface.
Traditionally, the highly porous nature of the electrodes is to maximize the concept of a
triple-phase boundary where reaction can occur between (1) the open pore for the reactant,
(2) the catalyst, and (3) the ionic conductor. Increasing the triple-phase boundary (TPB)
within the porous electrode structure leads to increased reaction site density for a given
superficial electrode area and therefore results in a higher performance electrode. In some
solid-phase electrode systems, the TPB concept is directly applicable, but in many systems
such as the PEFC, the concept of the TPB is not strictly accurate, because there is actually
5
The planform (geometric) area is the above view area.
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
54 Basic Electrochemical Principles
Figure 2.12 The simultaneous presence of reactant, catalyst, an ionic conductor with a continuous
path to the main electrolyte, and a continuous path of electrical conductivity is needed or a reaction
will not take place at a catalyst site. A catalyst rendered inactive by these situations is called an
“orphan” catalyst.
a thin film of electrolyte coating the catalyst structure that has a limited permeability to
the reactant. Thus, reaction can occur below the thin-film surface and is not strictly limited
to TPB locations. Nevertheless, the heuristic concept of a TPB is useful to understand the
engineering trade-offs in an electrode. If there is too little catalyst, or too much catalyst
isolated from the reactant (orphan catalyst), or insufficient pathways for ion or electron
transport exists, not enough reaction sites will be active and performance will suffer. If
the porosity is too low or the electrode is too thick, the reactant will not be as available
and performance will also suffer. Major losses in the catalyst layer can occur from lack
of electrical or ionic conductivity. Based on the parallel needs of high porosity and mixed
ionic and electronic conductivity, it is easy to understand that the catalyst structure is highly
complex, and there is a tenuous balance between the various phase distributions. Although
the structure is microscopic in nature, because the catalyst layer is typically manufactured
using macroscopic methods, such as tape casting, spray coating, or painting, there can be
a high number of orphan catalysts.
Proper catalyst selection is critical for optimal fuel cell performance. Low-temperature
fuel cells typically must utilize expensive noble metal catalysts such as platinum, which
has been an historical barrier in terms of cost. Since higher temperatures more readily
enable electrochemical reaction, less expensive catalysts such as nickel can be utilized for
high-temperature fuel cells such as the SOFC or MCFC.
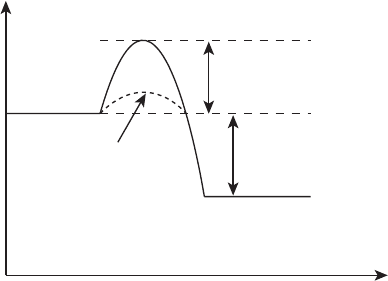
Consider a reaction coordinate plane for a given galvanic electrochemical reaction
shown in Figure 2.13. Even though the overall reaction is galvanic and will release elec-
trical energy in going from reactants to products, in order to proceed from an equilibrium
state of reactants to another equilibrium state of products, some activation energy is re-
quired to initiate a significant reaction. This is somewhat similar to a purely chemical
combustion reaction, where an ignition source is needed to initiate an exothermic reaction
from an initial nonreacting state. For an electrochemical reaction, this activation energy
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
2.6 The Generic Fuel Cell 55
Activation
energy
Energy
released
Energy
Stage of reaction
Path with
better catalyst
Total exothermic
release available
for work (electrical
or chemical)
Figure 2.13 Reaction plane for effective and ineffective catalysts.
lost is in the form of lost voltage potential. The more effective the catalyst, the lower the
activation energy barrier for reaction and the lower the voltage penalty. This activation
polarization loss is discussed in detail in Chapter 4. For now, consider this barrier as a mea-
sure of the quality of the electrode structure and materials in the promotion of the desired
reaction.
The catalyst layer must have a high degree of mixed conductivity for both electrons
and ions as well as a highly porous structure to promote reactant and product transport.
Ionic conduction in the catalyst layer is typically provided by addition of electrolyte in the
catalyst layer. This enables transport of ions through the catalyst layer to the main electrolyte
structure and to the opposing electrode. Without this ionically conductive material in the
catalyst layer, the circuit would not be complete. Concomitantly, if there were nothing
in the catalyst layer that conducted electrons, electron flow from the reaction site to the
current collector would not be possible. Electron conductivity is generally through catalyst
and other supporting materials.
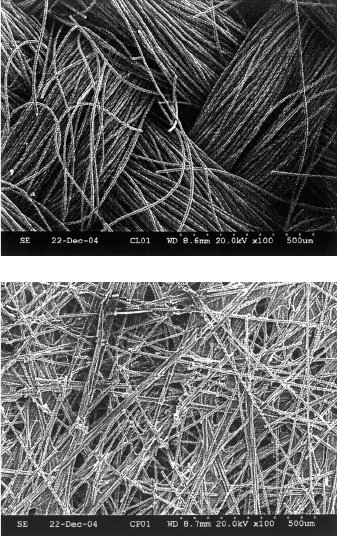
Several varieties of fuel cells use an electron-conducting porous DM as an interface
between the catalyst layer and the current collectors. This DM is not shown in Figure 2.9,
since it is not a universal feature of all fuel cells. For example, PEFCs use a carbon-based
porous media for this purpose, as shown in Figure 2.14. Either a woven carbon cloth or a
carbon fiber structure bonded with a graphitized thermoset resin is typically used for this
purpose. Alkaline fuel cells also use a similar porous media to aid electron conduction
between the porous electrodes and current collectors.
The electrolyte in a fuel cell (E in Figure 2.9) has three main purposes:
1. To physically separate the reactants.
2. To conduct the charge carrying ions from one electrode to the other.
3. To prevent electronic conduction between the anode and cathode.
The first purpose is unique to electrochemical reactions. The separation of reactants pro-
duces a thermodynamic activity difference between the anode and cathode that results in
the voltage potential difference. If there was air on both the anode and the cathode, there
would be no potential for reaction and no voltage difference between the two electrodes.
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
56 Basic Electrochemical Principles
Figure 2.14 Close-up scanning electron microscope images of (a) woven carbon cloth and (b)
nonwoven carbon paper bound with graphitized thermoset resin diffusion media used in PEFCs.
The second purpose is obvious but also includes a high resistance to flow of electrons. From
the basic circuit schematic of Figure 2.2, the electrolyte completes the flow of current by
conducting ions. However, the electrolyte must also be a strong electron insulator, or the
effect would be to short circuit the flow of electrons to the eternal circuit.
The cathode catalyst layer (F in Figure 2.9) is essentially the same function and purpose
as the anode catalyst layer; however the catalyst type and loading may be different than
that of the anode, as it is designed to promote the ORR. Additionally, the cathode flow field
and current collector, G and H, respectively, serve the same function on the cathode as the
anode, although the design may be different from that of the anode for a variety of reasons
discussed later in this text.
2.7 SUMMARY
The purpose of this chapter was to introduce the reader to the basics of electrochemical
reactions, provide a physical understanding of the basic parameters used in electrochemistry,
and introduce the general operation of a fuel cell. Future chapters will use this groundwork
to expand fundamental understanding and investigate the basic trade-offs in engineering
design. For an electrochemical reaction to take place, there must be an anode,acathode,
an electrolyte, and an external connection. Reactions producing and consuming electrical
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
2.7 Summary 57
energy are termed galvanic and electrolytic, respectively. Electrochemical reduction occurs
in a reaction that consumes electrons and electrochemical oxidation results in loss of
electrons from the reactant. The electrical current is the rate of the flow of charged species.
The total electrical charge passed is designated with the SI unit of coulomb.Thevolt
is defined as the work required to conduct one coulomb of charge. Electrical resistance,
measured in ohms, is a measure of the potential losses associated with moving a rate of
charge, and is not an intrinsic material property. Resistivity ρ and its inverse, conductivity,
are intrinsic properties of a material:
ρ =
RA
l
=
1
σ
Current, voltage, and resistance can be related through Ohm’s law in the absence of signif-
icant concentration gradients:
V = IR
Electrical power can be expressed as
P
e
= IV
Faraday’s constant F represents the charge per mole of equivalent electrons:
F =
6.023 × 10
23
electrons/mol eq
6.242 × 10
18
electrons/C
= 96,485 C/eq
The scaling factor n is defined as the number of electrons transferred per mole of species
of interest:
n =
number of electrons
mole of species of interest
=
eq
mol
Thus, the combination of nF is the charge passed per mole of specie of interest, and the
units of nF are coulombs per mole. Faraday’s second law of electrolysis can be written as
˙
n
x
=
iA
nF
=
I
nF
The Faradic efficiency is a measure of the percent utilization of reactant in a galvanic
process
ε
f
=
theoretical required rate of reactant supplied
actual rate of reactant supplied
Faradic efficiency is often called the fuel utilization efficiency (µ
f
) when applied to the fuel
in a galvanic redox reaction. The anode and cathode stoichiometries are defined as follows:
λ
c
=
actual rate of oxidizer delivered to cathode
theoretical rate of oxidizer required
λ
a
=
actual rate of fuel delivered to anode
theoretical rate of fuel required
c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
58 Basic Electrochemical Principles
In this chapter, the basic components, requirements, and functions of a generic fuel
cell were also discussed. The reader should be familiar with the functions of the current
collectors (also known as a bipolar plate or cell interconnect), the flow fields, the anodic
and cathode electrodes with the concept of the triple-phase boundary, and the electrolyte.
The reader should also understand the flow of current (ionic and electronic) through these
components.
APPLICATION STUDY: DESIGN OF FUEL CELL WITH
STORAGE TANKS AND TYPICAL
MATERIAL/PERFORMANCE PROPERTIES
In this assignment, you will design and compare various fuel storage systems for an
automotive fuel cell application. Assume you need 7 kg of hydrogen onboard to achieve
the desired range. There are several options to store hydrogen, including:
1. Liquefied hydrogen
2. Compressed gas-phase hydrogen
3. Stored as methanol in liquid form and reformed into hydrogen on-board
4. Stored in a metal hydride and released via heat addition
Find reliable sources online or in print and perform a feasibility study of the four options
listed above. Discuss in your report:
1. The volume and weight of the storage tanks required
2. The advantages and disadvantages of each design
3. How refueling would be accomplished in each option
Include the Internet resources consulted and copies of any reports or articles used in
preparation. Note that hydrogen storage is also discussed in Chapter 8.
PROBLEMS
Calculation/Short Answer Problems
2.1 Define the units of the following in terms of the most
basic SI units:
(a) Vo l t
(b) Ampere
(c) Ohm
(d) Faraday’s constant
(e) n (as in iA/nF)
2.2 Determine the theoretical open-circuit voltage of the
following fuel cells and determine which reactant would
be the oxidizer and which would be the fuel for a galvanic
reaction.
(a) Oxygen and hydrogen gas
(b) Lithium and oxygen gas
(c) Magnesium and oxygen gas
2.3 Determine the minimum theoretical open circuit volt-
age that would be required to generate hydrogen peroxide,
H
2
O
2
, with hydrogen gas and air.
2.4 Besides the desired hydrogen oxidation and oxygen re-
duction reactions, there are several other potential reactions
listed in Table 2.1 that can occur in a hydrogen/air fuel cell
stack (e.g., they only involve atomic hydrogen, oxygen, and
nitrogen species). List the potential reactions; then deter-
mine the theoretical voltage for these reactions and decide
if they could occur in a fuel cell or not. Could any of these
reactions occur normally? Note, the species besides H
2
,O
2
,
N
2
,andH
2
O must be generated and balanced by the over-
all reaction, so you will have to combine some reactions
to achieve this. Using your results, explain why the hydro-
gen oxidation and oxygen reduction reaction is in fact the

c02 JWPR067-Mench December 19, 2007 17:26 Char Count=
Problems 59
reaction that occurs, rather than other reactions along the
same potential series.
2.5 Demonstrate how Ohm’s law is consistent in units; for
example, show V = IR is self-consistent in terms of units.
2.6 Consider a 10-plate fuel cell stack at an anode stoi-
chiometry of 1.2 with 20 A current generated in the stack
and a stack voltage of 6.0 V. As an engineer, you have a
choice to install a recirculation pump to recycle the unused
hydrogen from the anode exhaust back into the anode to
increase the effective fuel utilization to 100%. However,
the pump required 60 W of parasitic power to operate con-
tinuously. Is installation of the pump justified? Explain. At
what value of parasitic power does the addition of the pump
become unjustified?
2.7 Consider a 300-plate fuel cell stack with 150 cm
2
active
area per plate:
(a) For an anode and a cathode stoichiometry of 1.4
and 2.5, respectively, determine the mass flow rate
of hydrogen and air into the fuel cell per ampere
of current.
(b) If the nominal operating point is an average of
0.6 V per plate with 1.2 A/cm
2
, determine the
stack voltage and electrical power output.
(c) How much total electrical work at 0.6 V per plate
could be performed with a storage tank contain-
ing 5 kg of hydrogen and limitless air? How much
more output could be achieved if the unused fuel
were recycled so that the effective fuel utilization
became 100%.
(d) Determine how many plates the fuel cell would
have to have at 0.6 V per plate, 1.2 A/cm
2
,to
generate 150 horsepower for an automotive appli-
cation.
2.8 A given fuel cell has continuous 150 A DC, an operat-
ing voltage of 0.55 V, and an overall internal resistance of
3m at 1.4 A/cm
2
current density. Calculate:
(a) The potential loss from ohmic resistance, in volts,
at this condition.
(b) The total electrical work produced in 2 h.
(c) The rate of ohmic heat dissipation from the cell in
watts.
2.9 Describe the concept of the TPB and how this is rele-
vant to fuel cell performance. A sketch will help.
2.10 Determine the single-pass fuel utilization efficiency
for a 150-plate fuel cell stack with 120 A current output
and a hydrogen flow rate through the stack of 0.2 g/s of
hydrogen.
2.11 We desire a fuel utilization efficiency of >95% on
the anode of a 300-plate, 100-cm
2
-active-area stack. Deter-
mine the hydrogen mass flow rate required in the stack as a
function of current density.
2.12 It is proposed to develop a fuel cell that runs directly
on propane (C
3
H
8g
) at a propane stoichiometry (λ
C
3
H
8
)of
2.5 and a cathode oxygen stoichiometry (λ
c
) of 2 [note the
cathode is running on air (79% N
2
, 21% O
2
by volume].
The cell operates at 0.3 V at a current density of 0.1 A/cm
2
.
The superficial active area of the cell is 25 cm
2
. The anode
electrochemical reaction is
(
C
3
H
8
)
g
+ 6
(
H
2
O
)
g
→ 20H
+
+ 20e
−
+ 3CO
2
The basic cathode electrochemical reaction is
O
2
+ 4e
−
+ 4H
+
→ 2H
2
O
The balanced overall electrochemical reaction is thus
(
C
3
H
8
)
g
+ 5O
2
→ 4H
2
O +3CO
2
with the following molecular weights:
C
3
H
8
44 g/mol
H
2
018g/mol
O
2
32 g/mol
air 28.85 g/mol
CO
2
28 g/mol
(a) Is the overall cell producing or consuming water—
at what rate in moles per second?
(b) What is the actual supply rate of air at the cathode
in grams per hour?
2.13 Consider a direct methanol fuel cell with a liquid
methanol and water solution in the anode and an air cath-
ode. The anode electrochemical oxidation reaction is
CH
3
OH +H
2
O → 6H
+
+ 6e
−
+ CO
2
The basic cathode electrochemical reduction reaction is
O
2
+ 4e
−
+ 4H
+
→ 2H
2
O
The balanced overall electrochemical reaction is
CH
3
OH +
3
2
O
2
→ 2H
2
O + CO
2
